Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula Roth. Seedlings Subjected to Defoliation
Abstract
:1. Introduction
2. Material and Methods
2.1. Growth of Plant Material and General Experiment Design
2.2. Inoculation of Plants with Pathogen Isolates
2.2.1. Phytophthora Cactorum
2.2.2. Armillaria Gallica
2.3. Verification of the Seedling Infection
2.4. Defoliation of Birch Seedlings
2.5. Evaluation of Birch Health Status
2.6. Measurement of Plant Response to Stress
2.6.1. Biometric Parameters
2.6.2. Fluorometric Analysis of Chlorophyll a
2.7. Secondary Metabolites Contained in Birch Leaves and Roots
2.8. Statistical Analysis
3. Results
3.1. Detection of P. cactorum and A. gallica
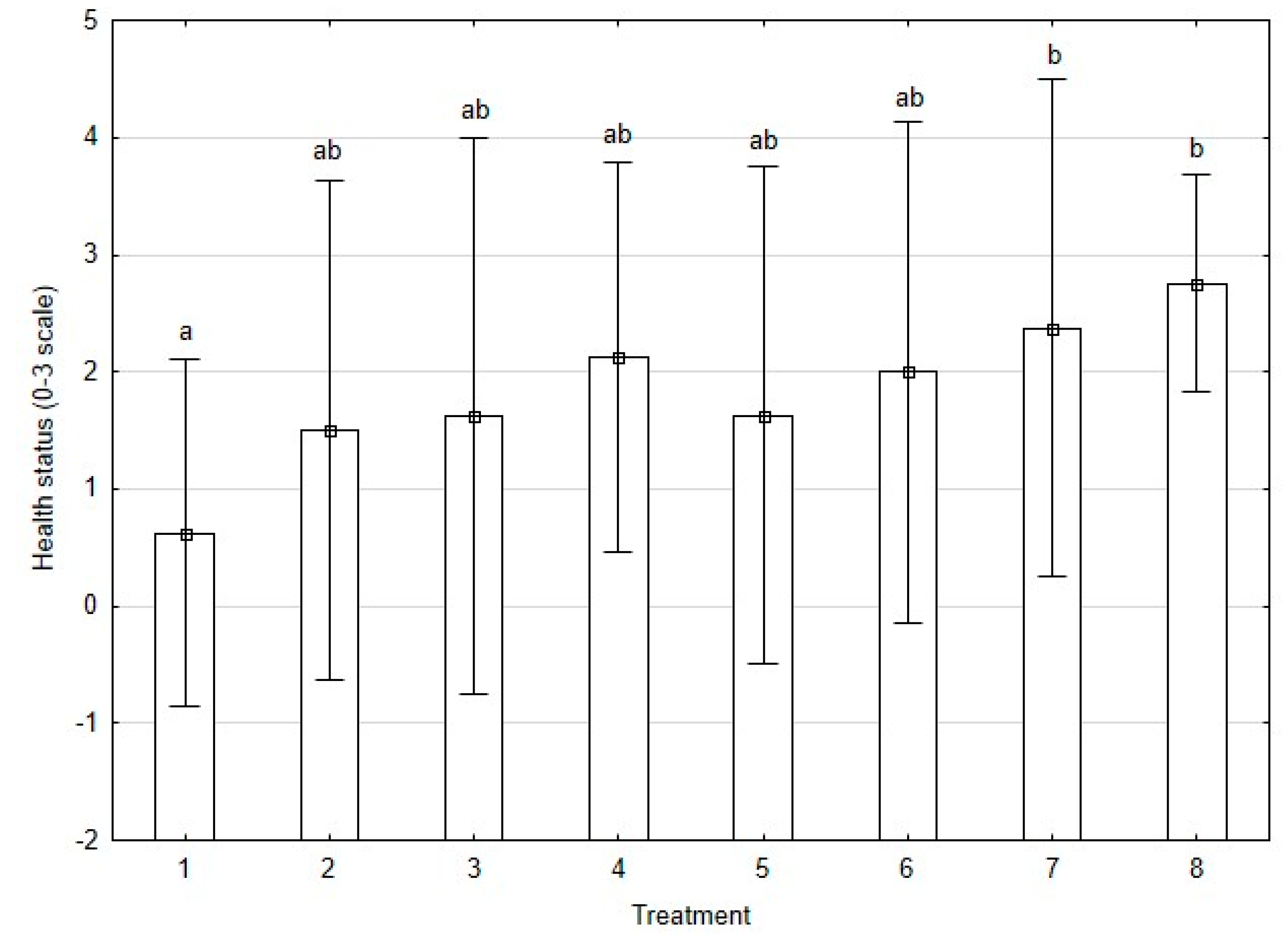
3.2. Health Status Assessment of Plants
3.3. Plants Response to Stress
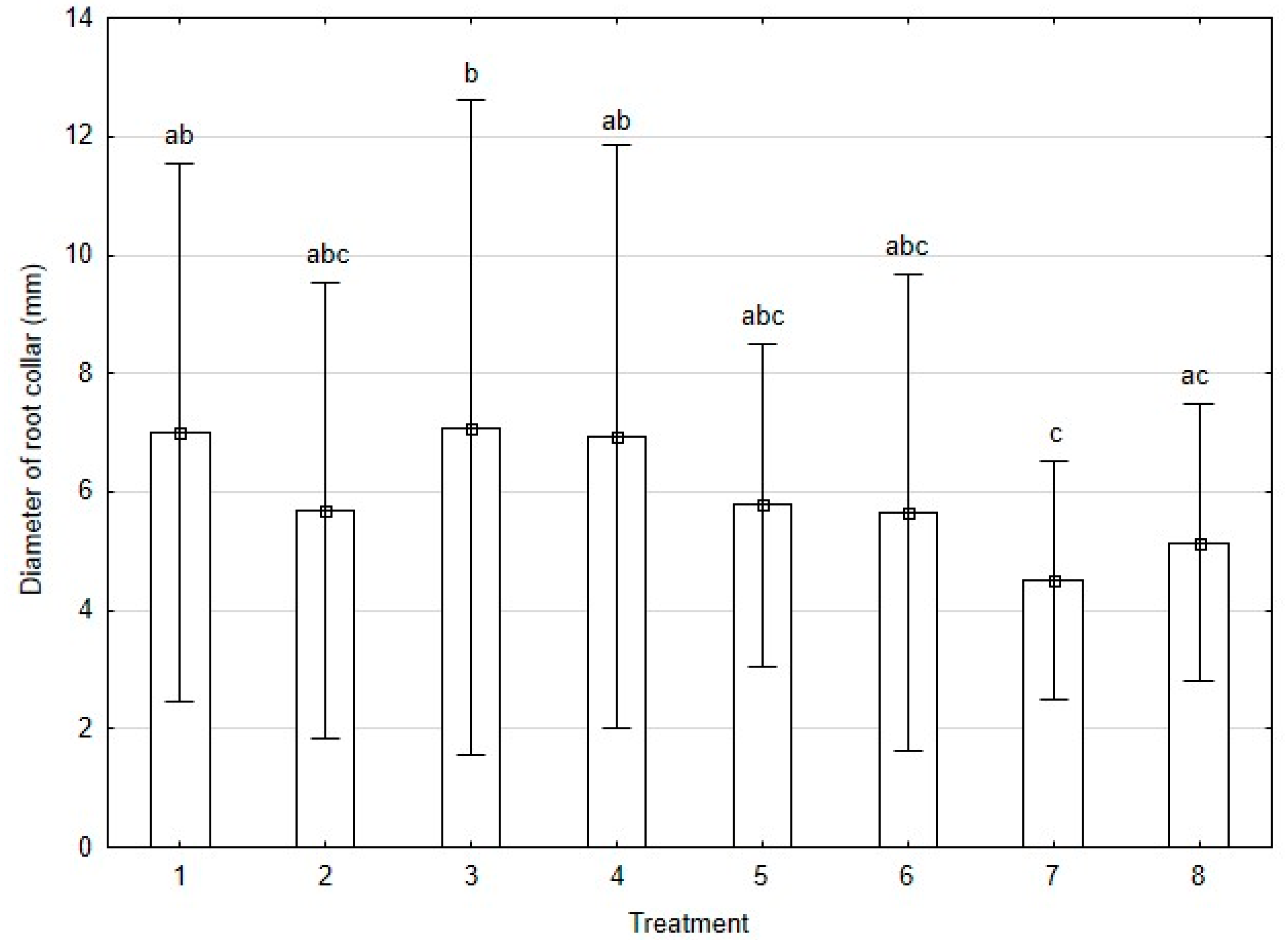
3.3.1. Growth Parameters
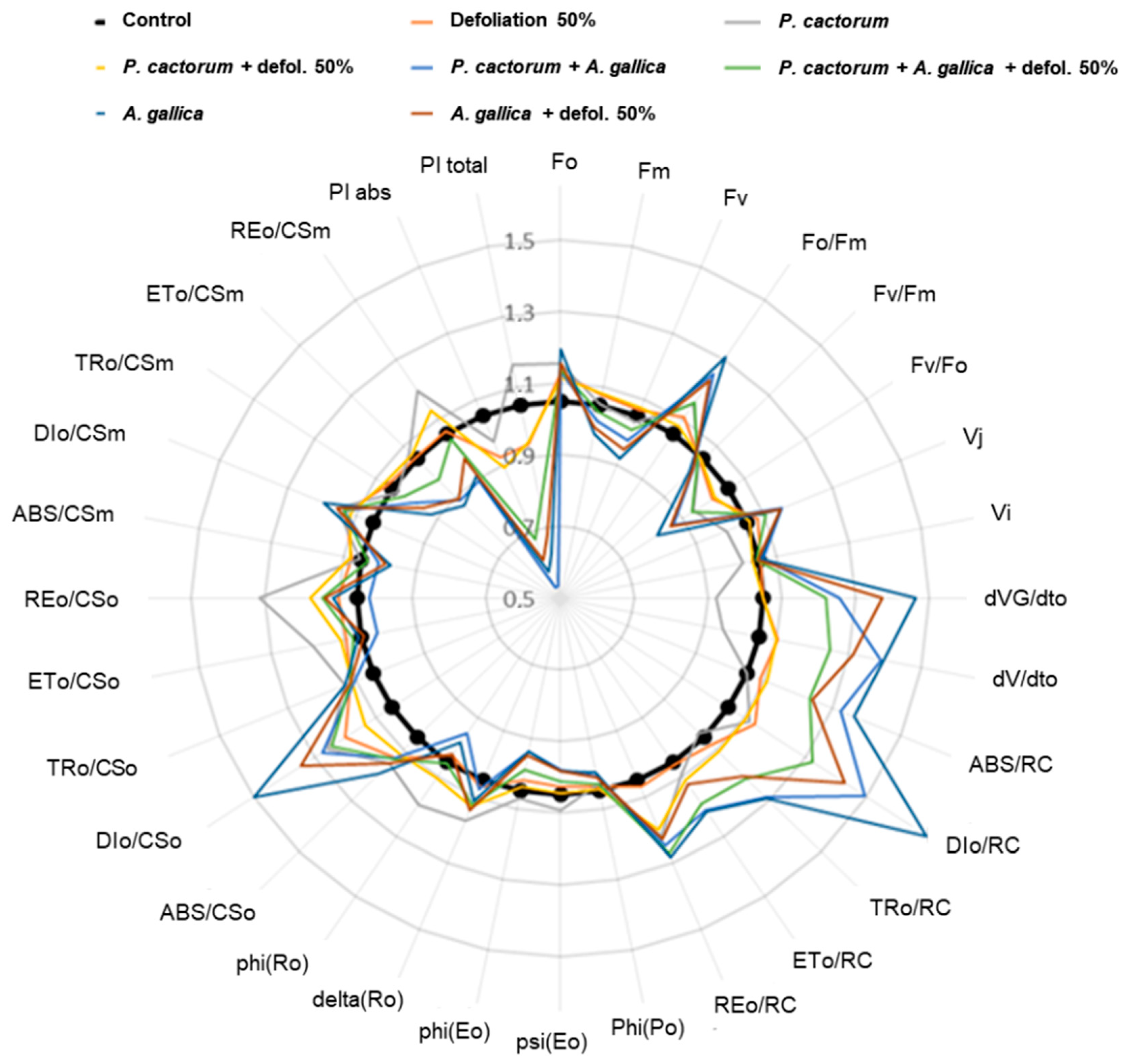
3.3.2. Chlorophyll Fluorescence
3.4. Secondary Metabolites of Leaves and Roots
4. Discussion
4.1. Birch Damage Caused by P. cactorum and A. gallica
4.2. Birch Damage (Defoliation) Caused by Insect Pests
4.3. Effects of Stress Factors on Physiological Response of Plants
4.3.1. Root Status of Treated Plants
4.3.2. Photosynthetic Activity
4.4. Effects of Stress Factors on Host Chemical Compound Production
5. Summing up and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rishbeth, J. Armillaria in an ancient broadleaved woodland. Eur. J. Plant. Pathol. 1991, 21, 238–249. [Google Scholar] [CrossRef]
- Keča, N.; Koufakis, I.; Dietershagen, J.; Nowakowska, J.A.; Oszako, T. European oak decline phenomenon in relation to climatic changes. Folia For. Pol. Series A 2016, 58, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.M. Phytophthora Biodiversity: How Many Phytophthora Species Are There? In Phytophthoras in Forests and Natural Ecosystems, Proceedings of the Fourth Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09; Monterey: Forest Service, CA, USA, 2009; pp. 101–115. [Google Scholar]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef] [Green Version]
- Aguayo, J.; Elegbede, F.; Husson, C.; Saintonge, F.X.; Marçais, B. Modeling climate impact on an emerging disease, the Phytophthora alni-induced alder decline. Glob. Chang. Biol. 2014, 20, 3209–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996; 562p. [Google Scholar]
- Brasier, C.M.; Robredo, F.; Ferraz, J.F.P. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathol. 1993, 42, 140–145. [Google Scholar] [CrossRef]
- Jung, T. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. For. Pathol. 2009, 39, 73–94. [Google Scholar] [CrossRef]
- Orlikowski, L.B.; Oszako, T. Phytophthorosis in Nurseries and Forest Stands; CILP: Warszawa, Polska, 2009. [Google Scholar]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densi-florus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.M. Sudden oak death: Phytophthora ramorum exhibits transatlantic differences. Mycol. Res. 2003, 107, 257–259. [Google Scholar] [CrossRef]
- Brasier, C.; Webber, J. Sudden larch death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef]
- Grűnwald, N.J.; LeBoldus, J.M.; Hamelin, R.C. Ecology and evolution of the sudden oak death pathogen Phytophthora ramorum. Annu. Rev. Phytopathol. 2019, 57, 301–321. [Google Scholar] [CrossRef]
- Ivors, K.; Garbelloto, M.; Vries, D.E.; Ruyter-Spira, C.; Hekkert, B.T.; Rosenzweig, N.; Bonants, P. Microsatellite markers identify three lineages of Phytophthora ramorum in US nurseries, yet single lineages in US forest and European nursery populations. Mol. Ecol. 2006, 15, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Blaschke, H. Phytophthora root rot in declining forest trees. Phyton 1996, 36, 95–102. [Google Scholar]
- Hantula, J.; Lilja, A.; Nuorteva, H.; Parikka, P.; Werres, S. Pathogenicity, morphology and genetic variation of Phytophthora cactorum from strawberry, apple, rhododendron, and silver birch. Mycol. Res. 2000, 104, 1062–1068. [Google Scholar] [CrossRef]
- Lilja, A.; Rikala, R.; Hietala, A.; Heinonen, R. Stem lesions on Betula pendula seedlings in Finnish forest nurseries and the pathogenicity of Phytophthora cactorum. Eur. J. For. Pathol. 1996, 26, 89–96. [Google Scholar] [CrossRef]
- Hantula, J.; Lilja, A.; Parikka, P. Genetic variation and host specificity of Phytophthora cactorum isolated in Europe. Mycol. Res. 1997, 101, 565–572. [Google Scholar] [CrossRef]
- Stępniewska, H. Occurrence of Phytophthora cactorum on tree seedlings with damping-off symptoms in some forest nurseries in south of Poland. Phytopathol. Pol. 2003, 29, 53–67. [Google Scholar]
- Brasier, C.M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann. For. Sci. 1996, 53, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Ruiz Gomez, F.J.; Pérez-de-Luque, A.; Sánchez-Cuesta, R.; Quero, J.L.; Navarro Cerrillo, R.M. Differences in the response to acute drought and Phytophthora cinnamomi rands infection in Quercus ilex L. seedlings. Forests 2018, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Orlikowski, L.B.; Ptaszek, M.; Trzewik, A.; Orlikowska, T. Relationship between source of water, surveying time and occurrence of Phytophthora spp. Prog. Plant Prot. 2008, 48, 246–251. [Google Scholar]
- Ptaszek, M.; Orlikowski, L.B. Occurrence of Phytophthora species in watercourses and reservoirs in Poland and the threat to cultivated plants by this genera. Prog. Plant Prot. 2015, 55, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Guillaumin, J.J.; Mohammed, C.; Anselmi, N.; Courtecuisse, R.; Gregory, S.C.; Holdenrieder, O.; Intini, M.; Lung, B.; Marxmüller, H.; Morrison, D.; et al. Geographical distribution and ecology of the Armillaria species in western Europe. Eur. J. For. Pathol. 1993, 23, 321–341. [Google Scholar] [CrossRef]
- Mauer, O.; Palátová, E. The role of root system in silver birch (Betula pendula Roth) dieback in the air-polluted area of Krušné hory Mts. J. For. Sci. 2003, 49, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Desprez-Loustau, M.L.; Marçais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef] [Green Version]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Chandelier, A.; Gerarts, F.; San Martin, G.; Herman, M.; Delahaye, L. Temporal evolution of collar lesions associated with ash dieback and the occurrence of Armillaria in Belgian forests. For. Pathol. 2016, 46, 289–297. [Google Scholar] [CrossRef]
- Jactel, H.; Desprez-Loustau, M.L.; Battisti, A.; Brockerhoff, E.; Santini, A.; Stenlid, J.; Björkman, C.; Branco, M.; Dehnen-Schmutz, K.; Douma, J.C.; et al. Pathologists and entomologists must join forces against forest pest and pathogen invasions. NeoBiota 2020, 58, 107–127. [Google Scholar] [CrossRef]
- Kula, E.; Pešlová, A.; Buchtová, D. Effects of nitrogen on the selection of food by Phyllobius arborator (Herbst). J. For. Sci. 2008, 54, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Urban, J. A contribution to the knowledge of biology and harmfulness of Deporaus betulae (L.) (Coleoptera, Attelabidae). Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Šimpraga, M.; Ghimire, R.P.; Van Der Straeten, D.; Blande, J.D.; Kasurinen, A.; Sorvari, J.; Holopainen, T.; Adriaenssens, S.; Holopainen, J.K.; Kivimäenpää, M. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. Eur. J. For. Res. 2019, 138, 763–787. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Grauer-Gray, K.; Holopainen, J.K.; Blande, J.D. Herbivore gender effects on volatile induction in Aspen and on olfactory responses in leaf beetles. Forests 2020, 11, 638. [Google Scholar] [CrossRef]
- Peghaire, E.; Hamdache, S.; Galien, A.; Sleiman, M.; ter Halle, A.; El Alaoui, H.; Kocer, A.; Richard, C.; Goupil, P. Inducing plant defense reactions in tobacco plants with phenolic-rich extracts from red maple leaves: A characterization of main active ingredients. Forests 2020, 11, 705. [Google Scholar] [CrossRef]
- Schoeneweiss, D.F. The influence of stress on diseases of nursery and landscape plants. J. Aboric. 1978, 4, 217–225. [Google Scholar]
- Oszako, T.; Sikora, K.; Belbahri, L.; Nowakowska, J.A. Molecular analysis of Phytophthora species found in Poland. Folia For. Pol. Series A 2017, 59, 321–328. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Milenković, I.; Nowakowska, J.A.; Borys, M.; Kałuski, T.; Gawlak, M.; Czyż, M.; Oszako, T. Morphological and molecular identification of Phytophthora species isolated from the rhizosphere of declining oak trees in Krotoszyn Plateau. Genetika 2017, 49, 203–215. [Google Scholar] [CrossRef]
- Oszako, T.; Żółciak, A.; Tulik, M.; Tkaczyk, M.; Stocki, M.; Nowakowska, J.A. Influence of Bacillus subtilis and Trichoderma asperellum on the development of birch seedlings infected with fine root pathogen. Phytophthora Plurivora Sylwan 2019, 163, 1006–1015. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H.; Neumann, P. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. Eur. J. For. Pathol. 1996, 26, 253–272. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Malewski, T.; Tereba, A.; Oszako, T. Rapid diagnosis of pathogenic Phytophthora species in soil by real-time PCR. For. Pathol. 2017, 47, e12303. [Google Scholar] [CrossRef]
- Oszako, T.; Voitka, D.; Tkaczyk, M.; Keča, N.; Belbahri, L.; Nowakowska, J.A. Assessment of interactions between defoliation and Phytophthora plurivora stem infections of birch seedlings. For. Chron. 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Stocki, M.; Vetchinikova, L. Inheritance of specific secondary volatile metabolites in buds of white birch Betula pendula and Betula pubescens hybrids. Trees Struct. Funct. 2019, 33, 1329–1344. [Google Scholar] [CrossRef] [Green Version]
- Stocki, M.; Zapora, E.; Rój, E.; Bakier, S. Recovering biologically active compounds from logging residue of birch (Betula spp.) with supercritical carbon dioxide. Przem. Chem. 2018, 97, 774–778. [Google Scholar]
- Stocki, M.; Bakier, S.; Isidorov, V. Study on extraction of biologically active compounds from birch (Betula) buds with supercritical carbon dioxide. Przem. Chem. 2019, 98, 1988–1991. [Google Scholar]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Series B 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Meszka, B.; Bielenin, A. Gooesberry—A new host of Phytophthora cactorum. Prog. Plant Prot. 2011, 51, 1184–1187. [Google Scholar]
- Lilja, A.; Karjalainen, R.; Parikka, P.; Kammiovirta, K.; Nuorteva, H. Pathogenicity and genetic variation of Phytophthora cactorum from silver birch and strawberry. Eur. J. Plant Pathol. 1998, 104, 529–535. [Google Scholar] [CrossRef]
- Wargo, P.M. Defoliation-induced chemical changes in sugar maple roots stimulate growth of Armillaria mellea. Phytopathology 1972, 62, 1278–1283. [Google Scholar] [CrossRef]
- Keča, N.; Tkaczyk, M.; Żółciak, A.; Stocki, M.; Kalaji, H.M.; Nowakowska, J.A.; Oszako, T. Survival of European ash seedlings treated with phosphite after infection with the Hymenoscyphus fraxineus and Phytophthora species. Forests 2018, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Rytkönen, A.; Lilja, A.; Petäistö, R.L.; Hantula, J. Irrigation water and Phytophthora cactorum in a forest nursery. Scand. J. For. Res. 2008, 23, 404–411. [Google Scholar] [CrossRef]
- Bernadzki, E.; Kowalski, M. Birch in formely arable land. Sylwan 1983, 12, 33–42. [Google Scholar]
- Ajchler, M.; Lobocka, M.; Oszako, T. Pathogenic oomycetes of Phytophthora genus—A new threat to forests in Europe. Sylwan 2017, 161, 870–880. [Google Scholar]
- Sturrock, R.N. Climate change and forest diseases: Using today’s knowledge to address future challenges. For. Syst. 2012, 21, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Denman, S.; Kirk, S.A.; Brasier, C.M.; Webber, J.F. In vitro leaf inoculation studies as an indication of tree foliage susceptibility to Phytophthora ramorum in the UK. Plant Pathol. 2005, 54, 512–521. [Google Scholar] [CrossRef]
- Wargo, P.M. Lysis of the cell wall of Armillaria mellea by enzymes from forest trees. Physiol. Plant Pathol. 1975, 5, 99–105. [Google Scholar] [CrossRef]
- Lamoure, D.; Guillaumin, J.J. The life cycle of the Armillaria mellea complex. Eur. J. Plant Pathol. 1985, 15, 288–293. [Google Scholar]
- Hoogesteger, J. Tree Ring Dynamics in Mountain Birch; SLU: Uppsala, Sweden, 2006. [Google Scholar]
- Beiger, M. Harmfulness of leaf mining insects to forest trees and shrubs. Sylwan 1985, 129, 83–92. [Google Scholar]
- Soika, G.; Labanowski, G. The most dangerous pests of birch and their control. Szkółkarstwo 2000, 4, 36–40. [Google Scholar]
- Spencer, K.A. The Agromyzidae (Diptera) of Fennoscandia and Denmark; Scandinavian Science Press: Vinderup, Denmark, 1976; 606p. [Google Scholar]
- Sznaider, Z. Atlas of Damage to Trees and Shrubs Caused by Insects and Arachnids, 2nd ed.; Wydawnictwo Naukowe PWN: Warszawa, Polska, 1991. [Google Scholar]
- Grodzki, W. Leafy admixture species and the threat of mountain stands caused by insect pests. Pr. IBL Series A 2001, 3, 917–922. [Google Scholar]
- Bussotti, F.; Pollastrini, M. Traditional and novel indicators of climate change impacts on European forest trees. Forests 2017, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Sierota, Z.; Grodzki, W.; Szczepkowski, A. Abiotic and biotic disturbances affecting forest health in Poland over the past 30 years: Impacts of climate and forest management. Forests 2019, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Oleksyn, J.; Karolewski, P.; Giertych, M.J.; Zytkowiak, R.; Reich, P.B.; Tjoelker, M.G. Primary and secondary host plants differ in leaf-level photosynthetic response to herbivory: Evidence from Alnus and Betula grazed by the alder beetle, Agelastica Alni. New Phytol. 1998, 140, 239–249. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; Frank, T.D.; DeLucia, E.H. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood saplings. Oecologia 2006, 149, 221–232. [Google Scholar] [CrossRef]
- Barry, K.M.; Pinkard, E.A. Growth and photosynthetic responses following defoliation and bud removal in Eucalypts. For. Ecol. Manag. 2013, 293, 9–16. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Gołaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bauriegel, E.; Herppich, W.B. Hyperspectral and chlorophyll fluorescence imaging for early detection of plant diseases, with special reference to Fusarium spec. infections on wheat. Agriculture 2014, 4, 32–57. [Google Scholar] [CrossRef] [Green Version]
- Fraenkel, G.S. The raison d’être of secondary plant substances. Science 1959, 129, 1466–1470. [Google Scholar] [CrossRef]
- Telford, A.; Cavers, S.; Ennos, R.A.; Cottrell, J.E. Can we protect forests by harnessing variation in resistance to pests and pathogens? Forestry 2015, 88, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Voda, K.; Boh, B.; Vrtačnik, M.; Pohleven, F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int. Biodeter. Biodegr. 2003, 51, 51–59. [Google Scholar] [CrossRef]
- Haukioja, E. Tree defenses against insects. In Multigenic and Induced Systemic Resistance in Plants; Tuzun, S., Bent, E., Eds.; Springer: Boston, MA, USA, 2006; pp. 279–295. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Reymond, P. Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. MPMI 2007, 20, 1406–1420. [Google Scholar] [CrossRef] [Green Version]
- Stotz, H.U.; Pieetendrigh, B.R.; Kroymann, J.; Weniger, K.; Fritsche, J.; Bauke, A.; Mitchell-Olds, T. Induced plant defense responses against chewing insects. Ethylene signaling reduces resistance of Arabidopsis against Egyptian cotton worm but not diamondback moth. Plant Physiol. 2000, 124, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Haukioja, E. Inducible defences of white birch to a geometrid defoliator, Epirrita autumnata. In Proceedings of the 5th International Symposium on Insect–Plant Relationships; Visser, J.H., Minks, A.K., Eds.; Pudoc: Wageningen, The Netherlands, 1982; pp. 199–203. [Google Scholar]
- Ruuhola, T.; Yang, S.Y.; Ossipov, V.; Haukioja, E. Foliar oxidases as mediators of the rapidly induced resistance of mountain birch against Epirrita autumnata. Oecologia 2008, 154, 725–730. [Google Scholar] [CrossRef]
- Jyothilakshmi, M.; Jyothis, M.; Narayanan, G.N.H.; Latha, M.S. Antidermatophytic and protease-inhibiting activities of zerumbone: A natural sesquiterpene from the rhizome of Zingiber zerumbet (L.) Roscoe ex JE.; Smith. Pharmacogn. Mag. 2017, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Shul’ts, E.E.; Bakhvalov, S.A.; Martem’yanov, V.V.; Petrova, T.N.; Syromyatnikova, I.N.; Shakirov, M.M.; Tolstikov, G.A. Effects of natural and artificial defoliation on the content and composition of extractive substances in birch leaves. Appl. Biochem. Microbiol. 2005, 41, 94–98. [Google Scholar] [CrossRef]
- Lahtinen, M.; Kapari, L.; Kenttä, J. Newly hatched neonate larvae can glycosylate: The fate of Betula pubescens bud flavonoids in first instar Epirrita autumnata. J. Chem. Ecol. 2006, 32, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Ádám, A.L.; Nagy, Z.Á.; Kátay, G.; Mergenthaler, E.; Viczián, O. Signals of systemic immunity in plants: Progress and open questions. Int. J. Mol. Sci. 2018, 19, 1146. [Google Scholar] [CrossRef] [Green Version]
- Haukioja, E.; Neuvonen, S. Induced long-term resistance of birch foliage against defoliators: Defensive or incidental? Ecology 1985, 66, 1303–1308. [Google Scholar] [CrossRef]
- Rossiter, M.; Schulz, J.C.; Baldwin, I.T. Relationships among defoliation, red oak phenolics, and gypsy moth growth and reproduction. Ecology 1988, 69, 267–277. [Google Scholar] [CrossRef]
- Lappalainen, J.H.; Koricheva, J.; Helander, M.L.; Haukioja, E. Densities of endophytic fungi and performance of leafminers (Lepidoptera: Eriocraniidae) on birch along a pollution gradient. Environ. Poll. 1999, 104, 99–105. [Google Scholar] [CrossRef]
- Liimatainen, J.; Karonen, M.; Sinkkonen, J.; Helander, M.; Salminen, J.-P. Characterization of phenolic compounds from inner bark of Betula pendula. Holzforschung 2012, 66, 171–181. [Google Scholar] [CrossRef]
- Pastor, V.; Balmer, A.; Gamir, J.; Flors, V.; Mauch-Mani, B. Preparing to fight back: Generation and storage of priming compounds. Front. Plant Sci. 2014, 5, 295. [Google Scholar] [CrossRef] [Green Version]
- Manion, P.D. Tree Disease Concepts; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1981. [Google Scholar]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lunzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–125. [Google Scholar] [CrossRef] [Green Version]
| T 1 | Interactions | Root Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. g.2 | P. c.3 | Def. 4 | NoT 5,* | FRL 6,* | MRL 7 | FRL/MRL 8 | TRL 9,* | FRSA 9,* | FRT 10,* | Dry Mass | |
| 1 | - | - | - | 4891.37b | 1485.10ab | 63.60 | 31.80 | 1577.01b | 166.94b | 4887.75b | 23.20 |
| 2 | + | - | - | 3362.87ab | 905.21ab | 48.82 | 18.06 | 988.57ab | 122.61ab | 3359.12ab | 16.78 |
| 3 | - | + | - | 2459.87ab | 725.40ab | 36.30 | 14.62 | 798.95ab | 96.60ab | 2456.50ab | 9.45 |
| 4 | - | - | + | 3740.37ab | 985.43ab | 49.60 | 19.69 | 1045.35ab | 134.58ab | 3738.12ab | 17.37 |
| 5 | + | - | + | 3770.87ab | 1164.54ab | 57.36 | 31.69 | 1226.70ab | 155.43b | 3767.62ab | 23.12 |
| 6 | - | + | + | 2659.87ab | 890.58ab | 41.45 | 15.32 | 965.20ab | 115.09ab | 2656.75ab | 14.26 |
| 7 | + | + | + | 3621.25ab | 1105.68ab | 51.73 | 21.08 | 1153.46ab | 124.79ab | 3618.75ab | 19.29 |
| 8 | + | + | - | 1670.87a | 446.61a | 26.25 | 14.05 | 483.77a | 70.74a | 1668.37a | 9.28 |
| Group of Compounds | Chemical Content (%) by Treatment | LSD 1) (p = 0.05) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Monoterpenes | 15.95 | 19.23 | 17.42 | 18.50 | 15.19 | 21.59 | 15.46 | 14.22 | 0.333 |
| Sesquiterpenes | 7.57 | 6.16 | 18.34 | 19.20 | 14.95 | 11.31 | 1.49 | 1.48 | 0.004 ** |
| Aromatic Esters | 5.52 | 5.91 | 8.05 | 3.96 | 7.12 | 6.68 | 4.98 | 4.66 | 0.998 |
| Aromatic Carbonyls | 1.32 | 3.87 | 4.03 | 2.47 | 4.37 | 7.12 | 5.46 | 6.98 | 0.805 |
| Aromatic Alcohols | 5.64 | 8.35 | 8.82 | 7.60 | 7.02 | 10.62 | 11.75 | 12.47 | 0.986 |
| Aliphatic Esters | 14.41 | 6.27 | 3.75 | 6.97 | 5.11 | 3.86 | 2.85 | 3.31 | 0.154 |
| Aliphatic Acids | 1.12 | - | - | 0.38 | - | 0.17 | 11.85 | 9.68 | 0.154 |
| Aliphatic Carbonyls | 21.58 | 22.47 | 17.12 | 17.21 | 20.79 | 18.32 | 20.66 | 18.30 | 0.711 |
| Aliphatic Alcohols | 15.36 | 15.31 | 12.76 | 13.38 | 15.78 | 8.43 | 17.13 | 21.41 | 0.873 |
| Alkanes and Alkenes | 7.12 | 8.65 | 7.04 | 7.44 | 6.62 | 9.62 | 2.73 | 1.83 | 0.189 |
| Other Compounds | 1.78 | 1.62 | 1.27 | 1.53 | 1.56 | 1.38 | 3.88 | 4.31 | 0.522 |
| Unidentified Compounds | 2.63 | 2.17 | 1.40 | 1.38 | 1.49 | 0.89 | 1.76 | 1.35 | 0.917 |
| Group of Compounds | Chemical Content (%) by Treatment | LSD (p = 0.05) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Phenolic Compounds | 0.87 | 15.35 | 5.02 | 11.91 | 4.83 | 4.36 | 7.85 | 13.32 | 0.711 |
| Triterpenes | 1.25 | 3.63 | 2.73 | 8.64 | 4.46 | 3.69 | 4.82 | 10.25 | 0.864 |
| Sterols | 33.53 | 39.58 | 28.37 | 41.83 | 28.94 | 29.61 | 23.30 | 35.80 | 0.998 |
| Fatty Acids | 44.47 | 21.47 | 32.30 | 15.65 | 32.10 | 33.93 | 35.82 | 15.50 | 0.086 |
| Fatty Alcohols | 4.23 | 5.45 | 6.43 | 6.29 | 9.27 | 6.15 | 7.28 | 5.67 | 0.954 |
| Other Compounds | 8.33 | 8.17 | 12.01 | 8.31 | 8.79 | 15.00 | 9.03 | 7.83 | 0.479 |
| Unidentified Compounds | 7.30 | 6.35 | 13.14 | 7.39 | 11.60 | 7.26 | 11.90 | 11.64 | 0.751 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowakowska, J.A.; Stocki, M.; Stocka, N.; Ślusarski, S.; Tkaczyk, M.; Caetano, J.M.; Tulik, M.; Hsiang, T.; Oszako, T. Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula Roth. Seedlings Subjected to Defoliation. Forests 2020, 11, 1107. https://doi.org/10.3390/f11101107
Nowakowska JA, Stocki M, Stocka N, Ślusarski S, Tkaczyk M, Caetano JM, Tulik M, Hsiang T, Oszako T. Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula Roth. Seedlings Subjected to Defoliation. Forests. 2020; 11(10):1107. https://doi.org/10.3390/f11101107
Chicago/Turabian StyleNowakowska, Justyna Anna, Marcin Stocki, Natalia Stocka, Sławomir Ślusarski, Miłosz Tkaczyk, João Maria Caetano, Mirela Tulik, Tom Hsiang, and Tomasz Oszako. 2020. "Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula Roth. Seedlings Subjected to Defoliation" Forests 11, no. 10: 1107. https://doi.org/10.3390/f11101107
APA StyleNowakowska, J. A., Stocki, M., Stocka, N., Ślusarski, S., Tkaczyk, M., Caetano, J. M., Tulik, M., Hsiang, T., & Oszako, T. (2020). Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula Roth. Seedlings Subjected to Defoliation. Forests, 11(10), 1107. https://doi.org/10.3390/f11101107








