Potential Interactions between Invasive Fusarium circinatum and Other Pine Pathogens in Europe
Abstract
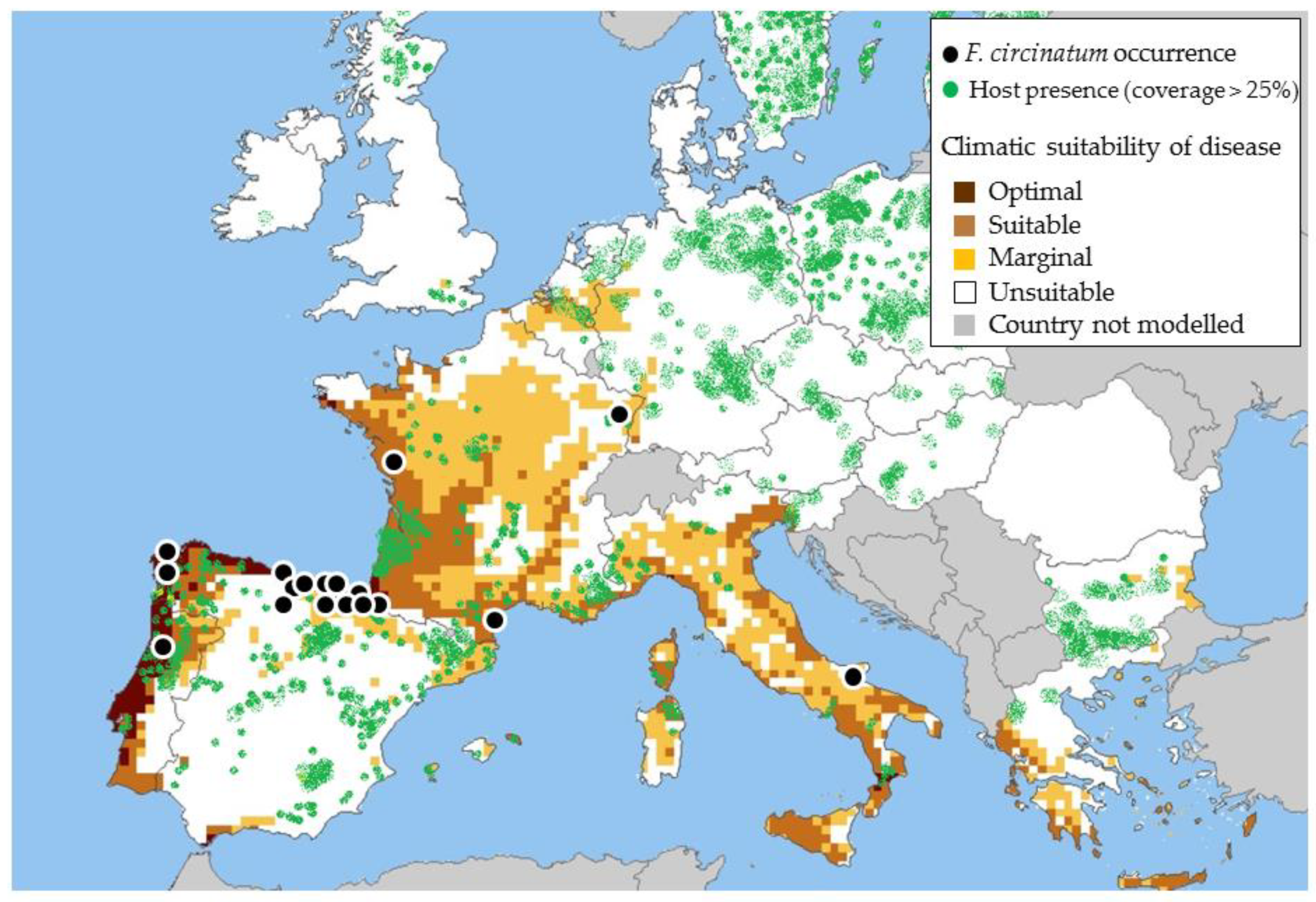
1. Introduction
2. Pine Pathogens Potentially Co-Occurring with Fusarium circinatum in Forests and Plantations
2.1. Root and Butt Rot Pathogens
2.2. Canker Pathogens
2.3. Foliar Pathogens
2.4. Vascular Pathogens
3. Pathogens Potentially Co-Occurring with Fusarium circinatum in Pine Nurseries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, D.M. Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 2000; ISBN 9780521789103. [Google Scholar]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Pinus Sylvestris in Europe: Distribution, Habitat, Usage and Threats; European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publ. Off. EU: Luxembourg, 2016; p. e016b94. [Google Scholar]
- Mead, D.J. Sustainable Management of Pinus Radiata Plantations; FAO Forestry Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; p. 170. [Google Scholar]
- Hermoso, E.; Carballo, J.; Fernandez-Golfin, J.I. Structural characterization of Pinus radiata D. Don timber from Pais Vasco (Spain) according to standard modifications. Tecnologia 2007, 9, 223–232. [Google Scholar] [CrossRef]
- Chavarriaga, D.; Bodles, W.J.A.; Leifert, C.; Belbahri, L.; Woodward, S. Phytophthora cinnamomi and other fine root pathogens in north temperate pine forests. FEMS Microbiol. Lett. 2007, 276, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Garbelotto, M.; Gonthier, P. Biology, epidemiology and control of Heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Drenkhan, R.; Tomešová-Haataja, V.; Fraser, S.; Vahalik, P.; Mullett, M.; Martín-García, J.; Bradshaw, R.E.; Bulman, L.; Wingfield, M.J.; Kirisits, T.; et al. Global geographic distribution and host range of Dothistroma species: A comprehensive review. For. Pathol. 2016, 46, 408–442. [Google Scholar] [CrossRef]
- Hansen, E.M.; Lewis, K.J.; Chastagner, G.A. Compendium of Conifer Diseases; American Phytopathological Society: Saint Paul, MN, USA, 2018. [Google Scholar]
- Santini, A.; Ghelardini, L.; de Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographic patterns and determinants of invasion by alien forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Mullett, M.S.; Brown, A.V. Effect of Dothistroma needle blight on needle and shoot lengths. For. Pathol. 2018, 48, e12382. [Google Scholar] [CrossRef]
- Woodward, S.; Stenlid, J.; Karjalainen, R.; Hüttermann, A. Preface. In Heterobasidion Annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hütterman, A., Eds.; CAB International: Wallingford, UK, 1998. [Google Scholar]
- Brasier, C.M. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; Landeras, E.; León, M.; Berbegal, M.; García Jiménez, J.; Armengol, J. Characterization of Fusarium circinatum from Pinus spp. in northern Spain. Mycol. Res. 2007, 111, 832–839. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Wright, J.; Heppe, E.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, M.J. A genetically homogenous population of Fusarium circinatum causes pitch canker of Pinus radiata in the basque country, Spain. Fungal Biol. 2011, 115, 288–295. [Google Scholar] [CrossRef]
- Landeras, E.; García, P.; Fernández, Y.; Braña, M.; Fernández Alonso, O.; Méndez-Lodos, S.; Pérez-Sierra, A.; León, M.; Abad-Campos, P.; Berbegal, M.; et al. Outbreak of pitch canker caused by Fusarium circinatum on Pinus spp. in northern Spain. Plant Dis. 2005, 89, 1015. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organisation). Efficacy evaluation of plant protection products. guidance on comparable climates. EPPO standard PP 1/241 (1). EPPO Bull. 2005, 35, 569–571. [Google Scholar]
- Collar Urquijo, J. Informe de la reunion del grupo de trabajo de laboratorios de diagnostico Y prospecciones fitosanitarias. Almeria 1995, 7, 26–33. [Google Scholar]
- Laucirica, J.M.; Muguruza, J.R. Presencia de Fusarium subglutinans f. sp. pini En Viveros De Pino radiata En Bizkaia. In Proceedings of the Actas XIV Reunión anualdel Grupo de Trabajo Fitosanitario de Forestales, Parques y Jardines, Spain, 18–20 November 1997; pp. 301–303. [Google Scholar]
- Dwinell, L.D.; Adams, D.; Guerra-Santos, J.J.; Aguirre, J.R.M. Pitch canker disease of Pinus radiata. In Proceedings of the VII International Congress of Plant Pathology, Edinburgh, UK, 9–16 August 1998; pp. 9–16. Available online: http://www.bspp.org.uk/icpp98/3.7/30.html (accessed on 4 October 2019).
- Dwinell, D. Global distribution of the pitch canker fungus. Current and potential impacts of pitch canker in Radiata pine. In Proceedings of the IMPACT Monterey Workshop, Monterey, CA, USA, 30 November–3 December 1998; pp. 54–57. [Google Scholar]
- Ridley, G.S.; Dick, M.A. Pine pitch canker disease: The name of the causal fungus and its distribution. Australas. Plant Pathol. 2000, 29, 263–266. [Google Scholar] [CrossRef]
- Bragança, H.; Diogo, E.; Moniz, F.; Amaro, P. First report of pitch canker on pines caused by Fusarium circinatum in Portugal. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef]
- EPPO. Update of the Situation of Fusarium circinatum In Portugal; EPPO Reporting Service: Paris, France, 2019; Num. article: 170. [Google Scholar]
- Carlucci, A.; Colatruglio, L.; Frisullo, S. First report of pitch canker caused by Fusarium circinatum on Pinus halepensis and P. pinea in Apulia (Southern Italy). Plant Dis. 2007, 91, 1683. [Google Scholar] [CrossRef]
- EPPO. First Report of Gibberella Circinata in France, EPPO Reporting Service; EPPO Global Database: Paris, France, 2006; Num. article: 104. [Google Scholar]
- Chapin, E.; Chauvel, G. Phytosanitary overview for 2006 of tree plantations, shrubs and clumps in green spaces. PHM Rev. Hortic. 2007, 490, 40–44. [Google Scholar]
- EPPO. Gibberella Circinata Detected Again in France, EPPO Reporting Service; EPPO Global Database: Paris, France, 2010; Num. article: 034. [Google Scholar]
- Gordon, T.R.; Okamoto, D.; Storer, A.J.; Wood, D.L. Susceptibility of five landscape pines to pitch canker disease, caused by Fusarium subglutinans f. sp. pini. Hortscience 1998, 33, 868–871. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Iturritxa, E.; Mesanza, N.; Elvira-Recuenco, M.; Serrano, Y.; Quintana, E.; Raposo, R. Evaluation of genetic resistance in Pinus to pitch canker in Spain. Australas. Plant Pathol. 2012, 41, 601–607. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Raposo, R.; García-Serna, I.; Mesanza, N.; Kirkpatrick, S.C.; Gordon, T.R. Resistance levels of Spanish conifers against Fusarium circinatum and Diplodia pinea. For. Pathol. 2013, 43, 488–495. [Google Scholar] [CrossRef]
- Martín-García, J.; Paraschiv, M.; Flores-Pacheco, J.A.; Chira, D.; Diea, J.J.; Fernández, M. Susceptibility of several northeastern conifers to Fusarium circinatum and strategies for biocontrol. Forests 2017, 8, 318. [Google Scholar] [CrossRef]
- Davydenko, K.; Nowakowska, J.; Kaluski, T.; Gawlak, M.; Sadowska, K.; García, J.; Diez, J.; Okorski, A.; Oszako, T. A comparative study of the pathogenicity of Fusarium circinatum and other Fusarium species in Polish provenances of P. sylvestris L. Forests 2018, 9, 560. [Google Scholar] [CrossRef]
- Vivas, M.; Zas, R.; Solla, A. Screening of maritime pine (Pinus pinaster) for resistance to Fusarium circinatum, the causal agent of pitch ca nker disease. Forestry 2012, 85, 185–192. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; Iturritxa, E.; Majada, J.; Alia, R.; Raposo, R. Adaptive potential of maritime pine (Pinus pinaster) populations to the emerging pitch canker pathogen, Fusarium circinatum. PLoS ONE 2014, 9, e114971. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, J.; Zas, R.; Solla, A.; Woodward, S.; Hantula, J.; Vainio, E.J.; Mullett, M.; Morales-Rodríguez, C.; Vannini, A.; Martínez-Álvarez, P.; et al. Environmentally friendly methods for controlling pine pitch canker. Plant Pathol. 2019, 68, 843–860. [Google Scholar] [CrossRef]
- Gordon, T.R.; Swett, C.L.; Wingfield, M.J. Management of Fusarium diseases affecting conifers. Crop Prot. 2015, 73, 28–39. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Potting, R.; Raposo, R. EU legislation on forest plant health: An overview with a focus on Fusarium circinatum. Forests 2018, 9, 568. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH). Risk assessment of Gibberella circinata for the EU territory and identification and evaluation of risk management options. EFSA J. 2010, 8, 1620. [Google Scholar] [CrossRef]
- Watt, M.S.; Ganley, R.J.; Kriticos, D.J.; Manning, L.K. Dothistroma needle blight and pitch canker: The current and future potential distribution of two important diseases of Pinus species. Can. J. For. Res. 2011, 41, 412–424. [Google Scholar] [CrossRef]
- Garbelotto, M.; Smith, T.; Schweigkofler, W. Variation in rates of spore deposition of Fusarium circinatum, the causal agent of pine pitch canker, over a 12-month-period at two locations in Northern California. Phytopathology 2008, 98, 137–143. [Google Scholar] [CrossRef]
- Dvořák, M.; Janoš, P.; Botella, L.; Rotková, G.; Zas, R. Spore dispersal patterns of Fusarium circinatum on an infested Monterey pine forest in North-Western Spain. Forests 2017, 8, 432. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Naves, P.; Witzell, J.; Musolin, D.L.; Selikhovkin, A.V.; Paraschiv, M.; Chira, D.; Martínez-Álvarez, P.; Martín-García, J.; Muñoz-Adalia, E.J.; et al. Pine pitch canker and insects: Relationships and implications for disease spread in Europe. Forests 2019, 10, 627. [Google Scholar] [CrossRef]
- Möykkynen, T.; Capretti, P.; Pukkala, T. Modelling the potential spread of Fusarium circinatum, the causal agent of pitch canker in Europe. Ann. For. Sci. 2015, 72, 169–181. [Google Scholar] [CrossRef]
- Christensen, O.B.; Goodess, C.M.; Harris, I.; Watkiss, P. European and global climate change projections: Discussion of climate change model outputs, scenarios and uncertainty in the EC RTD Climate Cost project. In The Climate Cost Project, Final Report; Watkiss, P., Ed.; Stockholm Environment Institute: Stockholm, Sweden, 2011; Volume 1, ISBN 978-91-86125-35-6. [Google Scholar]
- Storer, A.J.; Gordon, T.R.; Clark, S.L. Association of the pitch canker fungus, Fusarium subglutinans f. sp. pini, with Monterey pine seeds and seedlings in California. Plant Pathol. 1998, 47, 649–656. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; Iturritxa, E.; Raposo, R. Impact of seed transmission on the infection and development of pitch canker disease in Pinus radiata. Forests 2015, 6, 3353–3368. [Google Scholar] [CrossRef]
- Viljoen, A.; Wingfield, M.J.; Marasas, W.F.O. First report of Fusarium subglutinans f. sp. pini on pine seedlings in South Africa. Plant Dis. 1994, 78, 309–312. [Google Scholar] [CrossRef]
- Gordon, T.R.; Storer, A.J.; Wood, D.L. The pitch canker epidemic in California. Plant Dis. 2001, 85, 1128–1139. [Google Scholar] [CrossRef]
- Bezos, D.; Martìnez-Alvarez, P.; Fernadez, M.; Diez, J.J. Epidemiology and management of Pine pitch canker disease in Europe—A Review. Balt. For. 2017, 23, 279–293. [Google Scholar]
- Garbelotto, M.; Schweigkofler, W.; Shaw, D. First report of Fusarium circinatum, causal agent of Pitch canker disease, from the roots of mature Aleppo pines in California. Plant Health Prog. 2007. [CrossRef]
- Swett, C.L.; Gordon, T.R. Endophytic association of the pine pathogen Fusarium circinatum with corn (Zea mays). Fungal Ecol. 2015, 13, 120–129. [Google Scholar] [CrossRef]
- Swett, C.L.; Gordon, T.R. First report of grass species (Poaceae) as naturally occurring hosts of the pine pathogen Gibberella circinata. Plant Dis. 2012, 96, 908. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garcia, J.; Lukaćevićovŏ, A.; Pacheco, J.A.; Diez, J.J.; Dvořák, M. Evaluation of the susceptibility of several Czech conifer provenances to Fusarium circinatum. Forests 2018, 9, 72. [Google Scholar] [CrossRef]
- Garbelotto, M.; Gonthier, P. Variability and disturbances as key factors in forest pathology and plant health studies. Forests 2017, 8, 441. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejia, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, e15649–e15654. [Google Scholar] [CrossRef]
- Botella, L.; Santamaría, O.; Diez, J.J. Fungi associated with the decline of Pinus halepensis in Spain. Fungal Divers. 2010, 40, 1–11. [Google Scholar] [CrossRef]
- Botella, L.; Diez, J.J. Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Divers. 2011, 47, 9–18. [Google Scholar] [CrossRef]
- Agostinelli, M.; Cleary, M.; Martín, J.A.; Albrectsen, B.; Witzell, J. Pedunculate oaks (Quercus robur L.) differing in vitality as reservoirs for fungal biodiversity. Front. Microbiol. 2018, 9, 758. [Google Scholar] [CrossRef]
- Garbelotto, M.; Lowell, N.; Chen, I.; Osmundson, T. Evidence for inhibition of a fungal biocontrol agent by a plant microbiome. J. Plant Pathol. 2019. [Google Scholar] [CrossRef]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest tree microbiomes and associated fungal endophytes: Functional roles and impact on forest health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Doty, S.L. Functional Importance of the Plant Endophytic Microbiome; Springer International Publishing: Cham, Switzerland, 2017; Volume 1, p. 111. ISBN 978-3-319-65897-1. [Google Scholar]
- Kozanitas, M.; Osmundson, T.W.; Linzer, R.; Garbelotto, M. Interspecific interactions between the Sudden Oak Death pathogen Phytophthora ramorum and two sympatric Phytophthora species in varying ecological conditions. Fungal Ecol. 2017, 28, 86–96. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Bailey, B.J.; Fischer, D.G.; LeRoy, J.C.; Lonsdorf, E.V.; Whitham, T.G.; Hart, S.C. Plant–soil–microorganism interactions: Heritable relationship between plant genotype and associated soil microorganisms. Ecology 2008, 89, 773–781. [Google Scholar] [CrossRef]
- Cordier, T.; Robin, C.; Capdevielle, X.; Fabreguettes, O.; Desprez-Loustau, M.L.; Vacher, C. The composition of phyllosphere fungal assemblages of European beech (Fagus sylvatica) varies significantly along an elevation gradient. New Phytol. 2012, 196, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Bálint, M.; Tiffin, P.; Hallström, B.; O’Hara, R.B.; Olson, M.S.; Fankhauser, J.D.; Piepenbring, M.; Schmitt, I. Host genotype shapes the foliar fungal microbiome of Balsam poplar (Populus balsamifera). PLoS ONE 2013, 8, e53987. [Google Scholar] [CrossRef] [PubMed]
- Knief, C.; Ramette, A.; Frances, L.; Alonso-Blanco, C.; Vorholt, J.A. Site and plant species are improtant determinants of the Methylobacterium community composition in the plant phyllosphere. ISME J. 2010, 4, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef]
- Kembel, S.W.; Mueller, R.C. Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities. Botany 2014, 92, 303–311. [Google Scholar] [CrossRef]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Aguayo, J.; Dutech, C.; Hayden, K.J.; Husson, C.; Jakushkin, B.; Marçais, B.; Dominique, P.; Robin, C.; Vacher, C. An evolutionary ecology perspective to address forest pathology challenges of today and tomorrow. Ann. For. Sci. 2016, 73, 45–67. [Google Scholar] [CrossRef]
- Milanović, S.; Lazarević, J.; Karadžić, D.; Milenković, I.; Jankovský, L.; Vuleta, A.; Solla, A. Belowground infections of the invasive Phytophthora plurivora pathogen enhance the suitability of red oak leaves to the generalist herbivore Lymantria dispar. Ecol. Entomol. 2015, 40, 479–482. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Castagneyrol, B. Interactions between plant defence signalling pathways: Evidence from bioassays with insect herbivores and plant pathogens. J. Ecol. 2018, 106, 2353–2364. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Solla, A.; Ayres, M.P. Pine defences against the pitch canker disease are modulated by a native insect newly associated with the invasive fungus. For. Ecol. Manag. 2019, 437, 253–262. [Google Scholar] [CrossRef]
- Corcobado, T.; Miranda-Torres, J.J.; Martín-García, J.; Jung, T.; Solla, A. Early survival of Quercus ilex subspecies from different populations after infections and coinfections by multiple Phytophthora species. Plant Pathol. 2017, 66, 792–804. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, O.; Botella, L.; Diez, J.J. Gremmeniella abietina in North-western Spain: Distribution and associated mycoflora. Acta Silv. Lignaria Hung. (Spec. Edition) 2007, 137, 145. [Google Scholar]
- Barrett, D.K. Armillaria mellea as a possible factor predisposing roots to infection by Polyporus schweinitzii. Trans. Br. Mycol. Soc. 1970, 55, 459–462. [Google Scholar] [CrossRef]
- Biere, A.; Goverse, A. Plant-mediated systemic interactions between pathogens, parasitic nematodes, and herbivores above- and belowground. Annu. Rev. Phytopathol. 2016, 54, 499–527. [Google Scholar] [CrossRef]
- Enderle, R.; Sander, F.; Metzler, B. Temporal development of collar necroses and butt rot in association with ash dieback. iForest 2017, 10, 529–536. [Google Scholar] [CrossRef]
- Hernandez-Escribano, L.; Iturritxa, E.; Aragonés, A.; Mesanza, N.; Berbegal, M.; Raposo, R.; Elvira-Recuenco, M. Root infection of canker pathogens, Fusarium circinatum and Diplodia sapinea, in asymptomatic trees in Pinus radiata and Pinus pinaster plantations. Forests 2018, 9, 128. [Google Scholar] [CrossRef]
- Martín Rodrigues, N.; Sanchez Zabala, J.; Salcedo, I.; Majada, J.; González Murua, C.; Duñabeitia, M.K. New insights into radiata pine seedling root infection by Fusarium circinatum. Plant Pathol. 2015, 64, 1336–1348. [Google Scholar] [CrossRef]
- Marin-Pinto, P.; Pajares, J.; Díez, J. Pathogenicity of Fusarium verticillioides and Fusarium oxysporum on Pinus nigra seedlings in northwest Spain. For. Pathol. 2008, 38, 78–82. [Google Scholar] [CrossRef]
- Romón, P.; Troya, M.; Fernández de Gamarra, M.E.; Eguzkitza, A.; Iturrondobeitia, J.C.; Goldarazena, A. Fungal communities associated with pitch canker disease of Pinus radiata caused by Fusarium circinatum in northern Spain: Association with insects and pathogen-saprophyte antagonistic interactions. Can. J. Plant Pathol. 2008, 30, 241–253. [Google Scholar] [CrossRef]
- Dobreva, M.; Georgieva, M.; Dermedzhiev, P.; Nachev, R.; Velinov, V.; Terziev, P.; Georgiev, G. Fungal pathogens associated with Pinus species in the region of forest protection station Plovdiv in the period 2013–2016. For. Sci. 2016, 52, 103–116, (In Bulgarian with English summary). [Google Scholar]
- Hanso, S.; Hanso, M. Spread of Heterobasidion annosum in forests of Estonia. For. Stud. 1999, 31, 162–172, (In Estonian with English summary). [Google Scholar]
- Kutorga, E.; Adamonytė, G.; Iršėnaitė, R.; Juzėnas, S.; Kasparavičius, J.; Markovskaja, S.; Motiejūnaitė, J.; Treigienė, A. Wildfire and post-fire management effects on early fungal succession in Pinus mugo plantations located in the Curonian Spit (Lithuania). Geoderma 2012, 191, 70–79. [Google Scholar] [CrossRef]
- Taut, I.; Simonca, V. Pathogens identified in the forest culture in Transylvania in the 2010 year. Bull. UASVM Hortic. 2011, 68, 561. [Google Scholar]
- Neves, N.; Moniz, F.; De Azevedo, N.; Ferreira, M.C.; Ferreira, G.W.S. Present phytosanitory situation in Portuguese forests. EPPO Bull. 1986, 16, 505–508. [Google Scholar] [CrossRef]
- Piri, T.; Korhonen, K.; Sairanen, A. Occurrence of Heterobasidion annosum in pure and mixed spruce stands in Finland. Scand. J. For. Res. 1990, 5, 113–125. [Google Scholar] [CrossRef]
- Tsopelas, P.; Korhonen, K. Hosts and distribution of the intersterility groups of Heterobasidion annosum in the highlands of Greece. Eur. J. For. Pathol. 1996, 26, 4–11. [Google Scholar] [CrossRef]
- Chira, D.; Chira, F. Dynamics of wood fungi in wind-fallen stands from Oriental Carpathians. Ann. For. Res. 2001, 44, 54–59. [Google Scholar]
- Gonthier, P.; Garbelotto, M.; Nicolotti, G. Swiss stone pine trees and spruce stumps represent an important habitat for Heterobasidion spp. in subalpine forests. For. Pathol. 2003, 33, 191–203. [Google Scholar] [CrossRef]
- Kuz´michev, E.P.; Sokolova, E.S.; Mozolevskaya, E.G. Diseases and Insect Pests in Forests of Russia. Vol. 1. Diseases of Woody Plants; VNIILM: Moscow, Russia, 2004. (In Russian) [Google Scholar]
- Lygis, V.; Vasiliauskas, R.; Stenlid, J.; Vasiliauskas, A. Silvicultural and pathological evaluation of Scots pine afforestations mixed with deciduous trees to reduce the infections by Heterobasidion annosum ss. For. Ecol. Manag. 2004, 201, 275–285. [Google Scholar] [CrossRef]
- Asiegbu, F.O.; Adomas, A.; Stenlid, J. Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref. s.l. Mol. Plant Pathol. 2005, 6, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Bendel, M.; Kienast, F.; Bugmann, H.; Rigling, D. Incidence and distribution of Heterobasidion and Armillaria and their influence on canopy gap formation in unmanaged mountain pine forests in the Swiss Alps. Eur. J. For. Pathol. 2006, 116, 85–93. [Google Scholar] [CrossRef]
- Heydeck, P.; Knoche, D.; Dahms, C.; Rakel, T.; Bieler, T.; Sauermann, J.; Duhr, M. Prophylaktische maßnahmen zur bbwehr des Kiefern-Wurzelschwammes (H. annosum) in erstaufforstungen auf kippenstandorten im südlichen Brandenburg. Eberswalder Forstl. Schr. Band 2010, 61, 74. [Google Scholar]
- Szewczyk, W. Damage of selected young Scots Pine plantations by certain biotic factors in Drawsko forest district. Zarzdzanie Ochron Przyrody w Lasach 2012, 6, 170–176, (In Polish with English summary). [Google Scholar]
- Prieto-Recio, C.; Romeralo, C.; Bezos, D.; Martín-García, J.; Martínez-Álvarez, P.; Botella, L.; Diez, J.J. First report of Heterobasidion annosum on Pinus pinaster in Spain. Plant Dis. 2012, 96, 770. [Google Scholar] [CrossRef]
- Doğmuş-Lehtijärvi, H.T.; Erdoğan, R.C.; Lehtijärvi, A.; Woodward, S.; Aday Kaya, A.G. Pathogenicity of Heterobasidion annosum (Fr.) Bref. sensu stricto on coniferous tree species in Turkey. For. Pathol. 2016, 46, 22–28. [Google Scholar] [CrossRef]
- Sillo, F.; Gonthier, P.; Lockman, B.; Kasuga, T.; Garbelotto, M. Molecular analyses identify hybridization mediated nuclear evolution in newly discovered fungal hybrids. Ecol. Evol. 2019, 9, 6588–6605. [Google Scholar] [CrossRef]
- Linzer, R.E.; Otrosina, W.J.; Gonthier, P.; Bruhn, J.; Laflamme, G.; Bussiĕres, G.; Garbelotto, M. Inferences on the phylogeography of the fungal pathogen Heterobasidion annosum, including evidence of interspecific horizontal genetic transfer and of human-mediated, long range dispersal. Mol. Phylogenet. Evol. 2008, 46, 844–862. [Google Scholar] [CrossRef]
- Dalman, K.; Olson, A.; Stenlid, J. Evolutionary history of the conifer root rot fungus Heterobasidion annosum sensu lato. Mol. Ecol. 2010, 19, 4979–4993. [Google Scholar] [CrossRef]
- Olson, Å.; Aerts, A.; Asiegbu, F.; Belbahri, L.; Bouzid, O.; Broberg, A.; Canbäck, B.; Coutinho, P.M.; Cullen, D.; Dalman, K.; et al. Trade-off between wood decay and parasitism: Insights from the genome of a fungal forest pathogen. New Phytol. 2012, 194, 1001–1013. [Google Scholar] [CrossRef]
- Sillo, F.; Garbelotto, M.; Friedman, M.; Gonthier, P. Comparative genomics of sibling fungal pathogenic taxa identifies adaptive evolution without divergence in pathogenicity genes or genomic structure. Genome Biol. Evol. 2015, 7, 3190–3206. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Sun, H.; Vainio, H.; Raffaello, T.; Kovalchuk, A.; Morin, E.; Duplessis, S.; Asiegbu, F.O. Intraspecific comparative genomics of isolates of the Norway spruce pathogen (Heterobasidion parviporum) and identification of its potential virulence factors. BMC Genom. 2018, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, P.; Nicolotti, G.; Linzer, R.; Guglielmo, F.; Garbelotto, M. Invasion of European pine stands by a North American forest pathogen and its hybridization with a native interfertile taxon. Mol. Ecol. 2007, 16, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Motta, E.; Annesi, T.; Scire, M.; Luchi, N.; Hantula, J.; Korhonen, K.; Capretti, P. The North American P group of Heterobasidion annosum s. l. is widely distributed in Pinus pinea forests of the western coast of central Italy. For. Pathol. 2007, 37, 303–320. [Google Scholar]
- Gonthier, P.; Anselmi, N.; Capretti, P.; Bussotti, F.; Feducci, M.; Giordano, L.; Honorati, T.; Lione, G.; Luchi, N.; Michelozzi, M.; et al. An integrated approach to control the introduced forest pathogen Heterobasidion irregulare in Europe. Forestry 2014, 87, 471–481. [Google Scholar] [CrossRef]
- EPPO. Pest Risk Analysis for Heterobasidion Irregulare. EPPO, Paris. 2015. Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm (accessed on 11 October 2019).
- Martínez-Alvarez, P.; Pando, V.; Diez, J.J. Alternative species to replace Monterey pine plantations affected by pitch canker caused by Fusarium circinatum in northern Spain. Plant Pathol. 2014, 63, 1086–1094. [Google Scholar] [CrossRef]
- Prieto-Recio, C. Biotic, Abiotic and Management Factors Involved in Pinus Pinaster Decline in the Iberian Peninsula. Ph.D. Thesis, Universidad de Valladolid, Valladolid, Spain, 2016. [Google Scholar]
- Mesanza, N.; Iturritxa, E. Root and butt rot caused by Heterobasidion annosum in Atlantic coniferous ecosystems of Spain. For. Pathol. 2012, 42, 514–520. [Google Scholar] [CrossRef]
- Stark, R.W.; Cobb, F.W. Smog injury, root diseases and bark beetle damage in Ponderosa pine. Calif. Agric. 1969, 23, 13–15. [Google Scholar]
- Alexander, S.A.; Skelly, J.M.; Webb, R.S.; Bardinelli, R.R.; Bradford, B. Association of Heterobasidion annosum and the southern pine beetle on loblolly pine. Phytopathology 1980, 70, 510–513. [Google Scholar] [CrossRef]
- Bonello, P.; Capretti, P.; Luchi, N.; Martini, V.; Michelozzi, M. Systemic effects of Heterobasidion annosum s. s. infection on severity of Diplodia pinea tip blight and terpenoid metabolism in Italian stone pine (Pinus pinea). Tree Physiol. 2008, 28, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Drenkhan, T.; Voolma, K.; Adamson, K.; Sibul, I.; Drenkhan, R. The large pine weevil Hylobius abietis (L.) as a potential vector of the pathogenic fungus Diplodia sapinea (Fr.) Fuckel. Agric. For. Entomol. 2017, 19, 4–9. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Bowker, M.A.; Ochoa, V.; Carreira, J.A. Stand-structural effects on Heterobasidion abietinum-related mortality following drought events in Abies pinsapo. Oecologia 2010, 164, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Schmale, D.G., III; Gordon, T.R. Variation in susceptibility to pitch canker disease, caused by Fusarium circinatum, in native stands of Pinus muricata. Plant Pathol. 2003, 52, 720–725. [Google Scholar] [CrossRef]
- Tsopelas, P. Distribution and ecology of Armillaria species in Greece. Eur. J. For. Pathol. 1999, 29, 103–116. [Google Scholar] [CrossRef]
- Guillaumin, J.J.; Mohammed, C.; Anselmi, N.; Courtecuisse, R.; Gregory, S.C.; Holdenrieder, O.; Intini, M.; Lung, B.; Marxmüller, H.; Morrison, D.; et al. Geographical distribution and ecology of the Armillaria species in western Europe. Eur. J. For. Pathol. 1993, 23, 321–341. [Google Scholar] [CrossRef]
- Greig, B.J.W.; Gregory, S.C.; Strouts, R.G. Honey fungus. Forest. Comm. Bull. 1991, 100, 11. [Google Scholar]
- Wahlström, K.; Karlsson, J.O.; Holdenrieder, O.; Stenlid, J. Pectinolytic activity and isozymes in European Armillaria species. Can. J. Bot. 1991, 69, 2732–2739. [Google Scholar] [CrossRef]
- Szewczyk, W.; Macka, M. From the investigations on Armillaria root rot occurrence in young scots pine stands in Zielonka Forest District. Acta Agrobot. 2002, 55, 319–324, (In Polish with English summary). [Google Scholar] [CrossRef]
- Bragança, H.; Tenreiro, R.; Santos, N. Identification of portuguese Armillaria isolates by classic mating-tests and RFLP-PCR analysis of the ITS1 region of ribosomal DNA. Silva Lusit. 2004, 12, 67–75. [Google Scholar]
- Bagdžiūnaitė, A. Phytopatological monitoring and protection against fungal diseases in young pine Pinus sylvestris L. stands in Lithuania. J. Int. Sci. Publ. Agric. Food 2013, 1, 49–56. [Google Scholar]
- Mesanza, N.; Patten, C.L.; Iturritxa, E. Distribution and characterization of Armillaria complex in Atlantic forest ecosystems of Spain. Forests 2017, 8, 235. [Google Scholar] [CrossRef]
- Lehtijärvi, A.; Doğmuş Lehtijärvi, H.T.; Aday, A.G.; Unal, S.; Woodward, S. Armillaria ostoyae in managed coniferous forests in Kastamonu in Turkey. For. Pathol. 2017, 47. [Google Scholar] [CrossRef]
- Korhonen, K. Intersterility Groups of Heterobasidion Annosum; Commes Instituti Foralis Fenniae: Helsinki, Finland, 1978; Volume 94, p. 25. [Google Scholar]
- Roll-Hansen, F.; Roll-Hansen, H. On Diseases and Pathogens on Forest Trees in Norway 1966–1975; Communications of the Norwegian Forest Research Institute: Aas, Norway. For. Pathol. 1995, 479, 63. [Google Scholar]
- Kile, G.A.; McDonald, G.I.; Byler, J.W. Ecology and disease in natural forests. In Armillaria Root Disease; Shaw, C.G., Kile, G.A., Eds.; Agricultural Handbook: Washington, DC, USA, 1991; pp. 102–121. [Google Scholar]
- Lehtijärvi, A.; Doğmuş Lehtijärvi, H.T.; Aday, A.G. Armillaria ostoyae associated with dying 60-year-old Scots pines in northern Turkey. For. Pathol. 2012, 42, 267–269. [Google Scholar] [CrossRef]
- Wargo, P.M.; Shaw, C.G. Armillaria root rot: The puzzle is being solved. Plant Dis. 1985, 69, 826–832. [Google Scholar] [CrossRef]
- Mallett, K.; Maynard, D. Armillaria root disease, stand characteristics, and soil properties in young lodgepole pine. For. Ecol. Manag. 1998, 105, 37–44. [Google Scholar] [CrossRef]
- Solla, A.; Tomlinson, F.; Woodward, S. Penetration of Picea sitchensis root bark by Armillaria mellea, Armillaria ostoyae and Heterobasidion annosum. For. Pathol. 2002, 32, 55–70. [Google Scholar] [CrossRef]
- Hood, I.A.; Kimberley, M.O. Impact of Armillaria root disease and the effect of thinning in a late-rotation Pinus radiata plantation. For. Pathol. 2009, 39, 415–427. [Google Scholar] [CrossRef]
- Cleary, M.; van der Kamp, B.J.; Morrison, D.J. Effects of wounding and fungal infection with Armillaria ostoyae in three conifer species. II. Host response to the pathogen. For. Pathol. 2012, 42, 109–123. [Google Scholar] [CrossRef]
- Guillaumin, J.J.; Legrand, P. Armillaria root rots. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 159–177. [Google Scholar]
- Abad, G.Z. The taxonomy of Phytophthora: What is done and what is needed for the correct identification and diagnostics of species in the genus. In Proceedings of the 7th International Union of Forest Research Organizations, IUFRO Working Party 7-02-09 Meeting, Phytophthora in Forests and Natural Ecosystems, Esquel, Argentina, 10–14 November 2014. [Google Scholar]
- Ruano-Rosa, D.; Schena, L.; Agosteo, G.E.; Magnano di San Lio, G.; Cacciola, S.O. Phytophthora oleae sp. nov., causing fruit rot of olive in southern Italy. Plant Pathol. 2018, 67, 1362–1373. [Google Scholar] [CrossRef]
- Brasier, C.M. Phytophthora Pathogens of Trees: Their Rising Profile in Europe; Forestry Commission: Stockport, UK, 1999; p. 30.
- Zhukov, A.M.; Gninenko, Y.I.; Zhukov, P.D. Dangerous and Poorly Studied Diseases of Coniferous Trees in Forests of Russia, 2nd ed.; Pushkino: Moscow, Russia, 2013; p. 128. (In Russian) [Google Scholar]
- Jung, T.; Pérez-Sierra, A.; Duran, A.; Horta Jung, M.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil-and airborne Phytophthora species in forests and woodlands. Persoonia 2018, 40, 180–220. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: Saint Paul, MN, USA, 1996; p. 562. [Google Scholar]
- Migliorini, D.; Tondini, E.; Luchi, N.; Ghelardini, L.; Capretti, P.; Santini, A. Detection of Phytophthora species on different woody species in nurseries. In Proceedings of the 7th International Union of Forest Research Organizations, IUFRO Working Party 7-02-09 Meeting, Phytophthora in Forests and Natural Ecosystems, Esquel, Argentina, 10–14 November 2014. [Google Scholar]
- Marçais, B.; Caël, O.; Delatour, C. Interaction between root rot basidiomycetes and Phytophthora species on pedunculate oak. Plant Pathol. 2011, 60, 296–303. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday Kaya, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016. [Google Scholar] [CrossRef]
- Jung, T.; Burgess, T.I. Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia 2009, 22, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, M.; Sikora, K.; Nowakowska, J.; Aniśko, E.; Oszako, T.; Belbahri, L.; Milenković, I. Four different Phytophthora species that are able to infect Scots pine seedlings in laboratory conditions. Folia For. Pol. Ser. 2016, 58, 123–130. [Google Scholar] [CrossRef][Green Version]
- Cleary, M.; Blomquist, M.; Vetukuri, R.R.; Böhlenius, H.; Witzell, J. Susceptibility of common tree species in Sweden to Phytophthora cambivora, P. plurivora and P. cactorum. For. Pathol. 2017, 47, e12329. [Google Scholar] [CrossRef]
- Schoeneweiss, D.F. Predisposition, stress, and plant disease. Ann. Rev. Phytopathol. 1975, 13, 193–211. [Google Scholar] [CrossRef]
- Fabre, B.; Piou, D.; Desprez-Loustau, M.L.; Marcais, B. Can the emergence of pine Diplodia shoot blight in France be explained by changes in pathogen pressure linked to climate change? Glob. Chang. Biol. 2011, 17, 3218–3227. [Google Scholar] [CrossRef]
- Capretti, P. Caliciopsis pinea Peck parassita di Pinus pinaster e Pinus insignis. Phytopathol. Mediterr. 1978, 17, 101–104. [Google Scholar]
- Munck, I.A.; Livingston, W.; Lombard, K.; Luther, T.; Ostrofsky, W.D.; Weimer, J.; Wyka, S.; Broders, K. Extent and severity of Caliciopsis canker in New England, USA: An emerging disease of eastern white pine (Pinus strobus L.). Forests 2015, 6, 4360–4373. [Google Scholar] [CrossRef]
- Vanneste, J.; Hill, R.; Kay, S.; Farrell, R.; Holland, P. Biological control of sapstain fungi with natural products and biological control agents. A review of the work carried out in New Zealand. Mycol. Res. 2002, 106, 229–232. [Google Scholar] [CrossRef]
- Gardiner, B.; Berry, P.; Moulia, B. Wind impacts on plant growth, mechanics and damage. Plant Sci. 2016, 245, 94–118. [Google Scholar] [CrossRef] [PubMed]
- Varhola, A.; Coops, N.; Weiler, M.; Dan, M.R. Forest canopy effects on snow accumulation and ablation: An integrative review of empirical results. J. Hydrol. 2010, 392, 219–233. [Google Scholar] [CrossRef]
- Aglietti, C.; Luchi, N.; Capretti, P. Research about Dieback of Pinus Radiata in Tuscany Caused by Caliciopsis Pinea. Bachelor’s Thesis, University of Florence, School of Agriculture, Florence, Italy, 2014. [Google Scholar]
- Iturritxa, E.; Mesanza, N.; Brenning, A. Spatial analysis of the risk of major forest diseases in Monterey pine plantations. Plant Pathol. 2015, 64, 880–889. [Google Scholar] [CrossRef]
- Capretti, P.; Migliorini, D.; Diez Casero, J.; Martínez-Álvarez, P.; Luchi, N. Coexistence of Caliciopsis pinea and Fusarium circinatum on pine: Interactions among fungal pathogens. In Proceedings of the XXIV SIPaV Congress, Ancona, Italy, 5–7 September 2018. [Google Scholar]
- Bezos, D.; Martínez-Álvarez, P.; Fernández-Fernández, M.M.; Diez, J.J. Fungi and insect diversity associated with Pinus radiata in pitch-canker-affected stands. Int. For. Rev. 2014, 16, 336. [Google Scholar]
- Bezos, D.; Martínez-Álvarez, P.; Diez, J.J.; Fernández, M.M. Association levels between Pityophthorus pubescens and Fusarium circinatum in pitch canker disease affected plantations in northern Spain. Entomol. Gen. 2016, 36, 43–54. [Google Scholar] [CrossRef]
- Luchi, N.; Pepori, A.L.; Aglietti, C.; Migliorini, D.; Santini, A.; Capretti, P. Canker disease on Pinus radiata caused by Caliciopsis pinea in Italy. J. Plant Pathol. 2015, 97, 4. [Google Scholar]
- Jankovsky, L.; Palovcikova, D. Dieback of Austrian pine—The epidemic occurrence of Sphaeropsis sapinea in southern Moravia. J. For. Sci. 2003, 49, 389–394. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Marcais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effect of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; VonWeissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Baker, T.; Candresse, E.S.; Erzsebet Dormannsne, G.; Gilioli, J.; Gregoire, M.J. Risk assessment of Gibberella circinate for the EU territory and identification and evaluation of risk management options. EFSA 2010, 8, 1620. [Google Scholar]
- Serra-Varela, M.J.; Alía, R.; Pórtoles, J.; Gonzalo, J.; Soliño, M.; Grivet, D.; Raposo, R. Incorporating exposure to pitch canker disease to support management decisions of Pinus pinaster Ait. in the face of climate change. PLoS ONE 2017, 12, e0171549. [Google Scholar] [CrossRef] [PubMed]
- Munck, I.A.; Luther, T.; Wyka, S.; Keirstead, D.; McCracken, K.; Ostrofsky, W.; Searles, W.; Lombard, K.; Weimer, J.; Allen, B. Soil and stocking effects on Caliciopsis canker of Pinus strobus L. Forests 2016, 7, 269. [Google Scholar] [CrossRef]
- Burgess, T.I.; Gordon, T.R.; Wingfield, M.J.; Wingfield, B.D. Geographic isolation of Diplodia scrobiculata and its association with native Pinus radiate. Mycol. Res. 2004, 108, 1399–1406. [Google Scholar] [CrossRef]
- Butin, H. Tree Diseases and Disorders; Oxford University Press: New York, NY, USA, 1995; p. 84. [Google Scholar]
- Kunca, A.; Leontovy, R. Laboratory experiments with growth potential of Cenangium ferruginosum tested on natural nutrition soils. For. J. 2013, 59, 44–49. [Google Scholar] [CrossRef]
- Santamaría, O.; Tejerina, L.; Pajares, J.A.; Diez, J.J. Effects of associated fungi Sclerophoma pythiophila and Cenangium ferruginosum on Gremmeniella abietina dieback in Spain. For. Pathol. 2007, 37, 121–128. [Google Scholar] [CrossRef]
- OEPP/EPPO. Gremmeniella abietina. Bull. OEPP/EPPO 2009, 39, 310–317. [Google Scholar] [CrossRef]
- EPPO. EPPO Global Database Homepage. 2019. Available online: https://gd.eppo.int/taxon/PIURA/pests (accessed on 9 October 2019).
- Herron, D.A.; Wingfield, M.J.; Wingfield, B.D.; Rodas, C.A.; Marincowitz, S.; Steenkamp, E.T. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud. Mycol. 2015, 80, 131–150. [Google Scholar] [CrossRef]
- Bednářová, M.; Dvořák, M.; Janoušek, J.; Jankovský, L. Other foliar diseases of coniferous trees. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 458–487. [Google Scholar]
- Kowalski, T. Foliar diseases of broadleaved trees. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 488–518. ISBN 978-1-780640402. [Google Scholar]
- Hanso, M.; Drenkhan, R. Lophodermium needle cast, insect defoliation and growth responses of young Scots pines in Estonia. For. Pathol. 2012, 42, 124–135. [Google Scholar] [CrossRef]
- Tainter, F.H.; Baker, F.A. Principles of Forest Pathology; John Wiley and Sons: New York, NY, USA, 1996; ISBN 978-0471129523. [Google Scholar]
- Bulman, L.S.; Tubby, K.; Bradshaw, R.E.; Fraser, S.; Martín-García, J.; Musolin, D.L.; Barnes, I.; La Porta, N.; Diez-Casero, J.J.; Koltay, A.; et al. A worldwide perspective on the management and control of Dothistroma needle blight. For. Pathol. 2016, 46, 472–488. [Google Scholar] [CrossRef]
- Janoušek, J.; Wingfield, M.J.; Monsivais, J.G.; Jankovský, L.; Stauffer, C.; Konecný, A.; Barnes, I. Genetic analyses suggest separate introductions of the pine pathogen Lecanosticta acicola into Europe. Phytopathology 2016, 106, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Martín-García, J.; Bulman, L.; Vasconcelos, M.W.; Boberg, J.; La Porta, N.; Peredo, H.; Vergara, G.; Ahumada, R.; Brown, A.; et al. Dothistroma needle blight, weather and possible climatic triggers for the disease’s recent emergence. For. Pathol. 2016, 46, 443–452. [Google Scholar] [CrossRef]
- Cleary, M.; Laas, M.; Oskay, F.; Drenkhan, R. First report of Lecanosticta acicola on exotic Pinus mugo in southern Sweden. For. Pathol. 2019, 49, e12507. [Google Scholar] [CrossRef]
- Mullett, M.; Adamson, S.K.; Bragança, H.; Bulgakov, T.S.; Georgieva, M.; Henriques, J.; Jürisoo, L.; Laas, M.; Drenkhan, R. New country and regional records of the pine needle blight pathogens Lecanosticta acicola, Dothistroma septosporum and D. pini. For. Pathol. 2018, 48, e12440. [Google Scholar] [CrossRef]
- Broders, K.D.; Munck, I.; Wyka, S.; Iriarte, G.; Beaudoin, E. Characterization of fungal pathogens associated with white pine needle damage (WPND) in northeastern North America. Forests 2015, 6, 4088–4104. [Google Scholar] [CrossRef]
- Hintsteiner, M.; Cech, T.L.; Halmschlager, E.; Stauffer, C.; Kirisits, T. First report of Mycosphaerella dearnessii on Pinus nigra var. nigra in Austria. For. Pathol. 2012, 42, 437–440. [Google Scholar] [CrossRef]
- Piou, D.; Ioos, R. First report of Dothistroma pini, a recent agent of the Dothistroma needle blight (DNB), on Pinus radiata in France. Plant Dis. 2014, 98, 841. [Google Scholar] [CrossRef]
- Millberg, H.; Hopkins, A.J.M.; Boberg, J.; Davydenko, K.; Stenlid, J.; Woodward, S. Disease development of Dothistroma needle blight in seedlings of Pinus sylvestris and Pinus contorta under Nordic conditions. For. Pathol. 2016, 46, 515–521. [Google Scholar] [CrossRef]
- Perry, A.; Wachowiak, W.; Brown, A.V.; Ennos, R.A.; Cottrell, J.E.; Cavers, S. Substantial heritable variation for susceptibility to Dothistroma septosporum within populations of native British Scots pine (Pinus sylvestris). Plant Pathol. 2016, 65, 987–996. [Google Scholar] [CrossRef]
- Lazarević, J.; Davydenko, K.; Millberg, H. Dothistroma needle blight on high altitude pine forests in Montenegro. Balt. For. 2017, 23, 294–302. [Google Scholar]
- Mullett, M.S.; Brown, A.V.; Fraser, S.; Baden, R.; Tubby, K.V. Insights into the pathways of spread and potential origins of Dothistroma septosporum in Britain. Fungal Ecol. 2017, 26, 86–98. [Google Scholar] [CrossRef]
- Piotrowska, M.J.; Riddell, C.; Hoebe, P.N.; Ennos, R.A. Planting exotic relatives has increased the threat posed by Dothistroma septosporum to the Caledonian pine populations of Scotland. Evol. Appl. 2017, 11, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Adamson, K.; Mullett, M.S.; Solheim, H.; Barnes, I.; Müller, M.M.; Hantula, J.; Vuorinen, M.; Kacergius, A.; Markovskaja, S.; Musolin, D.L.; et al. Looking for relationship between the populations of Dothistroma septosporum in northern Europe and Asia. Fungal Genet. Biol. 2018, 110, 15–25. [Google Scholar] [CrossRef]
- Ortíz de Urbina, E.; Mesanza, N.; Aragonés, A.; Raposo, R.; Recuenco, M.E.; Boqué, R.; Patten, C.; Aitken, J.; Iturritxa, E. Emerging needle blight diseases in Atlantic Pinus ecosystems of Spain. Forests 2017, 8, 18. [Google Scholar] [CrossRef]
- Barnes, I.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Stud. Mycol. 2004, 50, 551–566. [Google Scholar]
- Barnes, I.; Wingfield, M.J.; Carbone, I.; Kirisits, T.; Wingfield, B.D. Population structure and diversity of an invasive pine needle pathogen reflects anthropogenic activity. Ecol. Evol. 2014, 4, 3642–3661. [Google Scholar] [CrossRef]
- Kais, A.G. Environmentl factors affecting brown-spot infection on longleaf pines. Phytopathology 1975, 65, 1389–1392. [Google Scholar] [CrossRef]
- Evans, H.C. The genus Mycosphaerella and its anamorphs Cercosetoria, Dothistroma and Leconosticta on pinea. Mycol. Pap. 1984, 153, 102. [Google Scholar]
- Kais, A.G. Dispersal of Schirrhia acicula spores in southern Mississippi. Plant Dis. Pap. 1971, 55, 309–311. [Google Scholar]
- Jankovsky, L.; Palovcikova, D.; Dvorak, M.; Tomsovsky, M. Records of brown spot needle blight related to Lecanosticta acicola in the Czech Republic. Plant Prot. Sci. 2009, 45, 16–18. [Google Scholar] [CrossRef]
- Markovskaja, S.; Kaćergius, A.; Treigien, A. Occurrence of new alien pathogenic fungus Mycosphaerella dearnessii in Lithuania. Bot. Lith. 2011, 17, 29–37. [Google Scholar]
- Adamson, K.; Drenkhan, R.; Hanso, M. Invasive brown spot needle blight caused by Lecanosticta acicola in Estonia. Scand. J. For. Res. 2015, 30, 587–593. [Google Scholar] [CrossRef]
- Martinez, J.B. Las micosis del Pinus insignis en Guipuzcoa. Publ. Inst. Investig. Exp. 1942, 13, 23. [Google Scholar]
- Wikler, K.; Gordon, T.R. An initial assessment of genetic relationships among populations of Fusarium circinatum in different parts of the world. Can. J. Bot. 2000, 78, 709–717. [Google Scholar]
- Berbegal, M.; Pérez-Sierra, A.; Armengol, J.; Grünwald, N.J. Evidence for multiple introductions and clonality in Spanish populations of Fusarium circinatum. Phytopathology 2013, 103, 851–861. [Google Scholar] [CrossRef]
- Blakeslee, G.M.; Oak, S.W.; Gregory, W.; Moses, C.S. Natural associations of Fusarium moniliforme var. subglutinans with Pissodes nemorensis. Phytopathology 1978, 12, 208. [Google Scholar]
- Barrows-Broaddus, J.; Dwinell, L.D. Variation in susceptibility to the pitch canker fungus among half-sib families of Virginia pine. Phytopathology 1984, 74, 438–444. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Pospiech, H.; Laukkanen, H.; Myllylä, R.; Hohtola, A. Two endophytic fungi in different tissues of Scots pine buds (Pinus sylvestris L.). Microb. Ecol. 2003, 45, 53–62. [Google Scholar] [CrossRef]
- Terhonen, E.; Marco, T.; Sun, H.; Jalkanen, R.; Kasanen, R.; Vuorinen, M.; Asiegbu, F. The effect of latitude, season and needle-age on the mycota of Scots pine (Pinus sylvestris) in Finland. Silva Fenn. 2011, 45, 301–317. [Google Scholar] [CrossRef]
- Talgø, V.; Chastagner, G.; Thomsen, I.M.; Cech, T.; Riley, K.; Lange, K.; Klemsdal, S.S.; Stensvand, A. Sydowia polyspora associated with current season needle necrosis (CSNN) on true fir (Abies spp.). Fungal Biol. 2010, 114, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Tinivella, F.; Dani, E.; Minuto, G.; Minuto, A. First Report of Sydowia polyspora on Aleppo Pine (Pinus halepensis) in Italy. Plant Dis. 2014, 98, 281. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Adalia, E.J.; Sanz-Ros, A.V.; Flores-Pacheco, J.A.; Hantula, J.; Diez, J.J.; Vainio, E.J.; Fernández, M. Sydowia polyspora dominates fungal communities carried by two Tomicus species in pine plantations threatened by Fusarium circinatum. Forests 2017, 8, 127. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef]
- Kirisits, T. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.-C., Evans, H.F., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 181–236. [Google Scholar]
- Linnakoski, R. Bark Beetle-Associated Fungi in Fennoscandia with Special Emphasis on Species of Ophiostoma and Grosmannia. Dissertationes Forestales 119. Ph. D. Thesis, School of Forest Sciences, University of Eastern Finland, Kuopio, Finland, 2011. [Google Scholar]
- Martín-Rodrigues, N.; Espinel, S.; Sanchez-Zabala, J.; Ortíz, A.; González-Murua, C.; Duñabeitia, M.K. Spatial and temporal dynamics of the colonization of Pinus radiata by Fusarium circinatum, of conidiophora development in pith and of traumatic resin duct formation. New Phytol. 2013, 198, 1215–1227. [Google Scholar] [CrossRef]
- De Beer, Z.W.; Wingfield, M.J. Emerging lineages in the Ophiostomatales. In The Ophiostomatoid Fungi: Expanding Frontiers; Seifert, K.A., de Beer, Z.W., Wingfi eld, M.J., Eds.; CBS: Utrecht, The Netherlands, 2013; pp. 21–46. [Google Scholar]
- Seifer, K.A. Sapstain of commercial lumber by species of Ophiostoma and Ceratocystis. In Ceratocystis and Ophiostoma. Taxonomy, Ecology, and Pathogenicity; Wingfield, M.J., Seifert, K.A., Webber, J.F., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 1993; pp. 141–151. [Google Scholar]
- Jankowiak, R. Fungi associated with Tomicus piniperda in Poland and assessment of their virulence using Scots pine seedlings. Ann. For. Sci. 2006, 63, 801–808. [Google Scholar] [CrossRef]
- Jankowiak, R. Ophiostomatoid fungi associated with Ips sexdentatus on Pinus sylvestris in Poland. Dendrobiology 2012, 68, 43–53. [Google Scholar]
- Álvarez, G.; Fernández, M.; Diez, J.J. Ophiostomatoid fungi associated with declined Pinus pinaster stands in Spain. For. Syst. 2015, 24, 1–9. [Google Scholar] [CrossRef]
- Långström, B.; Solheim, H.; Hellqvist, C.; Gref, R. Effects of pruning young Scots pines on host vigour and susceptibility to Leptographium wingfieldii and Ophiostoma minus, two blue-stain fungi associated with Tomicus piniperda. Eur. J. For. Pathol. 1993, 23, 400–415. [Google Scholar] [CrossRef]
- Solheim, H.; Langstrom, B.; Hellqvist, C. Pathogenicity of the blue-stain fungi Leptographium wingfieldii and Ophiostoma minus to Scots pine—Effect of tree pruning and inoculum density. Can. J. For. Res. 1993, 23, 1438–1443. [Google Scholar] [CrossRef]
- Solheim, H.; Krokene, P.; Långström, B. Effects of growth and virulence of associated blue-stain fungi on host colonization behaviour of the pine shoot beetles Tomicus minor and T. piniperda. Plant Pathol. 2001, 50, 111–116. [Google Scholar] [CrossRef]
- Davydenko, K.; Vasaitis, R.; Menkis, A. Fungi associated with Ips acuminatus (Coleoptera: Curculionidae) in Ukraine with a special emphasis on pathogenicity of ophiostomatoid species. Eur. J. Entomol. 2017, 114, 77–85. [Google Scholar] [CrossRef]
- Jacobs, K.; Wingfield, M.J. Leptographium Species: Tree Pathogens, Insect Associates, and Agents of Blue-Stain; American Phytopathological Society: St. Paul, MN, USA, 2001. [Google Scholar]
- Dori-Bachash, M.; Avrahami-Moyal, L.; Protasov, A.; Mendel, Z.; Freeman, S. The occurrence and pathogenicity of Geosmithia spp. and common blue-stain fungi associated with pine bark beetles in planted forests in Israel. Eur. J. Plant Pathol. 2015, 143, 627–639. [Google Scholar] [CrossRef]
- De Beer, Z.W.; Duang, T.A.; Barnes, I.; Wingfield, B.D.; Wingfield, M. Redefining Ceratocystis and allied genera. Stud. Mycol. 2014, 79, 187–219. [Google Scholar] [CrossRef]
- Romón, P.; De Beer, Z.W.; Fernández, M.; Diez, J.; Wingfield, B.D.; Wingfield, M.J. Ophiostomatoid fungi including two new fungal species associated with pine root-feeding beetles in northern Spain. Antonie van Leeuwenhoek 2014, 106, 1167–1184. [Google Scholar] [CrossRef]
- Owen, D.R.; Lindahl, K.Q., Jr.; Wood, D.L.; Parmeter, J.R., Jr. Pathogenicity of fungi isolated from Dendroctonus valens, D. brevicomis, and D. ponderosae to ponderosa pine seedlings. Phytopathology 1987, 77, 631–636. [Google Scholar] [CrossRef]
- Solheim, H.; Safranyik, L. Pathogenicity to Sitka spruce of Ceratocystis rufipenni and Leptographium abietinum blue-stain fungi associated with the spruce beetle. Can. J. For. Res. 1997, 27, 1336–1341. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Jacobs, A.; Coutinho, T.A.; Ahumada, R.; Wingfield, B.D. First report of the pitch canker fungus, Fusarium circinatum, on pines in Chile. Plant Pathol. 2002, 51, 397. [Google Scholar] [CrossRef]
- Alonso, R.; Bettucci, L. First report of the pitch canker fungus Fusarium circinatum affecting Pinus taeda seedlings in Uruguay. Australas. Plant Dis. Notes 2009, 4, 91–92. [Google Scholar]
- Steenkamp, E.T.; Rodas, C.A.; Kvas, M.; Wingfield, M.J. Fusarium circinatum and pitch canker of Pinus in Colombia. Australas. Plant Pathol. 2012, 41, 483–491. [Google Scholar] [CrossRef]
- Pfenning, L.H.; da Silva Costa, S.; Pereira de Melo, M.; Costa, H.; Ventura, J.A.; Auer, C.G.; Figueredo dos Santos, A. First report and characterization of Fusarium circinatum, the causal agent of pitch canker in Brazil. Trop. Plant Pathol. 2014, 39, 3. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Steenkamp, E.T.; Coutinho, T.A.; Wingfield, M.J. The pitch canker fungus, Fusarium circinatum: Implications for South African forestry. South. For. 2011, 73, 1–13. [Google Scholar] [CrossRef]
- Hurley, B.; Govender, P.; Coutinho, T.A.; Wingfield, B.; Wingfield, M.J. Fungus gnats and other Diptera in South African forestry nurseries and their possible association with the pitch canker fungus. S. Afr. J. Sci. 2007, 103, 43–46. [Google Scholar]
- Swett, C.L.; Porter, B.; Fourie, G.; Steenkamp, E.; Gordon, T.R.; Wingfield, M.J. Association of the pitch canker pathogen Fusarium circinatum with grass hosts in commercial pine production areas of South Africa. South. For. J. For. Sci. 2014, 76, 1–6. [Google Scholar]
- EPPO. Gibberella circinata. EPPO Bull. 2009, 93, 298–309. [Google Scholar]
- Landis, T.D.; Smith, R.S., Jr.; Toko, H.V. Tech. Coords. Forest Nursery Pests. Agriculture Handbook 680; USDA Forest Service: Washington, DC, USA, 2012; pp. 14–15.
- Ocamb, C.M.; Juzwik, J.; Martin, F.B. Fusarium spp. and Pinus strobus seedlings: Root disease pathogens and taxa associated with seed. New For. 2002, 24, 67–79. [Google Scholar] [CrossRef]
- Salerno, M.I.; Lori, G.A. Association of seed-borne Fusarium species on Pinus ponderosa with germination and seedling viability in Argentina. For. Pathol. 2007, 37, 263–271. [Google Scholar] [CrossRef]
- James, R.L. Fusarium root and stem diseases. In Forest Nursery Pests; Cram, M.M., Frank, M.S., Mallams, K.M., Eds.; Technical Coordinators; United States Department of Agriculture Forest Service: Colville, WA, USA, 2012; Handbook No. 680; pp. 117–120. Available online: http://www.rngr.net (accessed on 1 October 2019).
- González-Penalta, B.; Pintos-Varela, C.; Mansilla, J.P.; Aguín, O.; Pérez, R. Presencia de especies de Fusarium sobre semillas de Pinus spp. en Galicia. Sociedad Española de Ciencias Forestales 2008, 26, 149–154. [Google Scholar]
- Stewart, J.E.; Kim, M.S.; James, R.L.; Dumroese, R.K.; Klopfenstein, N.B. Molecular characterization of Fusarium oxysporum and Fusarium commune isolates from a conifer nursery. Phytopathology 2006, 96, 1124–1233. [Google Scholar] [CrossRef]
- James, R.L. Cylindrocarpon root disease. In Forest Nursery Pests; Cram, M.M., Frank, M.S., Mallams, K.M., Eds.; United States Department of Agriculture Forest Service: Colville, WA, USA, 2012; Handbook No. 680; pp. 31–32. Available online: http://www.rngr.net (accessed on 3 October 2019).
- Lombard, L.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Phylogeny and systematics of the genus Calonectria. Stud. Mycol. 2010, 66, 31–69. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, O.; Smith, D.R.; Stanosz, G.R. Interaction between Diplodia pinea and D. scrobiculata in Red and Jack pine seedlings. Phytopathology 2011, 101, 334–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cram, M.M.; Fraedrich, S.W. Seed diseases and seedborne pathogens of north America. Tree Plant. Notes 2009, 53, 35–44. [Google Scholar]
- Smith, D.R.; Stanosz, G.R. A species-specific PCR assay for detection of Diplodia pinea and D. scrobiculata in dead red and jack pines with collar rot symptoms. Plant Dis. 2006, 90, 307–313. [Google Scholar] [CrossRef]
- Sánchez, M.E.; Andicoberry, S.; Trapero, A. Phytophthora root rot of Aleppo pine seedlings in a forest nursery in Spain. Plant Dis. 2002, 86, 563. [Google Scholar] [CrossRef]
- Lehtijärvi, A.; Aday Kaya, A.G.; Woodward, S.; Jung, T.; Doğmuş Lehtijärvi, H.T. Oomycota species associated with deciduous and coniferous seedlings in forest tree nurseries of Western Turkey. For. Pathol. 2017, 47, e12363. [Google Scholar] [CrossRef]
- Durán, A.; Gryzenhout, M.; Slippers, B.; Ahumada, R.; Rotella, A.; Flores, F.; Wingfield, B.D.; Wingfield, M.J. Phytophthora pinifolia sp. nov. associated with a serious needle disease of Pinus radiata in Chile. Plant Pathol. 2008, 57, 715–727. [Google Scholar] [CrossRef]
- Schwingle, B.W.; Smith, J.A.; Blanchette, R.A. Phytophthora species associated with diseased woody ornamentals in Minnesota nurseries. Plant Dis. 2007, 91, 97–102. [Google Scholar] [CrossRef]
- Moralejo, E.; Pérez-Sierra, A.M.; Álvarez, L.A.; Belbahri, L.; Lefort, F.; Descals, E. Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathol. 2009, 58, 100–110. [Google Scholar] [CrossRef]
- Bienapfl, J.C.; Balci, Y. Movement of Phytophthora spp. in Maryland’s Nursery Trade. Plant Dis. 2014, 98, 134–144. [Google Scholar] [CrossRef]
- Prigigallo, M.I.; Mosca, S.; Cacciola, S.O.; Cooke, D.E.L.; Schena, L. Molecular analysis of Phytophthora diversity in nursery-grown ornamental and fruit plants. Plant Pathol. 2015, 64, 1308–1319. [Google Scholar] [CrossRef]
- Sims, L.; Tjosvold, S.; Chambers, D.; Garbelotto, M. Control of Phytophthora species in plant stock for habitat restoration through best management practices. Plant Pathol. 2018, 68, 196–204. [Google Scholar] [CrossRef]
- Reuveni, R.; Madar, Z. The role of Macrophomina phaseolina in mortality of pine seedlings in forest nurseries. J. Phytopathol. 1985, 112, 161–164. [Google Scholar] [CrossRef]
- Cordell, C.E.; Anderson, R.L.; Hoffard, W.H.; Landis, T.D.; Smith, R.S.; Toko, H.V., Jr. Charcoal root rot and black root rot. In Forest Nursery Pests; U. S. Department of Agriculture, Forest Service: Colville, WA, USA, 1989; Agriculture Handbook No. 680. [Google Scholar]
- Lilja, A.; Poteri, M.; Petäistö, R.L.; Rikala, R.; Kurkela, T.; Kasanen, R. Fungal diseases in forest nurseries in Finland. Silva Fenn. 2010, 44, 525–545. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Alves-Santos, F.M.; Diez, J.J. In vitro and in vivo interactions between Trichoderma viride and Fusarium circinatum. Silva Fenn. 2012, 46, 303–316. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Fernández-González, R.A.; Sanz-Ros, A.V.; Pando, V.; Diez, J.J. Two fungal endophytes reduce the severity of pitch canker disease in Pinus radiata seedlings. Biol. Control 2016, 94, 1–10. [Google Scholar] [CrossRef]
- Enebak, S.A. Pestalotiopsis Foliage Blight. In Forest Nursery Pests; Cram, M.M., Frank, M.S., Mallams, K.M., Eds.; United States Department of Agriculture Forest Service: Colville, WA, USA, 2012; Handbook No. 680; pp. 52–53. [Google Scholar]
- Brown, A.; Webber, J. Red Band Needle Blight of Conifers in Britain; Forestry Commission: Edinburgh, UK, 2008; pp. 1–8.
- Cram, M.M.; Frank, M.S.; Mallams, K.M. Forest Nursery Pests; Cram, M.M., Frank, M.S., Mallams, K.M., Eds.; United States Department of Agriculture Forest Service: Colville, WA, USA, 2012; Handbook 680; pp. 5–12.
- Ostry, M.E.; Juzwik, J. Selected Forest and Shade Tree Diseases of Significance in the 20th Century. APSnet Features 2008, 1–12. [Google Scholar] [CrossRef]
- Santamaria, O.; Pando, V.; Diez, J.J. Susceptibility of six pine species to Gremmeniella abietina isolates from Spain. For. Pathol. 2006, 36, 349–359. [Google Scholar] [CrossRef]
- Amaral, J.; Pinto, G.; Flores-Pacheco, J.A.; Díez-Casero, J.J.; Cerqueira, A.; Monteiro, P.; Gómez-Cadenas, A.; Alves, A.; Martín-García, J. Effect of Trichoderma viride pre-inoculation in pine species with different levels of susceptibility to Fusarium circinatum: Physiological and hormonal responses. Plant Pathol. 2019. [Google Scholar] [CrossRef]
- Susi, H.; Barres, B.; Vale, P.F.; Laine, A.L. Co-infection alters population dynamics of infectious disease. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host-multi-pathogen warfare: Pathogen interactions in co-infected plants. Front. Plant Sci. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, P.E. Three-way interactions between plant pathogenic fungi, herbivorous insects and their host plants. Biol. Rev. 1995, 70, 639–694. [Google Scholar] [CrossRef]
- Childs, T.W. Elytroderma Disease of Ponderosa Pine; Department of Agriculture, Forest Service: Colville, WA, USA, 1971; p. 42.
- Ioos, R.; Aloi, F.; Piškur, B.; Guinet, C.; Mullett, M.; Berbegal, M.; Bragança, H.; Cacciola, S.O.; Oskay, F.; Cornejo, C.; et al. Transferability of PCR-based diagnostic protocols: An international collaborative case study assessing protocols targeting the quarantine pine pathogen Fusarium circinatum. Sci. Rep. 2019, 9, 8195. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.; Oskay, F.; Doğmuş-Lehtijärvi, T.; Lehtijarvi, A.; Woodward, S.; Vettraino, A.M. Cryptic risks to forest biosecurity associated with the global movement of commercial seed. Forests 2019, 10, 459. [Google Scholar] [CrossRef]
- Vainio, E.J.; Bezos, D.; Bragança, H.; Cleary, M.; Fourie, G.; Georgieva, M.; Ghelardini, L.; Hannunen, S.; Ioos, R.; Martín-García, J.; et al. Sampling and Detection Strategies for the Pine Pitch Canker (PPC) Disease Pathogen Fusarium circinatum in Europe. Forests 2019, 10, 723. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.S.; Upadhyay, R.S.; Vieira Gomes, E.; Kin-Ming Tsui, C.; Nayak, C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2015, 40, 182–207. [Google Scholar]
- Abdelfattah, A.; Wisniewski, M.; Li Destri Nicosia, M.G.; Cacciola, S.O.; Schena, L. Metagenomic analysis of fungal diversity on strawberry plants and the effect of management practices on the fungal community structure of aerial organs. PLoS ONE 2016, 11, e0160470. [Google Scholar] [CrossRef]
- Feau, N.; Hamelin, R.C. Say hello to my little friends: How microbiota can modulate tree health. New Phytol. 2017, 215, 508–510. [Google Scholar] [CrossRef]
- Reis-Gonçalves, D.; Romeralo, C.; Martinez-Álvarez, G.; Martín-García, J.; Muñoz, J.; Diez, J.J. Next Generation Sequencing (NGS) and Quantitative PCR (qPCR) as Tools to Detect Fusarium Circinatum in Different Pine Species. Master’s Thesis, University of Valladolid, Valladolid, Spain, 2018. [Google Scholar]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Ann. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 63, 863–883. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Malacrinò, A.; Wisniewski, M.; Cacciola, S.O.; Schena, L. Metabarcoding: A powerful tool to investigate microbial communities and shape future plant protection strategies. Biol. Control 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Abrantes, I.; Barra Caracciolo, A.; Bevivino, A.; Ciancio, A.; Grenni, P.; Hrynkiewicz, K.; Kredics, L.; Proença, D.N. Belowground microbiota and the health of tree groops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Aimé, S.; Alabouvette, C.; Steinberg, C.; Olivain, C. The endophytic strain Fusarium oxysporum Fo47: A good candidate for priming the defence responses in tomato roots. Mol. Plant Microbe Interact 2013, 26, 918–926. [Google Scholar] [CrossRef]
- Bonello, P.; Gordon, T.R.; Storer, A.J. Systemic induced resistance in Monterey pine. For. Pathol. 2001, 31, 99–106. [Google Scholar] [CrossRef]
- Gordon, T.R.; Kirkpatrick, S.C.; Aegerter, B.J.; Fisher, A.J.; Storer, A.J.; Wood, D.L. Evidence for the occurrence of induced resistance to pitch canker, caused by Gibberella circinata (anamorph Fusarium circinatum), in populations of Pinus radiata. For. Pathol. 2011, 41, 227–232. [Google Scholar] [CrossRef]
- Moret, A.; Munoz, Z. Control of Diplodia pinea and D. scrobiculata in Pinus halepensis by 5-chloro-salicylic acid. Phytopathol. Mediterr. 2007, 46, 150–156. [Google Scholar]
- Aleandri, M.P.; Chilosi, G.; Bruni, N.; Tomassini, A.; Vettraino, A.M.; Vannini, A. Use of nursery potting mixes amended with local Trichoderma strains with multiple complementary mechanisms to control soil-borne disease. Crop Prot. 2015, 67, 269–278. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elvira-Recuenco, M.; Cacciola, S.O.; Sanz-Ros, A.V.; Garbelotto, M.; Aguayo, J.; Solla, A.; Mullett, M.; Drenkhan, T.; Oskay, F.; Aday Kaya, A.G.; et al. Potential Interactions between Invasive Fusarium circinatum and Other Pine Pathogens in Europe. Forests 2020, 11, 7. https://doi.org/10.3390/f11010007
Elvira-Recuenco M, Cacciola SO, Sanz-Ros AV, Garbelotto M, Aguayo J, Solla A, Mullett M, Drenkhan T, Oskay F, Aday Kaya AG, et al. Potential Interactions between Invasive Fusarium circinatum and Other Pine Pathogens in Europe. Forests. 2020; 11(1):7. https://doi.org/10.3390/f11010007
Chicago/Turabian StyleElvira-Recuenco, Margarita, Santa Olga Cacciola, Antonio V. Sanz-Ros, Matteo Garbelotto, Jaime Aguayo, Alejandro Solla, Martin Mullett, Tiia Drenkhan, Funda Oskay, Ayşe Gülden Aday Kaya, and et al. 2020. "Potential Interactions between Invasive Fusarium circinatum and Other Pine Pathogens in Europe" Forests 11, no. 1: 7. https://doi.org/10.3390/f11010007
APA StyleElvira-Recuenco, M., Cacciola, S. O., Sanz-Ros, A. V., Garbelotto, M., Aguayo, J., Solla, A., Mullett, M., Drenkhan, T., Oskay, F., Aday Kaya, A. G., Iturritxa, E., Cleary, M., Witzell, J., Georgieva, M., Papazova-Anakieva, I., Chira, D., Paraschiv, M., Musolin, D. L., Selikhovkin, A. V., ... Díez, J. J. (2020). Potential Interactions between Invasive Fusarium circinatum and Other Pine Pathogens in Europe. Forests, 11(1), 7. https://doi.org/10.3390/f11010007













