Functional Role of Extrafloral Nectar in Boreal Forest Ecosystems under Climate Change
Abstract
:1. Introduction
2. EFN Production
3. Usage of Non-Floral Carbohydrate Sources by Mutualists
4. Ants and EFN in Boreal Forests
5. Ecosystem Services Provided by EFN in Boreal Forest
6. Effects of Climate Change on EFN Production and the Distribution of EFN-Producing Plants
6.1. EFN Production
6.2. Physiological Response to Climate Change Factors
6.3. Range Shift of EFN-Bearing Forest Plants
7. Effects of Climate Change on EFN-Dependent Communities
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Family | EFN Position | References |
|---|---|---|---|
| Native Tree Species | |||
| Populus tremula L. | Salicaea | Leaf base | [28,34] |
| Salix pentandra L. | Salicaea | Petiole, leaf base | [115] |
| Prunus padus L. | Rosaceae | Petiole | [28,34] |
| Viburnum opulus L. | Oleaceae | Petiole | [34] |
| Native Understory Species | |||
| Pteridium aquilinum L. (Kuhn) | Dennstaedtiaceae | Rachis stem junction | [28,34] |
| Vaccinium uliginosum L. | Ericaceae | Petiole | [28] |
| Vicia cracca L. | Fabaceae | Stipules | [34] |
| V. sepium L. | Fabaceae | Stipules | [34] |
| Impatiens noli-tangere L. | Balsaminaceae | Petiole, leaf margin | [28] |
| Melampyrum pratense L. | Orobanchaceae | Bracts | [34] |
| M. nemorosum L. | Orobanchaceae | Bracts | [34] |
| Introduced Tree Species | |||
| Sambucus racemosa L. | Adoxaceae | Stipules | [34] |
| Populus × wettsteinii1 Hämet-Ahti | Salicaea | Leaf base | [19] |
| Prunus pensylvanica L. f. | Rosacea | Petiole | [182] |
| Introduced Understory Species | |||
| Impatiens glandulifera Royle | Balsaminaceae | Stipules, petiole, | [34] |
| I. parviflora DC. | Balsaminaceae | petiole | [34] |
| Reynoutria japonica Houtt. | Polygonaceae | Petiole near stem | [34] |
| Reynoutria sachalinensis (F. Schmidt) Nakai | Polygonaceae | Petiole near stem | [34] |
| Reynoutria × bohemica Chrtek and Chrtková | Polygonaceae | Petiole near stem | [142] |
References
- Giuliani, C.; Lastrucci, L.; Cresti, L.; Santini, G.; Fogg, B.; Lippi, M.M. The morphology and activity of the extrafloral nectaries in Reynoutria x bohemica (Polygonaceae). Plant Biol. 2019, 21, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.G.; Keeler, K.H. The phylogenetic distribution of extrafloral nectaries in plants. Ann. Bot. 2013, 111, 1251–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heil, M. Extrafloral Nectar at the Plant-Insect Interface: A Spotlight on Chemical Ecology, Phenotypic Plasticity, and Food Webs. Annu. Rev. Entomol. 2015, 60, 213–232. [Google Scholar] [CrossRef]
- Mathur, V.; Wagenaar, R.; Caissard, J.; Reddy, A.S.; Vet, L.E.M.; Cortesero, A.; van Dam, N.M. A novel indirect defence in Brassicaceae: Structure and function of extrafloral nectaries in Brassica juncea. Plant Cell Environ. 2013, 36, 528–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marazzi, B.; Bronstein, J.L.; Koptur, S. The diversity, ecology and evolution of extrafloral nectaries: Current perspectives and future challenges. Ann. Bot. 2013, 111, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.G.; Agrawal, A.A. Defense mutualisms enhance plant diversification. Proc. Natl. Acad. Sci. USA 2014, 111, 16442–16447. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, S.A.; Holland, J.N. Quantitative synthesis of context dependency in ant-plant protection mutualisms. Ecology 2009, 90, 2384–2392. [Google Scholar] [CrossRef] [Green Version]
- Mayer, V.E.; Frederickson, M.E.; McKey, D.; Blatrix, R. Current issues in the evolutionary ecology of ant-plant symbioses. New Phytol. 2014, 202, 749–764. [Google Scholar] [CrossRef]
- Del-Claro, K.; Rico-Gray, V.; Torezan-Silingardi, H.M.; Alves-Silva, E.; Fagundes, R.; Lange, D.; Dattilo, W.; Vilela, A.A.; Aguirre, A.; Rodriguez-Morales, D. Loss and gains in ant-plant interactions mediated by extrafloral nectar: Fidelity, cheats, and lies. Insectes Soc. 2016, 63, 207–221. [Google Scholar] [CrossRef]
- Stone, G.N.; Raine, N.E.; Prescott, M.; Willmer, P.G. Pollination ecology of acacias (Fabaceae, Mimosoideae). Aust. Syst. Bot. 2003, 16, 103–118. [Google Scholar] [CrossRef]
- Wackers, F.L.; Wunderlin, R. Induction of cotton extrafloral nectar production in response to herbivory does not require a herbivore-specific elicitor. Entomol. Exp. Appl. 1999, 91, 149–154. [Google Scholar] [CrossRef]
- Nyffeler, M.; Olson, E.J.; Symondson, W.O.C. Plant-eating by spiders. J. Arachnol. 2016, 44, 15–27. [Google Scholar] [CrossRef]
- Heil, M.; Karban, R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evolut. 2010, 25, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Aljbory, Z.; Chen, M. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Yamawo, A.; Suzuki, N. Induction and relaxation of extrafloral nectaries in response to simulated herbivory in young Mallotus japonicus plants. J. Plant Res. 2018, 131, 255–260. [Google Scholar] [CrossRef]
- Huntzinger, M.; Karban, R.; Young, T.P.; Palmer, T.M. Relaxation of induced indirect defenses of acacias following exclusion of mammalian herbivores. Ecology 2004, 85, 609–614. [Google Scholar] [CrossRef]
- Wäckers, F.L.; van Rijn, P.C.J.; Bruin, J. (Eds.) Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and its Applications; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Blande, J.D.; Holopainen, J.K.; Li, T. Air pollution impedes plant-to-plant communication by volatiles. Ecol. Lett. 2010, 13, 1172–1181. [Google Scholar] [CrossRef]
- Li, T.; Holopainen, J.K.; Kokko, H.; Tervahauta, A.I.; Blande, J.D. Herbivore-induced aspen volatiles temporally regulate two different indirect defences in neighbouring plants. Funct. Ecol. 2012, 26, 1176–1185. [Google Scholar] [CrossRef]
- Ness, J.H. Catalpa bignonioides alters extrafloral nectar production after herbivory and attracts ant bodyguards. Oecologia 2003, 134, 210–218. [Google Scholar] [CrossRef]
- Smith, L.L.; Lanza, J.; Smith, G.C. Amino-Acid-Concentrations in Extrafloral Nectar of Impatiens sultani Increase After Simulated Herbivory. Ecology 1990, 71, 107–115. [Google Scholar] [CrossRef]
- Kost, C.; Heil, M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J. Ecol. 2006, 94, 619–628. [Google Scholar] [CrossRef]
- Camara, T.; Leal, I.R.; Bluethgen, N.; Oliveira, F.M.P.; de Queiroz, R.T.; Arnan, X. Effects of chronic anthropogenic disturbance and rainfall on the specialization of ant-plant mutualistic networks in the Caatinga, a Brazilian dry forest. J. Anim. Ecol. 2018, 87, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Fiala, B.; Linsenmair, K.E. Distribution and Abundance of Plants with Extrafloral Nectaries in the Woody Flora of a Lowland Primary Forest in Malaysia. Biodivers. Conserv. 1995, 4, 165–182. [Google Scholar] [CrossRef]
- Bluthgen, N.; Stork, N.E.; Fiedler, K. Bottom-up control and co-occurrence in complex communities: Honeydew and nectar determine a rainforest ant mosaic. Oikos 2004, 106, 344–358. [Google Scholar] [CrossRef]
- Bluethgen, N.; Stork, N.E. Ant mosaics in a tropical rainforest in Australia and elsewhere: A critical review. Austral Ecol. 2007, 32, 93–104. [Google Scholar] [CrossRef]
- Keeler, K.H. Distribution of Plants with Extrafloral Nectaries in Temperate Communities. Am. Midl. Nat. 1980, 104, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Pemberton, R.W. The occurrence and abundance of plants with extrafloral nectaries, the basis for antiherbivore defensive mutualisms, along a latitudinal gradient in east Asia. J. Biogeogr. 1998, 25, 661–668. [Google Scholar] [CrossRef]
- Lange, D.; Del-Claro, K. Ant-Plant Interaction in a Tropical Savanna: May the Network Structure Vary over Time and Influence on the Outcomes of Associations? PLoS ONE 2014, 9, e105574. [Google Scholar] [CrossRef]
- Morellato, L.; Oliveira, P.S. Distribution of Extrafloral Nectaries in Different Vegetation Types of Amazonian Brazil. Flora 1991, 185, 33–38. [Google Scholar] [CrossRef]
- Ohm, J.R.; Miller, T.E.X. Balancing anti-herbivore benefits and anti-pollinator costs of defensive mutualists. Ecology 2014, 95, 2924–2935. [Google Scholar] [CrossRef] [Green Version]
- Holland, J.N.; Chamberlain, S.A.; Horn, K.C. Temporal variation in extrafloral nectar secretion by reproductive tissues of the senita cactus, Pachycereus schottii (Cactaceae), in the Sonoran Desert of Mexico. J. Arid Environ. 2010, 74, 712–714. [Google Scholar] [CrossRef]
- Aranda-Rickert, A.; Diez, P.; Marazzi, B. Extrafloral nectar fuels ant life in deserts. AoB Plants 2014, 6, plu068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offenberg, J. Correlated evolution of the association between aphids and ants and the association between aphids and plants with extrafloral nectaries. Oikos 2000, 91, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Wenninger, A.; Hollingsworth, T.; Wagner, D. Predatory hymenopteran assemblages in boreal Alaska: Associations with forest composition and post-fire succession. Ecoscience 2019, 26, 205–220. [Google Scholar] [CrossRef]
- Doak, P.; Wagner, D.; Watson, A. Variable extrafloral nectary expression and its consequences in quaking aspen. Can. J. Bot. Rev. Can. Bot. 2007, 85, 1–9. [Google Scholar] [CrossRef]
- Mortensen, B.; Wagner, D.; Doak, P. Defensive effects of extrafloral nectaries in quaking aspen differ with scale. Oecologia 2011, 165, 983–993. [Google Scholar] [CrossRef]
- Newman, J.R.; Wagner, D.; Doak, P. Impact of extrafloral nectar availability and plant genotype on ant (Hymenoptera: Formicidae) visitation to quaking aspen (Salicaceae). Can. Entomol. 2016, 148, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Bluthgen, N.; Verhaagh, M.; Goitia, W.; Jaffe, K.; Morawetz, W.; Barthlott, W. How plants shape the ant community in the Amazonian rainforest canopy: The key role of extrafloral nectaries and homopteran honeydew. Oecologia 2000, 125, 229–240. [Google Scholar] [CrossRef]
- Taggart, R.E.; Cross, A.T. Global greenhouse to icehouse and back again: The origin and future of the Boreal Forest biome. Glob. Planet. Chang. 2009, 65, 115–121. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sietio, O.; Strakoya, P.; Prommer, J.; Wild, B.; Hagner, M.; Pihlatie, M.; Fritze, H.; Richter, A.; Heinonsalo, J. Plant roots increase both decomposition and stable organic matter formation in boreal forest soil. Nat. Commun. 2019, 10, 3982. [Google Scholar] [CrossRef] [Green Version]
- Tikhonova, E.; Tikhonov, G.; Shevchenko, N.; Knyazeva, S.; Plotnikova, A.; Lukina, N.; Shashkov, M. Tree diversity patterns along the latitudinal gradient in the northwestern Russia. For. Ecosyst. 2017, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, A.P.; Riggio, J.; Tait, A.M.; Baillie, J.E.M. Global areas of low human impact (‘Low Impact Areas’) and fragmentation of the natural world. Sci. Rep. 2019, 9, 14179. [Google Scholar] [CrossRef]
- Korhonen, K.T.; Ihalainen, A.; Ahola, A.; Heikkinen, J.; Henttonen, H.M.; Hotanen, J.P.; Nevalainen, S.; Pitkänen, J.; Strandström, M.; Viiri, H. Suomen metsät 2009–2013 ja niiden kehitys 1921–2013. LUKE Luonnonvara Biotalouden Tutk. 2017, 59, 1–86. [Google Scholar]
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.L.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.M.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, aaf7671. [Google Scholar] [CrossRef] [PubMed]
- Kellomäki, S.; Rouvinen, I.; Peltola, H.; Strandman, H.; Steinbrecher, R. Impact of global warming on the tree species composition of boreal forests in Finland and effects on emissions of isoprenoids. Glob. Chang. Biol. 2001, 7, 531–544. [Google Scholar] [CrossRef]
- Torssonen, P.; Strandman, H.; Kellomaki, S.; Kilpelainen, A.; Jylha, K.; Asikainen, A.; Peltola, H. Do we need to adapt the choice of main boreal tree species in forest regeneration under the projected climate change? Forestry 2015, 88, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Brice, M.; Cazelles, K.; Legendre, P.; Fortin, M. Disturbances amplify tree community responses to climate change in the temperate-boreal ecotone. Glob. Ecol. Biogeogr. 2019, 28, 1668–1681. [Google Scholar] [CrossRef]
- Hiura, T.; Go, S.; Iijima, H. Long-term forest dynamics in response to climate change in northern mixed forests in Japan: A 38-year individual-based approach. For. Ecol. Manag. 2019, 449, 117469. [Google Scholar] [CrossRef]
- Karhu, K.J. Effects of ant exclusion during outbreaks of a defoliator and a sap-sucker on birch. Ecol. Entomol. 1998, 23, 185–194. [Google Scholar] [CrossRef]
- Punttila, P.; Haila, Y.; Niemelä, J.; Pajunen, T. Ant Communities in Fragments of Old-Growth Taiga and Managed Surroundings. Ann. Zool. Fenn. 1994, 31, 131–144. [Google Scholar]
- Rudgers, J.A.; Strauss, S.Y. A selection mosaic in the facultative mutualism between ants and wild cotton. Proc. R. Soc. B Biol. Sci. 2004, 271, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Koptur, S.; Palacios-Rios, M.; Diaz-Castelazo, C.; Mackay, W.P.; Rico-Gray, V. Nectar secretion on fern fronds associated with lower levels of herbivore damage: Field experiments with a widespread epiphyte of Mexican cloud forest remnants. Ann. Bot. 2013, 111, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Rosumek, F.B.; Bluthgen, N.; Brueckner, A.; Menzel, F.; Gebauer, G.; Heethoff, M. Unveiling community patterns and trophic niches of tropical and temperate ants using an integrative framework of field data, stable isotopes and fatty acids. PeerJ 2018, 6, e5467. [Google Scholar] [CrossRef] [PubMed]
- Frouz, J.; Jilková, V.; Sorvari, J. Contribution of wood ants to nutrient cycling and ecosystem function. In Wood Ant Ecology and Conservation; Stockan, J.A., Robinson, E.J.H., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 207–220. [Google Scholar]
- Finer, L.; Jurgensen, M.F.; Domisch, T.; Kilpeläinen, J.; Neuvonen, S.; Punttila, P.; Risch, A.C.; Ohashi, M.; Niemelä, P. The Role of Wood Ants (Formica rufa group) in Carbon and Nutrient Dynamics of a Boreal Norway Spruce Forest Ecosystem. Ecosystems 2013, 16, 196–208. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Wan, J. Potential invasive plant expansion in global ecoregions under climate change. PeerJ 2019, 7, e6479. [Google Scholar] [CrossRef] [Green Version]
- Bentley, B.L. Extra-Floral Nectaries and Protection by Pugnacious Bodyguards. Annu. Rev. Ecol. Syst. 1977, 8, 407–427. [Google Scholar] [CrossRef]
- Staab, M.; Methorst, J.; Peters, J.; Bluethgen, N.; Klein, A. Tree diversity and nectar composition affect arthropod visitors on extrafloral nectaries in a diversity experiment. J. Plant Ecol. 2017, 10, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Escalante-Perez, M.; Jaborsky, M.; Lautner, S.; Fromm, J.; Mueller, T.; Dittrich, M.; Kunert, M.; Boland, W.; Hedrich, R.; Ache, P. Poplar Extrafloral Nectaries: Two Types, Two Strategies of Indirect Defenses against Herbivores. Plant Physiol. 2012, 159, 1176–1191. [Google Scholar] [CrossRef] [Green Version]
- Gish, M.; Mescher, M.C.; De Moraes, C.M. Targeted predation of extrafloral nectaries by insects despite localized chemical defences. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151835. [Google Scholar] [CrossRef] [Green Version]
- Gaylor, M.O.; Juntunen, H.L.; Hazelwood, D.; Videau, P. Assessment of Multiple Solvents for Extraction and Direct GC-MS Determination of the Phytochemical Inventory of Sansevieria Extrafoliar Nectar Droplets. J. Chromatogr. Sci. 2018, 56, 293–299. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, D.J. Foliar Nectar Production and Ant Activity on a Neotropical Tree, Ochroma pyramidale. Oecologia 1979, 43, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Free, J.B. The behaviour of honeybees visiting field beans (Vicia faba). J. Anim. Ecol. 1962, 31, 497–502. [Google Scholar] [CrossRef]
- Dennis, R.; Doak, P.; Wagner, D. Aspen leaf miner (Phyllocnistis populiella) oviposition site selection mediated by aspen (Populus tremuloides) extrafloral nectaries. Arthropod-Plant Interact. 2015, 9, 405–413. [Google Scholar] [CrossRef]
- Geneau, C.E.; Waeckers, F.L.; Luka, H.; Balmer, O. Effects of extrafloral and floral nectar of Centaurea cyanus on the parasitoid wasp Microplitis mediator: Olfactory attractiveness and parasitization rates. Biol. Control 2013, 66, 16–20. [Google Scholar] [CrossRef]
- Kirmse, S.; Chaboo, C.S. Extrafloral nectaries mediate the arboreal beetle community (Coleoptera) in a Neotropical rainforest. J. Nat. Hist. 2019, 53, 1313–1349. [Google Scholar] [CrossRef]
- Davis, A.R.; Peterson, R.L.; Shuel, R.W. Vasculature and Ultrastructure of the Floral and Stipular Nectaries of Vicia faba (Leguminosae). Can. J. Bot. Rev. Can. Bot. 1988, 66, 1435–1448. [Google Scholar] [CrossRef]
- Terrab, A.; Berjano, R.; Alberto Sanchez, J.; Gomez Pajuelo, A.; Josefa Diez, M. Palynological and geographical characterisation of Spanish oak honeydew honeys. Grana 2019, 58, 63–77. [Google Scholar] [CrossRef]
- Teraoka, T.; Numata, H. Effects of feeding on reproduction and overwintering in female adults of Ooencyrtus nezarae Ishii (Hymenoptera: Encyrtidae). Appl. Entomol. Zool. 2000, 35, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Grasso, D.A.; Pandolfi, C.; Bazihizina, N.; Nocentini, D.; Nepi, M.; Mancuso, S. Extrafloral-nectar-based partner manipulation in plant-ant relationships. AoB Plants 2015, 7, plv002. [Google Scholar] [CrossRef] [Green Version]
- Douglas, A.E. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Madsen, N.E.L.; Sorensen, P.B.; Offenberg, J. Sugar and amino acid preference in the black garden ant Lasius niger (L.). J. Insect Physiol. 2017, 100, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Villamil, N.; Marquez-Guzman, J.; Boege, K. Understanding ontogenetic trajectories of indirect defence: Ecological and anatomical constraints in the production of extrafloral nectaries. Ann Bot 2013, 112, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villamil, N. Why are flowers sweeter than fruits or buds? Variation in extrafloral nectar secretion throughout the floral ontogeny of a myrmecophile. Biotropica 2017, 49, 581–585. [Google Scholar] [CrossRef]
- Villamil, N.; Boege, K.; Stone, G.N. Testing the Distraction Hypothesis: Do extrafloral nectaries reduce ant-pollinator conflict? J. Ecol. 2019, 107, 1377–1391. [Google Scholar] [CrossRef] [Green Version]
- Iakovlev, I.K.; Novgorodova, T.A.; Tiunov, A.V.; Reznikova, Z.I. Trophic position and seasonal changes in the diet of the red wood ant Formica aquilonia as indicated by stable isotope analysis. Ecol. Entomol. 2017, 42, 263–272. [Google Scholar] [CrossRef]
- Rosengren, R.; Pamilo, P. The evolution of polygyny and polydomy in mound-building Formica ants. Acta Entomol. Fenn. 1983, 42, 65–77. [Google Scholar]
- Punttila, P.; Kilpeläinen, J. Distribution of mound-building ant species (Formica spp., Hymenoptera) in Finland: Preliminary results of a national survey. Ann. Zool. Fenn. 2009, 46, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Punttila, P. Succession, forest fragmentation, and the distribution of wood ants. Oikos 1996, 75, 291–298. [Google Scholar] [CrossRef]
- Seifert, B. The Ants of Central and Northern Europe; Lutra Verlags- und Vertriebsgeschellschaft: Boxberg, Germany, 2018; pp. 1–408. [Google Scholar]
- Fisher, B.L.; Cover, S.P. Ants of North America—A Guide to the Genera; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA; London, UK, 2007; p. 194. [Google Scholar]
- Jurgensen, M.F.; Storer, A.J.; Risch, A.C. Red wood ants in North America. Ann. Zool. Fenn. 2005, 42, 235–242. [Google Scholar]
- Weseloh, R.M. Forest characteristics associated with abundance of foraging ants (Hymenoptera: Formicidae) in Connecticut. Environ. Entomol. 1995, 24, 1453–1457. [Google Scholar] [CrossRef]
- Korochkina, N.I.; Konopleva, E.E.; Zryanina, T.A. Population Structure of Formica aquilonia (Hymenoptera, Formicidae) at the Border of Boreal and Subboreal Landscapes in the Volga River Region. Zool. Zhurnal 2014, 93, 559–569. [Google Scholar]
- Sondej, I.; Domisch, T.; Finer, L.; Czechowski, W. Wood ants in the Bialowieza Forest and factors affecting their distribution. Ann. Zool. Fenn. 2018, 55, 103–114. [Google Scholar] [CrossRef]
- Hansen, L.D.; Klotz, J.H. Carpenter Ants of the United States and Canada; Cornell University Press: Ithaca, NY, USA, 2005; p. 204. [Google Scholar]
- Locatelli, B.; Lavorel, S.; Sloan, S.; Tappeiner, U.; Geneletti, D. Characteristic trajectories of ecosystem services in mountains. Front. Ecol. Environ. 2017, 15, 150–159. [Google Scholar] [CrossRef]
- Simpraga, M.; Ghimire, R.P.; Van Der Straeten, D.; Blande, J.D.; Kasurinen, A.; Sorvari, J.; Holopainen, T.; Adriaenssens, S.; Holopainen, J.K.; Kivimaenpaa, M. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. Eur. J. For. Res. 2019, 138, 763–787. [Google Scholar] [CrossRef] [Green Version]
- Affek, A.N. Indicators of ecosystem potential for pollination and honey production. Ecol. Ind. 2018, 94, 33–45. [Google Scholar] [CrossRef]
- Thurman, J.H.; Northfield, T.D.; Snyder, W.E. Weaver Ants Provide Ecosystem Services to Tropical Tree Crops. Front. Ecol. Evol. 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Wardle, D.A.; Hyodo, F.; Bardgett, R.D.; Yeates, G.W.; Nilsson, M. Long-term aboveground and belowground consequences of red wood ant exclusion in boreal forest. Ecology 2011, 92, 645–656. [Google Scholar] [CrossRef]
- Giovanetti, M.; Lippi, M.M.; Foggi, B.; Giuliani, C. Exploitation of the invasive Acacia pycnantha pollen and nectar resources by the native bee Apis mellifera. Ecol. Res. 2015, 30, 1065–1072. [Google Scholar] [CrossRef]
- Salonen, A.; Virjamo, V.; Tammela, P.; Fauch, L.; Julkunen-Tiitto, R. Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem. 2017, 237, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.M.; Fellowes, M.D.E.; Sage, R.B.; Leather, S.R. Host selection and performance of the giant willow aphid, Tuberolachnus salignus Gmelin—Implications for pest management. Agric. For. Entomol. 2001, 3, 183–189. [Google Scholar] [CrossRef]
- Salle, A.; Jerger, R.; Vincent-Barbaroux, C.; Baubet, O.; Dahuron, D.; Bourgerie, S.; Lieutier, F. Tree-killing aphid dramatically reduces bark contents in carbohydrates and nitrogen compounds. For. Ecol. Manag. 2018, 407, 23–30. [Google Scholar] [CrossRef]
- Whittaker, J.B.; Warrington, S. An Experimental Field-Study of Different Levels of Insect Herbivory Induced by Formica rufa Predation on Sycamore (Acer pseudoplatanus). Effects on Tree Growth. J. Appl. Ecol. 1985, 22, 797–811. [Google Scholar] [CrossRef]
- Kilpelainen, J.; Finer, L.; Neuvonen, S.; Niemela, P.; Domisch, T.; Risch, A.C.; Jurgensen, M.F.; Ohashi, M.; Sundstrom, L. Does the mutualism between wood ants (Formica rufa group) and Cinara aphids affect Norway spruce growth? For. Ecol. Manag. 2009, 257, 238–243. [Google Scholar] [CrossRef]
- Lindstedt, C.; Mappes, J.; Paivinen, J.; Varama, M. Effects of group size and pine defence chemicals on Diprionid sawfly survival against ant predation. Oecologia 2006, 150, 519–526. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Forest Management and Biodiversity Conservation Based on Natural Ecosystem Dynamics in Northern Europe: The Complexity Challenge. Ambio 2009, 38, 309–315. [Google Scholar] [CrossRef]
- Corlett, R.T. The Anthropocene concept in ecology and conservation. Trends Ecol. Evol. 2015, 30, 36–41. [Google Scholar] [CrossRef]
- Levin, D.A. Alkaloid-Bearing Plants—Ecogeographic Perspective. Am. Nat. 1976, 110, 261–284. [Google Scholar] [CrossRef]
- Anstett, D.N.; Ahern, J.R.; Johnson, M.T.J.; Salminen, J. Testing for latitudinal gradients in defense at the macroevolutionary scale. Evolution 2018, 72, 2129–2143. [Google Scholar] [CrossRef]
- Johnson, M.T.J.; Rasmann, S. The latitudinal herbivory-defence hypothesis takes a detour on the map. New Phytol. 2011, 191, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Bonser, S.P.; Poore, A.G.B.; Wallis, I.R.; Foley, W.J. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct. Ecol. 2011, 25, 380–388. [Google Scholar] [CrossRef]
- Hämet-Ahti, L.; Suominen, J.; Ulvinen, T.; Uotila, P. (Eds.) Retkeilykasvio (Field Flora of Finland); Finnish Museum of Natural History, Botanical Museum: Helsinki, Finland, 1998. [Google Scholar]
- Lampinen, R.; Lahti, T. Kasviatlas 2018; Distibution Maps; University of Helsinki, Finnish Museum of Natural History: Helsinki, Finland, 2019; Available online: http://koivu.luomus.fi/kasviatlas (accessed on 30 October 2019).
- Kellomaki, S.; Peltola, H.; Nuutinen, T.; Korhonen, K.T.; Strandman, H. Sensitivity of managed boreal forests in Finland to climate change, with implications for adaptive management. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2341–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wooley, S.C.; Donaldson, J.R.; Gusse, A.C.; Lindroth, R.L.; Stevens, M.T. Extrafloral nectaries in aspen (Populus tremuloides): Heritable genetic variation and herbivore-induced expression. Ann. Bot. 2007, 100, 1337–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyman, T.; Julkunen-Tiitto, R. Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc. Natl. Acad. Sci. USA 2000, 97, 13184–13187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lersten, N.R.; Czlapinski, A.R.; Curtis, J.D.; Freckmann, R.; Horner, H.T. Oil bodies in leaf mesophyll cells of angiosperms: Overview and a selected survey. Am J Bot 2006, 93, 1731–1739. [Google Scholar] [CrossRef]
- Wilkinson, H.P. Leaf teeth in certain Salicaceae and ‘Flacourtiaceae’. Bot. J. Linn. Soc. 2007, 155, 241–256. [Google Scholar] [CrossRef] [Green Version]
- Ianishevskii, D.E. The extrafloral nectar-glands of Salix. Trudy Bot. Inst. Akad. Nauk SSSR Ser4 1941, 5, 258–294. [Google Scholar]
- Curtis, J.D.; Lersten, N.R. Morphology and Anatomy of Resin Glands in Salix lucida (Salicaceae). Am. J. Bot. 1980, 67, 1289–1296. [Google Scholar] [CrossRef]
- Barkalov, V.Y.; Kozyrenko, M.M. Phylogenetic Relationships of Salix L. subg. Salix Species (Salicaceae) according to Sequencing Data of Intergenic Spacers of the Chloroplast Genome and ITS rDNA. Russ. J. Gen. 2014, 50, 940–949. [Google Scholar]
- Weber, M.G.; Clement, W.L.; Donoghue, M.J.; Agrawal, A.A. Phylogenetic and Experimental Tests of Interactions among Mutualistic Plant Defense Traits in Viburnum (Adoxaceae). Am. Nat. 2012, 180, 450–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of forest insect pests to climate change: Not so simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kudo, G.; Cooper, E.J. When spring ephemerals fail to meet pollinators: Mechanism of phenological mismatch and its impact on plant reproduction. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef] [Green Version]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Penuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef]
- Blande, J.D.; Holopainen, J.K.; Niinemets, U. Plant volatiles in polluted atmospheres: Stress responses and signal degradation. Plant Cell Environ. 2014, 37, 1892–1904. [Google Scholar] [CrossRef] [Green Version]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect Odorscapes: From Plant Volatiles to Natural Olfactory Scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef]
- Saikkonen, K.; Taulavuori, K.; Hyvonen, T.; Gundel, P.E.; Hamilton, C.E.; Vanninen, I.; Nissinen, A.; Helander, M. Climate change-driven species’ range shifts filtered by photoperiodism. Nat. Clim. Chang. 2012, 2, 239–242. [Google Scholar] [CrossRef]
- Ward, S.F.; Moon, R.D.; Herms, D.A.; Aukema, B.H. Determinants and consequences of plant-insect phenological synchrony for a non-native herbivore on a deciduous conifer: Implications for invasion success. Oecologia 2019, 190, 867–878. [Google Scholar] [CrossRef]
- Liberloo, M.; Calfapietra, C.; Lukac, M.; Godbold, D.; Luos, Z.B.; Polle, A.; Hoosbeek, M.R.; Kull, O.; Marek, M.; Raines, C.; et al. Woody biomass production during the second rotation of a bio-energy Populus plantation increases in a future high CO2 world. Glob. Chang. Biol. 2006, 12, 1094–1106. [Google Scholar] [CrossRef]
- Calfapietra, C.; Mugnozza, G.S.; Karnosky, D.F.; Loreto, F.; Sharkey, T.D. Isoprene emission rates under elevated CO2 and O3 in two field-grown aspen clones differing in their sensitivity to O3. New Phytol. 2008, 179, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, A.; Rusterholz, H.P.; Stocklin, J. Elevated carbon dioxide increases nectar production in Epilobium angustifolium L. Oecologia 2005, 146, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Fabian, B.; Atwell, B.J.; Hughes, L. Response of extrafloral nectar production to elevated atmospheric carbon dioxide. Aust. J. Bot. 2018, 66, 479–488. [Google Scholar] [CrossRef]
- Vuorinen, T.; Nerg, A.M.; Ibrahim, M.A.; Reddy, G.; Holopainen, J.K. Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol. 2004, 135, 1984–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamont, M.; Crepelliere, S.; Jaloux, B. Effect of extrafloral nectar provisioning on the performance of the adult parasitoid Diaeretiella rapae. Biol. Control 2013, 65, 271–277. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Mutanda, I.; Inafuku, M.; Saitoh, S.; Iwasaki, H.; Fukuta, M.; Watanabe, K.; Oku, H. Temperature controls on the basal emission rate of isoprene in a tropical tree Ficus septica: Exploring molecular regulatory mechanisms. Plant Cell Environ. 2016, 39, 2260–2275. [Google Scholar] [CrossRef]
- Boudouris, J.; Queenborough, S.A. Diversity and distribution of extra-floral nectaries in the cerrado savanna vegetation of Brazil. PeerJ 2013, 1, e219. [Google Scholar] [CrossRef] [Green Version]
- Bristow, C.M.; Yanity, E. Seasonal response of workers of the Allegheny mound ant, Formica exsectoides (Hymenoptera: Formicidae) to artificial honeydews of varying nutritional content. Great Lakes Entomol. 1999, 32, 15–27. [Google Scholar]
- Newman, J.R.; Wagner, D. The influence of water availability and defoliation on extrafloral nectar secretion in quaking aspen (Populus tremuloides). Botany 2013, 91, 761–767. [Google Scholar] [CrossRef]
- Jones, I.M.; Koptur, S. Quantity over quality: Light intensity, but not red/far-red ratio, affects extrafloral nectar production in Senna mexicana var. chapmanii. Ecol. Evol. 2015, 5, 4108–4114. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.M.; Koptur, S.; Gallegos, H.R.; Tardanico, J.P.; Trainer, P.A.; Pena, J. Changing light conditions in pine rockland habitats affect the intensity and outcome of ant-plant interactions. Biotropica 2017, 49, 83–91. [Google Scholar] [CrossRef]
- ALRahahleh, L.; Kilpelainen, A.; Ikonen, V.P.; Strandman, H.; Asikainen, A.; Venalainen, A.; Kaurola, J.; Kangas, J.; Peltola, H. Effects of using certain tree species in forest regeneration on volume growth, timber yield, and carbon stock of boreal forests in Finland under different CMIP5 projections. Eur. J. For. Res. 2018, 137, 573–591. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.R.; Breger, B.; Drummond, F. Novel plant–insect interactions in an urban environment: Enemies, protectors, and pollinators of invasive knotweeds. Ecosphere 2019, 10, 2885. [Google Scholar] [CrossRef]
- Radhika, V.; Kost, C.; Mithoefer, A.; Boland, W. Regulation of extrafloral nectar secretion by jasmonates in lima bean is light dependent. Proc. Natl. Acad. Sci. USA 2010, 107, 17228–17233. [Google Scholar] [CrossRef] [Green Version]
- Yi, K.; Liu, J.; Ban-Weiss, G.; Zhang, J.; Tao, W.; Cheng, Y.; Tao, S. Response of the global surface ozone distribution to Northern Hemisphere sea surface temperature changes: Implications for long-range transport. Atmos. Chem. Phys. 2017, 17, 8771–8788. [Google Scholar] [CrossRef] [Green Version]
- Jeon, W.; Lee, S.; Lee, H.; Park, C.; Kim, D.; Park, S. A study on high ozone formation mechanism associated with change of NOx/VOCs ratio at a rural area in the Korean Peninsula. Atmos. Environ. 2014, 89, 10–21. [Google Scholar] [CrossRef]
- Bronstein, J.L.; Alarcon, R.; Geber, M. The evolution of plant-insect mutualisms. New Phytol. 2006, 172, 412–428. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Chen, I.; Hill, J.K.; Ohlemueller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Takolander, A.; Hickler, T.; Meller, L.; Cabeza, M. Comparing future shifts in tree species distributions across Europe projected by statistical and dynamic process-based models. Reg. Environ. Chang. 2019, 19, 251–266. [Google Scholar] [CrossRef] [Green Version]
- Boisvert-Marsh, L.; Perie, C.; de Blois, S. Divergent responses to climate change and disturbance drive recruitment patterns underlying latitudinal shifts of tree species. J. Ecol. 2019, 107, 1956–1969. [Google Scholar] [CrossRef]
- Screen, J.A.; Simmonds, I. The central role of diminishing sea ice in recent Arctic temperature amplification. Nature 2010, 464, 1334–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevey, J.S.; Rixen, C.; Rueger, N.; Hoye, T.T.; Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Ashton, I.W.; Cannone, N.; Chisholm, C.L.; et al. Warming shortens flowering seasons of tundra plant communities. Nat. Ecol. Evol. 2019, 3, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, L.; Fretwell, S.D.; Arruda, J.; Niemela, P. Exploitation Ecosystems in Gradients of Primary Productivity. Am. Nat. 1981, 118, 240–261. [Google Scholar] [CrossRef]
- Bleiker, K.P.; Smith, G.D.; Humble, L.M. Cold Tolerance of Mountain Pine Beetle (Coleoptera: Curculionidae) Eggs from the Historic and Expanded Ranges. Environ. Entomol. 2017, 46, 1165–1170. [Google Scholar] [CrossRef]
- Veteli, T.O.; Lahtinen, A.; Repo, T.; Niemela, P.; Varama, M. Geographic variation in winter freezing susceptibility in the eggs of the European pine sawfly (Neodiprion sertifer). Agric. For. Entomol. 2005, 7, 115–120. [Google Scholar] [CrossRef]
- Pellissier, L.; Moreira, X.; Danner, H.; Serrano, M.; Salamin, N.; van Dam, N.M.; Rasmann, S. The simultaneous inducibility of phytochemicals related to plant direct and indirect defences against herbivores is stronger at low elevation. J. Ecol. 2016, 104, 1116–1125. [Google Scholar] [CrossRef]
- Kergunteuil, A.; Roeder, G.; Rasmann, S. Environmental gradients and the evolution of tri-trophic interactions. Ecol. Lett. 2019, 22, 292–301. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Mofikoya, A.O.; Bui, T.N.T.; Kivimäenpää, M.; Holopainen, J.K.; Himanen, S.J.; Blande, J.D. Foliar behaviour of biogenic semi-volatiles: Potential applications in sustainable pest management. Arthropod-Plant Interact. 2019, 13, 193–212. [Google Scholar] [CrossRef] [Green Version]
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate Change and the Past, Present, and Future of Biotic Interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeder, K.A.; Kaspari, M. From cryptic herbivore to predator: Stable isotopes reveal consistent variability in trophic levels in an ant population. Ecology 2017, 98, 297–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trager, M.D.; Bhotika, S.; Hostetler, J.A.; Andrade, G.V.; Rodriguez-Cabal, M.A.; McKeon, C.S.; Osenberg, C.W.; Bolker, B.M. Benefits for plants in ant-plant protective mutualisms: A meta-analysis. PLoS ONE 2010, 5, e14308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D. Cherries, ants and tent caterpillars: Timing of nectar production in relation to susceptibility of caterpillars to ant predation. Ecology 1978, 59, 686–692. [Google Scholar] [CrossRef]
- Essiamah, S.K. Spring Sap of Trees. Berichte Deutsch. Bot. Ges. 1980, 93, 257–267. [Google Scholar]
- Kallio, H.; Ahtonen, S. Seasonal-Variations of the Sugars in Birch Sap. Food Chem. 1987, 25, 293–304. [Google Scholar] [CrossRef]
- Westhoff, M.; Schneider, H.; Zimmermann, D.; Mimietz, S.; Stinzing, A.; Wegner, L.H.; Kaiser, W.; Krohne, G.; Shirley, S.; Jakob, P.; et al. The mechanisms of refilling of xylem conduits and bleeding of tall birch during spring. Plant Biol. 2008, 10, 604–623. [Google Scholar] [CrossRef]
- Hölttä, T.; Dominguez Carrasco, M.D.R.; Salmon, Y.; Aalto, J.; Vanhatalo, A.; Back, J.; Lintunen, A. Water relations in silver birch during springtime: How is sap pressurised? Plant Biol. 2018, 20, 834–847. [Google Scholar] [CrossRef]
- Kallio, H.; Ahtonen, S.; Raulo, J.; Linko, R.R. Identification of the Sugars and Acids in Birch Sap. J. Food Sci. 1985, 50. [Google Scholar] [CrossRef]
- Luczaj, L.; Bilek, M.; Stawarczyk, K. Sugar content in the sap of birches, hornbeams and maples in southeastern Poland. Cent. Eur. J. Biol. 2014, 9, 410–416. [Google Scholar] [CrossRef]
- Vihera-Aarnio, A.; Velling, P. Growth, wood density and bark thickness of silver birch originating from the Baltic countries and Finland in two Finnish provenance trials. Silva Fenn. 2017, 51, 7731. [Google Scholar] [CrossRef] [Green Version]
- Kankaanhuhta, V. Lehtitikaskuoriainen (Trypodendron signatum); MetINFO Service: Luke, Finland, 2003; Available online: http://www.metla.fi.ezproxy.uef.fi:2048/metinfo/metsienterveys/lajit_kansi/trsign-n.htm (accessed on 30 October 2019).
- Elton, C. Territory among wood ants (Formica rufa L.) at Picket Hill. J. Anim. Ecol. 1932, 1, 69–76. [Google Scholar] [CrossRef]
- Rosengren, R.; Sundström, L. The Foraging System of a Red Wood Ant Colony (Formica s. str.)—Collecting and Defending Food Through an Extended Phenotype. In From Individual to Collective Behavior in Social Insects; Pasteels, K.M., Deneubourg, J.L., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1987; pp. 117–137. [Google Scholar]
- Staab, M.; Fornoff, F.; Klein, A.; Bluethgen, N. Ants at Plant Wounds: A Little-Known Trophic Interaction with Evolutionary Implications for Ant-Plant Interactions. Am. Nat. 2017, 190, 442–450. [Google Scholar] [CrossRef]
- Domisch, T.; Finer, L.; Neuvonen, S.; Niemelä, P.; Risch, A.C.; Kilpeläinen, J.; Ohashi, M.; Jurgensen, M.F. Foraging activity and dietary spectrum of wood ants (Formica rufa group) and their role in nutrient fluxes in boreal forests. Ecol. Entomol. 2009, 34, 369–377. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Peltonen, P. Bright autumn colours of deciduous trees attract aphids: Nutrient retranslocation hypothesis. Oikos 2002, 99, 184–188. [Google Scholar] [CrossRef]
- Peltonen, P.A.; Julkunen-Tiitto, R.; Vapaavuori, E.; Holopainen, J.K. Effects of elevated carbon dioxide and ozone on aphid oviposition preference and birch bud exudate phenolics. Glob. Chang. Biol. 2006, 12, 1670–1679. [Google Scholar] [CrossRef]
- Wagner, D.L.; Gagliardi, B.L. Hairstreaks (and Other Insects) Feeding at Galls, Honeydew, Extrafloral Nectaries, Sugar Bait, Cars, and Other Routine Substrates. Am. Entomol. 2015, 61, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Pierce, M.P. The ecological and evolutionary importance of nectar-secreting galls. Ecosphere 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Shanin, V.; Komarov, A.; Makipaa, R. Tree species composition affects productivity and carbon dynamics of different site types in boreal forests. Eur. J. For. Res. 2014, 133, 273–286. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.; Lutz, S.; Wen, J.; Potter, D. The Bitter and the Sweet: Inference of Homology and Evolution of Leaf Glands in Prunus (Rosaceae) through Anatomy, Micromorphology, and Ancestral-Character State Reconstruction. Int. J. Plant Sci. 2013, 174, 27–46. [Google Scholar] [CrossRef]
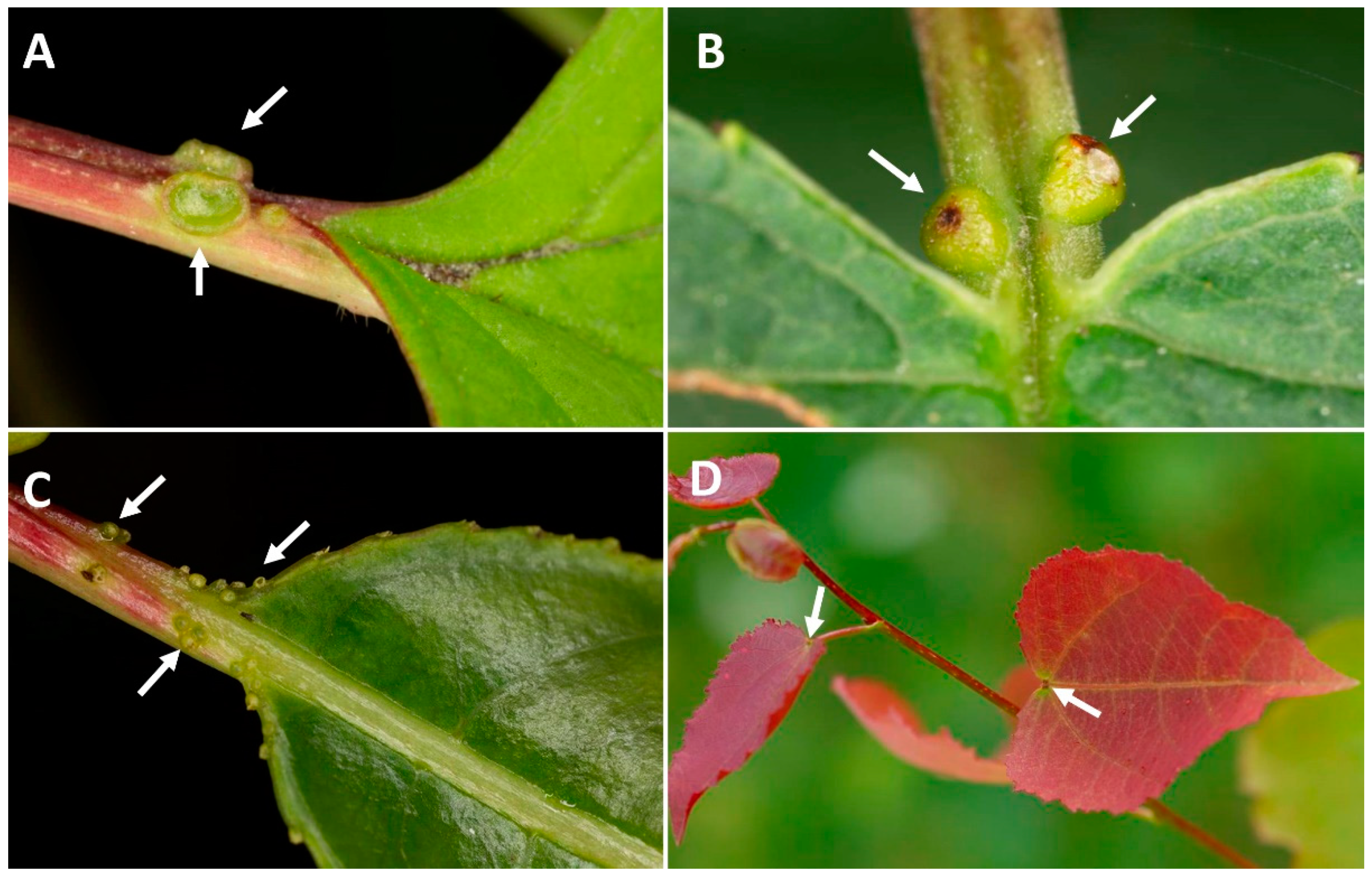
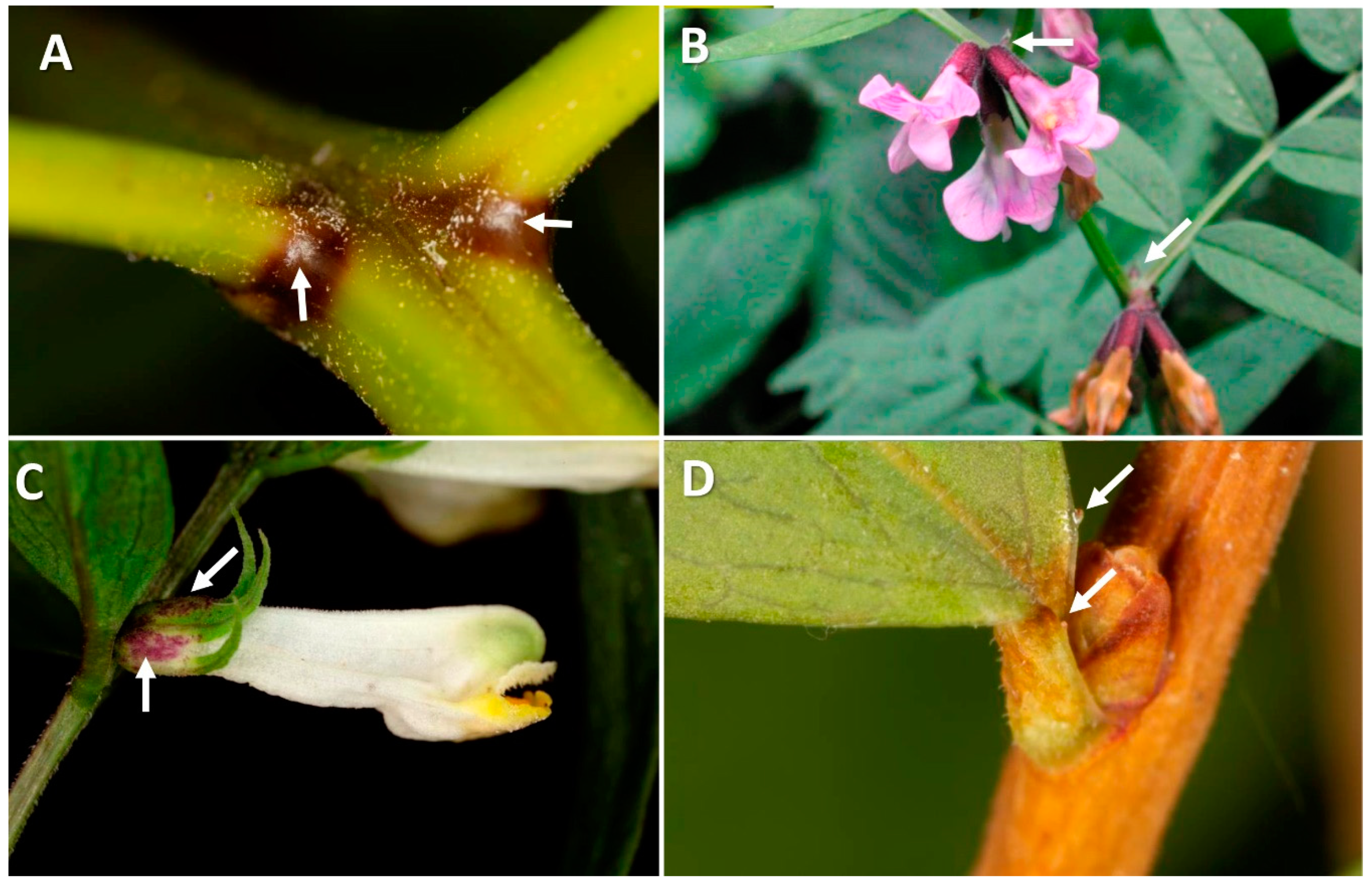
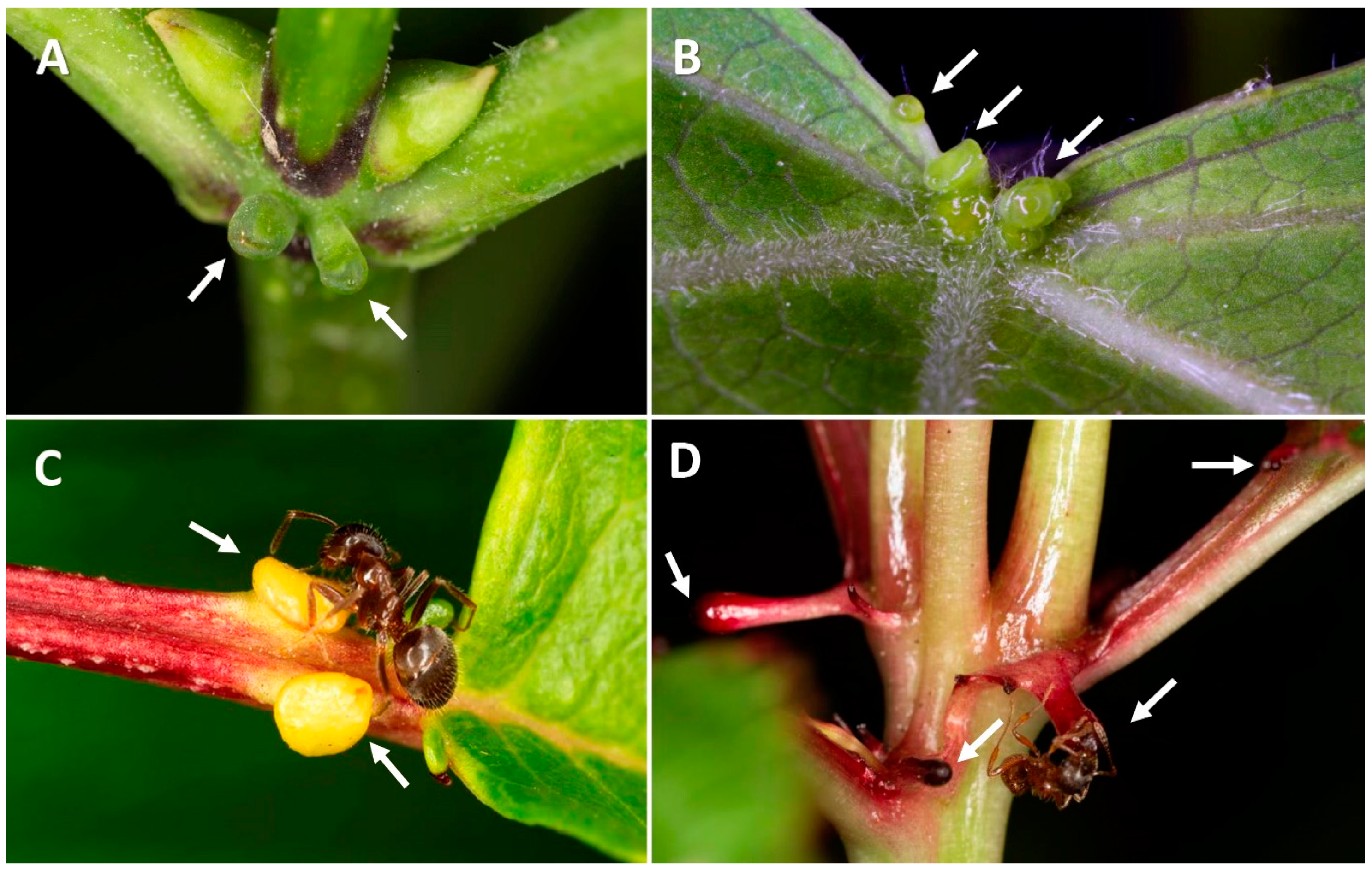
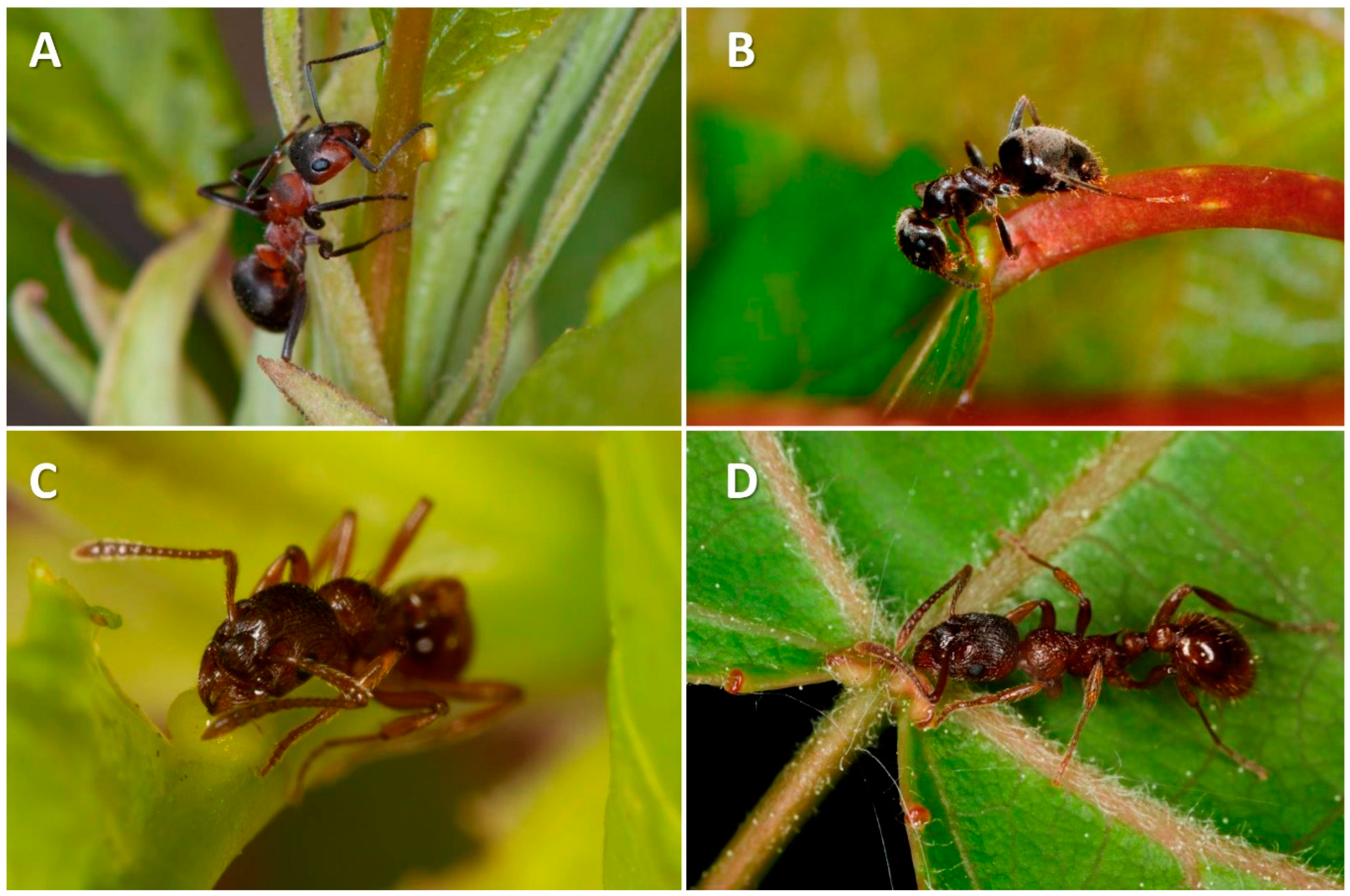
| Type of Disturbance | EFN Production Response | Ref. | Potential Effects Affecting Ants and Other Mutualists | Ref. |
|---|---|---|---|---|
| Plant physiological level responses | ||||
| Elevated CO2 | A higher number of leaves producing EFN, no quality changes | [131] | Improved EFN availability | [131] |
| Warming | Accelerates leaf maturation leading to earlier ceasing of EFN production | [59] | Possible sucrose shortage later in the growth season | [137] |
| Drought | Reduced sugar secretion | [138] | Reduced EFN quality | [139] |
| Cloudiness/shading | Reduced EFN production | [140] | Reduced EFN availability, lower herbivore predation rate, reduced plant fitness | [140] |
| Forest community-level responses | ||||
| Warming | Deciduous trees become more dominant in conifer forests | [141] | Improved EFN availability → change in ant species composition | [85] |
| Warming | Among alien invasive plant species more efficient EFN producers | [142] | Ants can move to invasive plant species | [142] |
| Elevated O3 | Atmospheric degradation of volatile organic compound (VOC) signal reduces EFN induction in neighboring plants | [18] | Less EFN available for natural enemies of herbivores → higher damage risk | [18] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holopainen, J.K.; Blande, J.D.; Sorvari, J. Functional Role of Extrafloral Nectar in Boreal Forest Ecosystems under Climate Change. Forests 2020, 11, 67. https://doi.org/10.3390/f11010067
Holopainen JK, Blande JD, Sorvari J. Functional Role of Extrafloral Nectar in Boreal Forest Ecosystems under Climate Change. Forests. 2020; 11(1):67. https://doi.org/10.3390/f11010067
Chicago/Turabian StyleHolopainen, Jarmo K., James D. Blande, and Jouni Sorvari. 2020. "Functional Role of Extrafloral Nectar in Boreal Forest Ecosystems under Climate Change" Forests 11, no. 1: 67. https://doi.org/10.3390/f11010067
APA StyleHolopainen, J. K., Blande, J. D., & Sorvari, J. (2020). Functional Role of Extrafloral Nectar in Boreal Forest Ecosystems under Climate Change. Forests, 11(1), 67. https://doi.org/10.3390/f11010067







