Podophyllotoxin Isolated from Podophyllum peltatum Induces G2/M Phase Arrest and Mitochondrial-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. MTS Assay
2.4. Soft Agar Assay
2.5. Cell Cycle Distribution Analysis
2.6. Annexin V/7-Aminoactinomycin D (7-AAD) Stained Cell Counting
2.7. Western Blots
2.8. ROS Assay
2.9. Measurement of Mitochondrial Membrane Potential
2.10. Cytosolic and Mitochondrial Fractionation
2.11. Caspase Activity
2.12. Statistical Analysis
3. Results
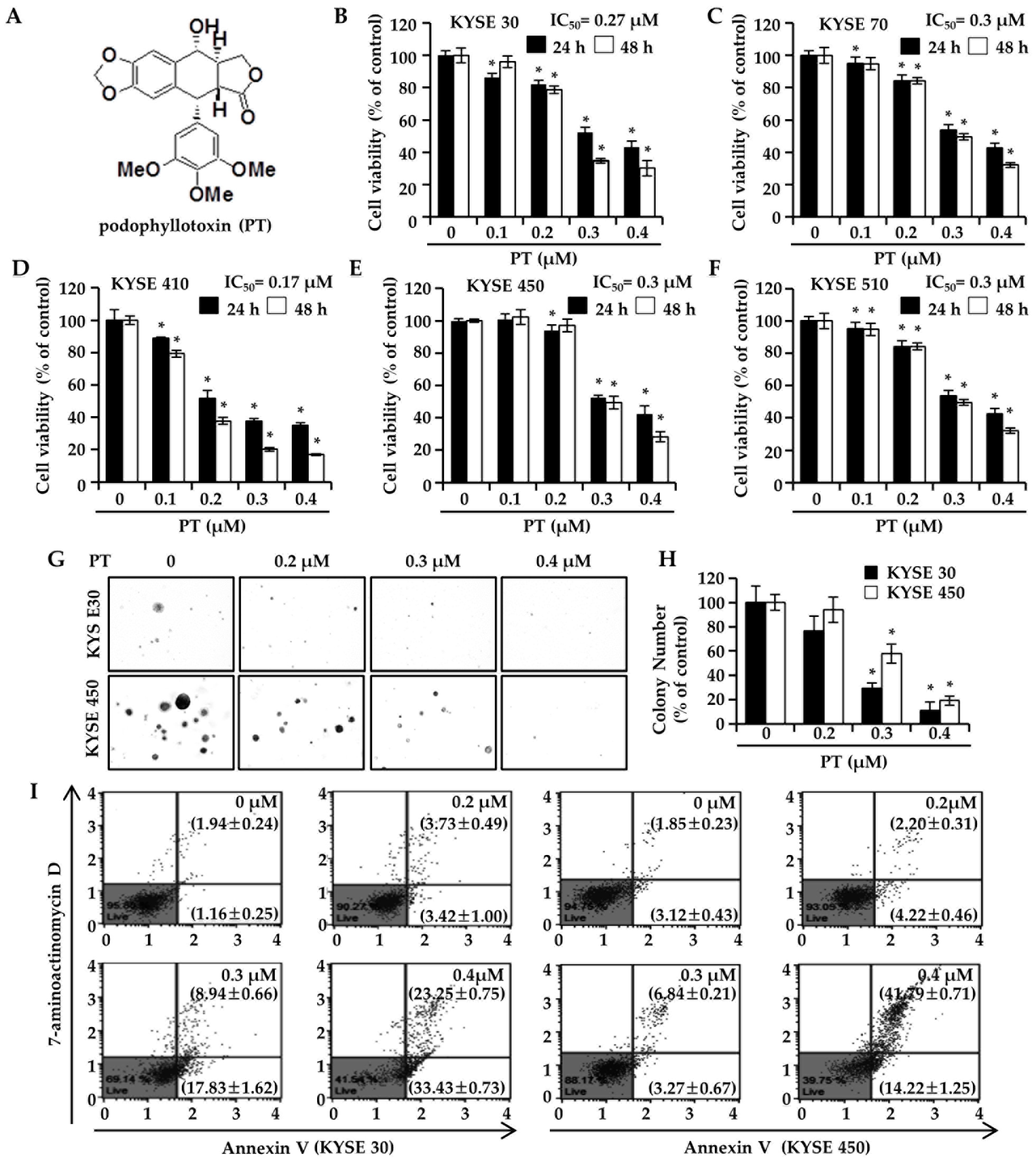
3.1. PT Suppresses ESCC Cell Proliferation and Colony-Forming Ability
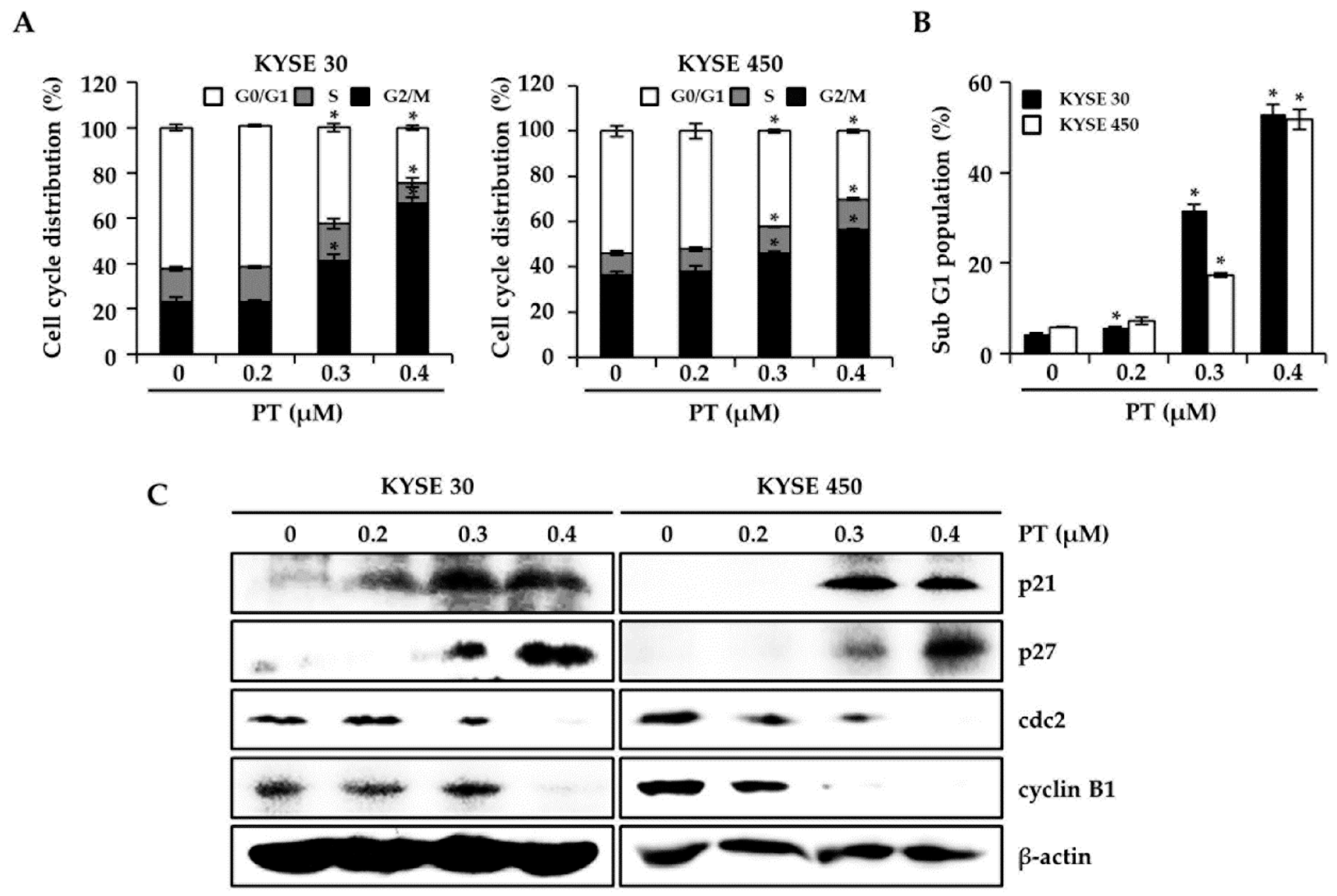
3.2. PT Induces G2/M Phase Arrest of Cell Cycle in KYSE 30 and KYSE 450 Cells
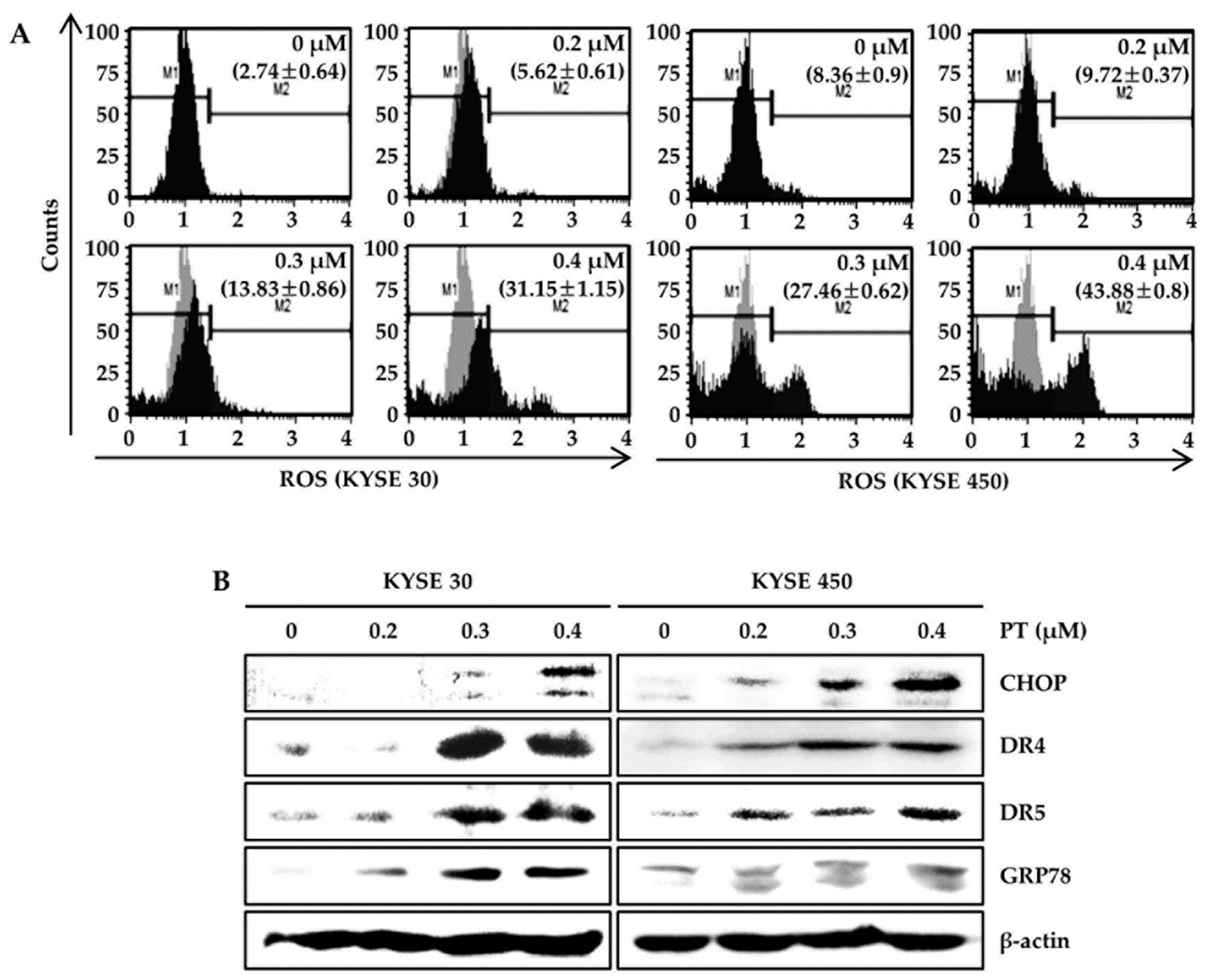
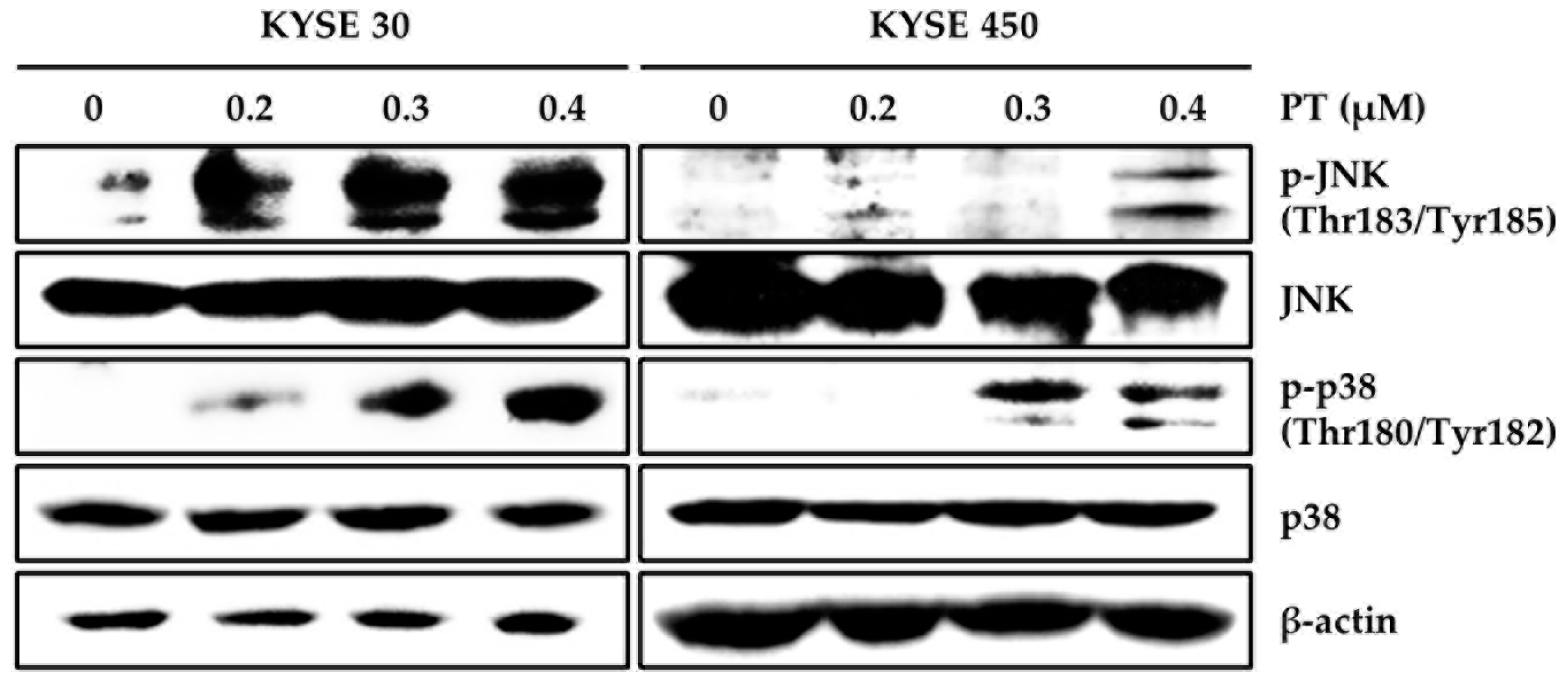
3.3. PT Induces Intracellular ROS Generation and Activates ROS-Dependent MAPK Pathway
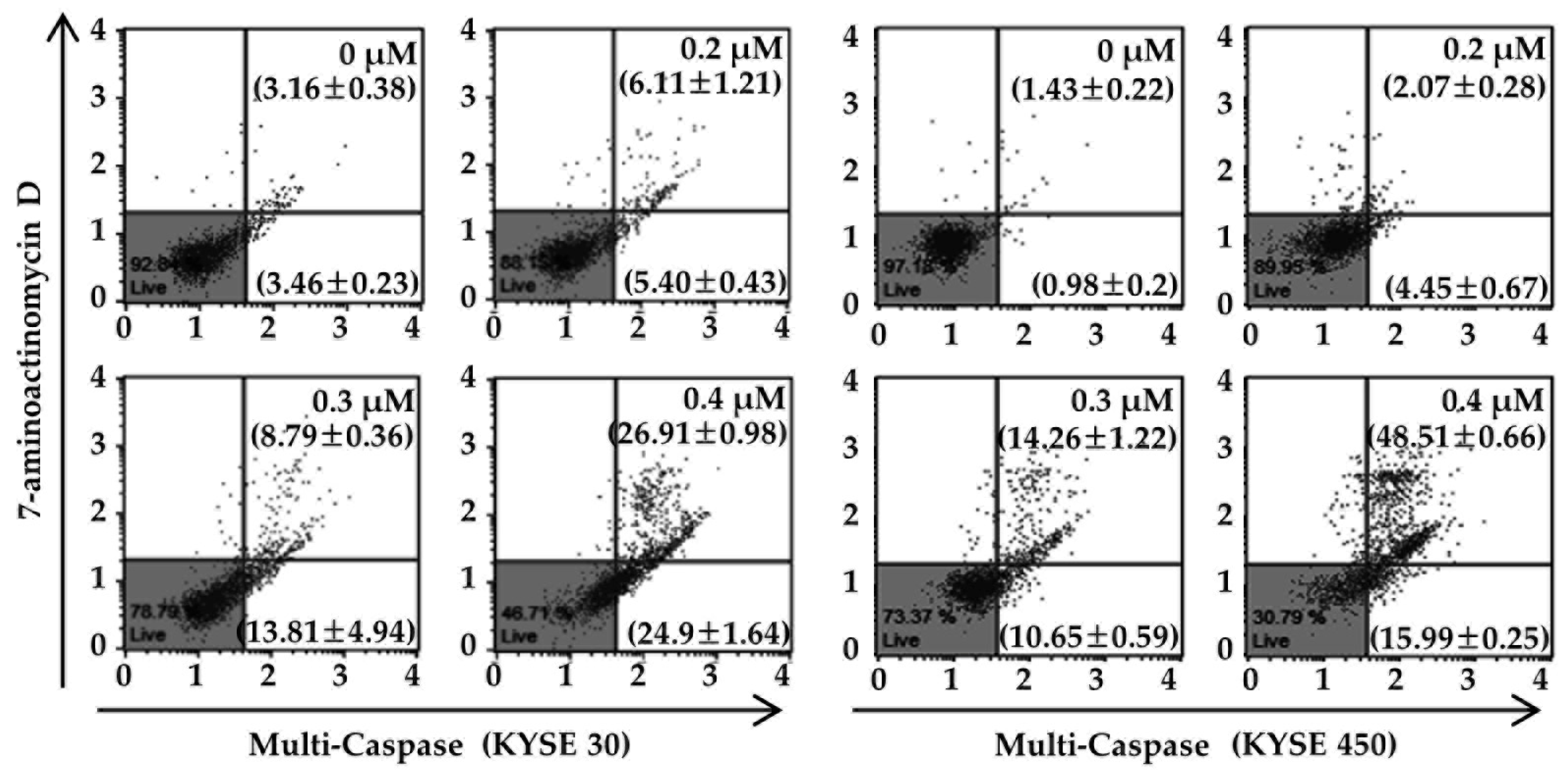
3.4. PT Induces Apoptosis of ESCC Cells via Reduction of Mitochondrial Membrane Potential and its Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Zhou, X.; Bao, W.; Chen, Y.; Cheng, L.; Qiu, G.; Sheng, L.; Ji, Y.; Du, X. Plasma fibrinogen levels are correlated with postoperative distant metastasis and prognosis in esophageal squamous cell carcinoma. Oncotarget 2015, 6, 38410–38420. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Johnson, A.; Ali, S.M.; Klempner, S.J.; Bekaii-Saab, T.; Vacirca, J.L.; Khaira, D.; Yelensky, R.; Chmielecki, J.; Elvin, J.A.; et al. Comprehensive Genomic Profiling of Advanced Esophageal Squamous Cell Carcinomas and Esophageal Adenocarcinomas Reveals Similarities and Differences. Oncologist 2015, 20, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Sugase, T.; Takahashi, T.; Serada, S.; Nakatsuka, R.; Fujimoto, M.; Ohkawara, T.; Hara, H.; Nishigaki, T.; Tanaka, K.; Miyazaki, Y.; et al. Suppressor of cytokine signaling-1 gene therapy induces potent antitumor effect in patient-derived esophageal squamous cell carcinoma xenograft mice. Int. J. Cancer 2017, 140, 2608–2621. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.L.; Faheina-Martins, G.V.; Maia, R.C.; Araujo, D.A. Compound A398, a novel podophyllotoxin analogue: Cytotoxicity and induction of apoptosis in human leukemia cells. PLoS ONE 2014, 9, e107404. [Google Scholar] [CrossRef] [PubMed]
- Zupko, I.; Jaeger, W.; Topcu, Z.; Wu, C.C. Anticancer Properties of Natural Products. Biomed. Res. Int. 2015, 2015, 242070. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhaes, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Alternat. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Shang, H.; Niu, C.; Zhang, Z.H.; Zhang, L.M.; Chen, H.; Zou, Z.M. Synthesis and Evaluation of New Podophyllotoxin Derivatives with in Vitro Anticancer Activity. Molecules 2015, 20, 12266–12279. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Tian, J.; Qian, K.; Zhao, X.B.; Morris-Natschke, S.L.; Yang, L.; Nan, X.; Tian, X.; Lee, K.H. Recent progress on C-4-modified podophyllotoxin analogs as potent antitumor agents. Med. Res. Rev. 2015, 35, 1–62. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, F.; Chen, Y.; Lin, Y.; Wang, J. Aromatic heterocyclic esters of podophyllotoxin exert anti-MDR activity in human leukemia K562/ADR cells via ROS/MAPK signaling pathways. Eur. J. Med. Chem. 2016, 123, 226–235. [Google Scholar] [CrossRef]
- Singh, A.; Yashavarddhan, M.H.; Kalita, B.; Ranjan, R.; Bajaj, S.; Prakash, H.; Gupta, M.L. Podophyllotoxin and Rutin Modulates Ionizing Radiation-Induced Oxidative Stress and Apoptotic Cell Death in Mice Bone Marrow and Spleen. Front. Immunol. 2017, 8, 183. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, F.; Li, Y.; Xue, D.; Zhao, N.; Zhang, J.; Deng, G.; Li, M.; Liu, X.; Wang, Y. Capsular Polysaccharide of Mycoplasma ovipneumoniae Induces Sheep Airway Epithelial Cell Apoptosis via ROS-Dependent JNK/P38 MAPK Pathways. Oxid. Med. Cell Longev. 2017, 2017, 6175841. [Google Scholar] [CrossRef]
- Lin, C.L.; Lee, C.H.; Chen, C.M.; Cheng, C.W.; Chen, P.N.; Ying, T.H.; Hsieh, Y.H. Protodioscin Induces Apoptosis Through ROS-Mediated Endoplasmic Reticulum Stress via the JNK/p38 Activation Pathways in Human Cervical Cancer Cells. Cell Physiol. Biochem. 2018, 46, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, J.; Fu, Y.; Hu, X.; Sun, L.Q.; Huang, Y.; Fan, X. Hepatitis B virus X protein inhibits apoptosis by modulating endoplasmic reticulum stress response. Oncotarget 2017, 8, 96027–96034. [Google Scholar] [CrossRef] [PubMed]
- Onyeagucha, B.; Subbarayalu, P.; Abdelfattah, N.; Rajamanickam, S.; Timilsina, S.; Guzman, R.; Zeballos, C.; Eedunuri, V.; Bansal, S.; Mohammad, T.; et al. Novel post-transcriptional and post-translational regulation of pro-apoptotic protein BOK and anti-apoptotic protein Mcl-1 determine the fate of breast cancer cells to survive or die. Oncotarget 2017, 8, 85984–85996. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, X.; Hu, D. Furanodienone induces G0/G1 arrest and causes apoptosis via the ROS/MAPKs-mediated caspase-dependent pathway in human colorectal cancer cells: A study in vitro and in vivo. Cell Death Dis. 2017, 8, e2815. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, W.; Liu, B.; Wang, Y.; Shao, J.; Wang, J.; Xia, K.; Liang, C.; Fang, W.; Zhou, C.; et al. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2017, 8, e3113. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, J.; Sun, L.L.; Li, B.H.; Gao, H.L.; Xie, T.; Zhang, N.; Ye, Z.M. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: An in vitro and in vivo study. Cell Death Dis. 2015, 6, e1604. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Wang, S.; Li, T.; Xiao, Z.; Lan, W.; Huang, G.; Cai, X. alpha-Mangostin, A Natural Xanthone, Induces Apoptosis and ROS Accumulation in Human Rheumatoid Fibroblast-Like Synoviocyte MH7A Cells. Curr. Mol. Med. 2017, 17, 375–380. [Google Scholar] [CrossRef]
- Oh, H.N.; Oh, K.B.; Lee, M.H.; Seo, J.H.; Kim, E.; Yoon, G.; Cho, S.S.; Cho, Y.S.; Choi, H.W.; Chae, J.I.; et al. JAK2 regulation by licochalcone H inhibits the cell growth and induces apoptosis in oral squamous cell carcinoma. Phytomedicine 2019, 52, 60–69. [Google Scholar] [CrossRef]
- Han, G.; Wu, Z.; Zhao, N.; Zhou, L.; Liu, F.; Niu, F.; Xu, Y.; Zhao, X. Overexpression of stathmin plays a pivotal role in the metastasis of esophageal squamous cell carcinoma. Oncotarget 2017, 8, 61742–61760. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, S.; Zhang, D.; Deng, M.; Zhang, X.; Tang, Z.; Chen, X. A polypeptide based podophyllotoxin conjugate for the treatment of multi drug resistant breast cancer with enhanced efficiency and minimal toxicity. Acta Biomater. 2018, 73, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.L.; Zhou, X.; Zhang, H.L.; Wu, L.L.; Tang, L.S.; Chen, L.L.; Duan, J.L. Exposure to podophyllotoxin inhibits oocyte meiosis by disturbing meiotic spindle formation. Sci. Rep. 2018, 8, 10145. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, G.; Qu, L.; Zhong, R.; Chen, P.; Lu, Z.; Zhou, J.; Guo, X.; Li, Z.; Ma, A.; et al. Podophyllotoxin Extracted from Juniperus sabina Fruit Inhibits Rat Sperm Maturation and Fertility by Promoting Epididymal Epithelial Cell Apoptosis. Evid. Based Complement. Alternat. Med. 2017, 2017, 6958982. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Tower, J. Programmed cell death in aging. Ageing Res. Rev. 2015, 23, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.J.; Shi, Z.Z.; Zhao, Z.X.; Zhang, Y.; Gong, T.; Li, C.X.; Zhan, T.; Cai, Y.; Dong, J.T.; Fu, S.B.; et al. Characterization of genetic rearrangements in esophageal squamous carcinoma cell lines by a combination of M-FISH and array-CGH: Further confirmation of some split genomic regions in primary tumors. BMC Cancer 2012, 12, 367. [Google Scholar] [CrossRef]
- Siedlecka-Kroplewska, K.; Wronska, A.; Stasilojc, G.; Kmiec, Z. The Designer Drug 3-Fluoromethcathinone Induces Oxidative Stress and Activates Autophagy in HT22 Neuronal Cells. Neurotox. Res. 2018. [Google Scholar] [CrossRef]
- Yu, H.; Yin, S.; Zhou, S.; Shao, Y.; Sun, J.; Pang, X.; Han, L.; Zhang, Y.; Gao, X.; Jin, C.; et al. Magnolin promotes autophagy and cell cycle arrest via blocking LIF/Stat3/Mcl-1 axis in human colorectal cancers. Cell Death Dis. 2018, 9, 702. [Google Scholar] [CrossRef]
- Chang, C.C.; Hung, C.M.; Yang, Y.R.; Lee, M.J.; Hsu, Y.C. Sulforaphane induced cell cycle arrest in the G2/M phase via the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J. Ovarian Res. 2013, 6, 41. [Google Scholar] [CrossRef]
- Rouault-Pierre, K.; Lopez-Onieva, L.; Foster, K.; Anjos-Afonso, F.; Lamrissi-Garcia, I.; Serrano-Sanchez, M.; Mitter, R.; Ivanovic, Z.; de Verneuil, H.; Gribben, J.; et al. HIF-2alpha protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell Stem Cell 2013, 13, 549–563. [Google Scholar] [CrossRef]
- Chen, W.Y.; Hsieh, Y.A.; Tsai, C.I.; Kang, Y.F.; Chang, F.R.; Wu, Y.C.; Wu, C.C. Protoapigenone, a natural derivative of apigenin, induces mitogen-activated protein kinase-dependent apoptosis in human breast cancer cells associated with induction of oxidative stress and inhibition of glutathione S-transferase pi. Investig. New Drugs 2011, 29, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.L.; Shen, Z.L.; Li, Y.L.; Bao, Y.Q.; Lu, H. Oxymatrine Causes Hepatotoxicity by Promoting the Phosphorylation of JNK and Induction of Endoplasmic Reticulum Stress Mediated by ROS in LO2 Cells. Mol. Cells 2018, 41, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wang, L.; Wang, B.; Wang, T.; Yang, G.; Shen, L.; Wang, T.; Guo, X.; Liu, Y.; Xia, Y.; et al. Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: Involvement of CHOP through Wnt. Cell Death Dis. 2014, 5, e1528. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.S.; Sharma, A.K.; Soni, H.; Ali, D.M.; Tews, B.; Konig, R.; Eibl, H.; Berger, M.R. Induction of ER and mitochondrial stress by the alkylphosphocholine erufosine in oral squamous cell carcinoma cells. Cell Death Dis. 2018, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, H.Y.; Wu, B.; Cheng, C.Y.; Xiao, W.; Wang, Z.Z.; Yang, Y.Y.; Li, P.; Yang, H. Ginkgolide K attenuates neuronal injury after ischemic stroke by inhibiting mitochondrial fission and GSK-3beta-dependent increases in mitochondrial membrane permeability. Oncotarget 2017, 8, 44682–44693. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.H.; Liu, K.L.; Shih, Y.L.; Chuang, Y.Y.; Chou, J.; Lu, H.F.; Jair, H.W.; Lee, M.Z.; Au, M.K.; Chung, J.G. Ouabain Induces Apoptotic Cell Death Through Caspase- and Mitochondria-dependent Pathways in Human Osteosarcoma U-2 OS Cells. Anticancer Res. 2018, 38, 169–178. [Google Scholar] [CrossRef]
- Su, L.Y.; Shi, Y.X.; Yan, M.R.; Xi, Y.; Su, X.L. Anticancer bioactive peptides suppress human colorectal tumor cell growth and induce apoptosis via modulating the PARP-p53-Mcl-1 signaling pathway. Acta Pharmacol. Sin. 2015, 36, 1514–1519. [Google Scholar] [CrossRef]
- Wang, R.; Ma, L.; Weng, D.; Yao, J.; Liu, X.; Jin, F. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol. Rep. 2016, 35, 3075–3083. [Google Scholar] [CrossRef]
- Huang, X.; Zou, L.; Yu, X.; Chen, M.; Guo, R.; Cai, H.; Yao, D.; Xu, X.; Chen, Y.; Ding, C.; et al. Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J. Mol. Cell Cardiol. 2015, 82, 153–166. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, G.; Lee, M.-H.; Kwak, A.-W.; Oh, H.-N.; Cho, S.-S.; Choi, J.-S.; Liu, K.; Chae, J.-I.; Shim, J.-H. Podophyllotoxin Isolated from Podophyllum peltatum Induces G2/M Phase Arrest and Mitochondrial-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma Cells. Forests 2020, 11, 8. https://doi.org/10.3390/f11010008
Yoon G, Lee M-H, Kwak A-W, Oh H-N, Cho S-S, Choi J-S, Liu K, Chae J-I, Shim J-H. Podophyllotoxin Isolated from Podophyllum peltatum Induces G2/M Phase Arrest and Mitochondrial-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma Cells. Forests. 2020; 11(1):8. https://doi.org/10.3390/f11010008
Chicago/Turabian StyleYoon, Goo, Mee-Hyun Lee, Ah-Won Kwak, Ha-Na Oh, Seung-Sik Cho, Joon-Seok Choi, Kangdong Liu, Jung-Il Chae, and Jung-Hyun Shim. 2020. "Podophyllotoxin Isolated from Podophyllum peltatum Induces G2/M Phase Arrest and Mitochondrial-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma Cells" Forests 11, no. 1: 8. https://doi.org/10.3390/f11010008
APA StyleYoon, G., Lee, M.-H., Kwak, A.-W., Oh, H.-N., Cho, S.-S., Choi, J.-S., Liu, K., Chae, J.-I., & Shim, J.-H. (2020). Podophyllotoxin Isolated from Podophyllum peltatum Induces G2/M Phase Arrest and Mitochondrial-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma Cells. Forests, 11(1), 8. https://doi.org/10.3390/f11010008






