Temporal Effects of Thinning on the Leaf C:N:P Stoichiometry of Regenerated Broadleaved Trees in Larch Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Survey Methods
2.3. Sampling and Chemical Analysis
2.4. Data Analysis
3. Results
3.1. Canopy Openness
3.2. Soil C:N:P Stoichiometry
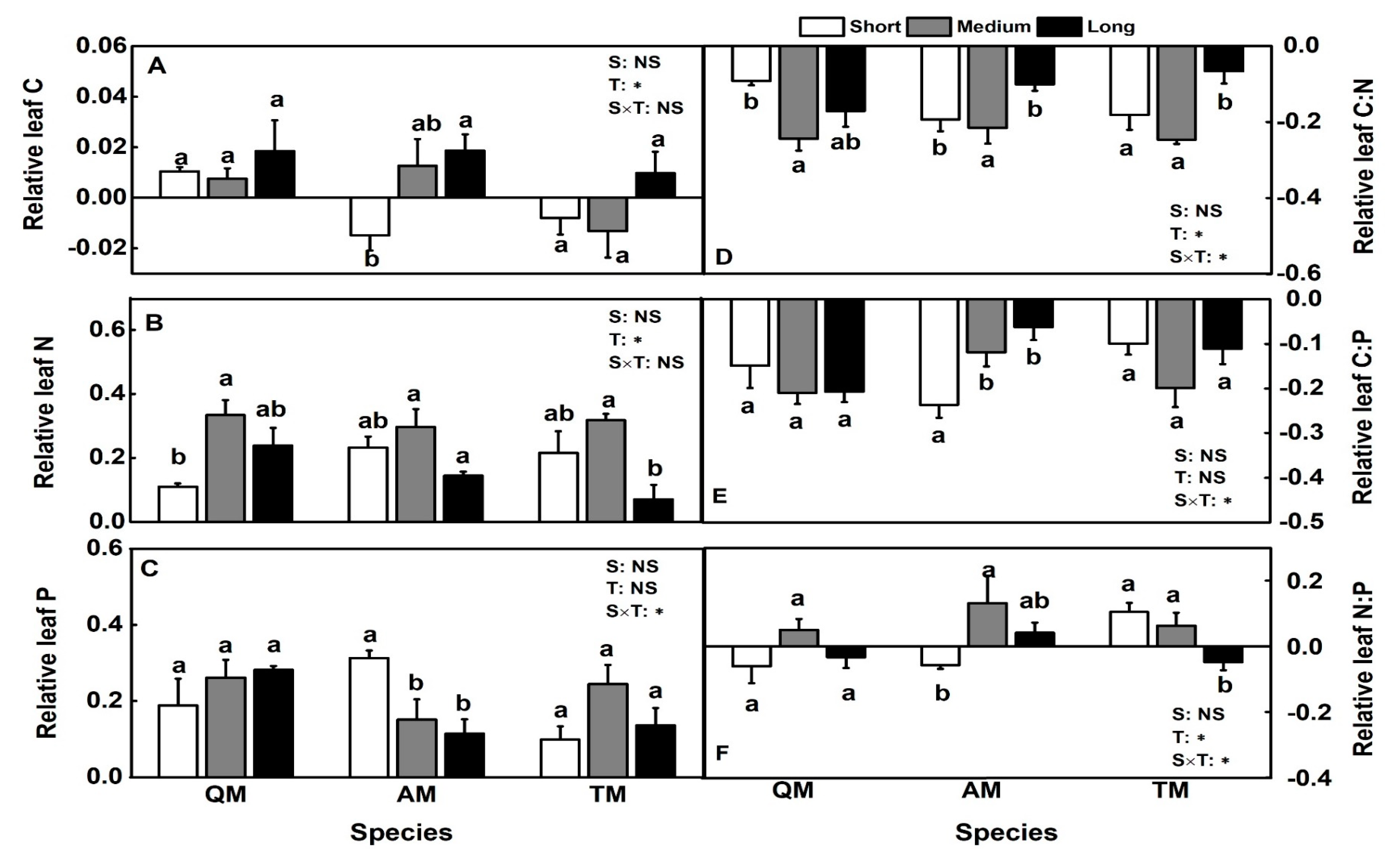
3.3. Leaf C:N:P Stoichiometry
3.4. Relationship between Plant Stoichiometry and Light Availability and Soil Stoichiometry
4. Discussion
4.1. Temporal Effects of Thinning on Light Availability and Soil C:N:P Characteristics
4.2. Temporal Effects of Thinning on Leaf C:N:P Characteristics of Different Species
4.3. Implication for Determining the Thinning Interval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, J. Planted Forests: Uses, Impacts and Sustainability; FAO and CAB International: Wallingford, UK, 2009. [Google Scholar]
- Spracklen, B.D.; Lane, J.V.; Spracklen, D.V.; Williams, N.; Kunin, W.E. Regeneration of native broadleaved species on clearfelled conifer plantations in upland Britain. For. Ecol. Manag. 2013, 310, 204–212. [Google Scholar] [CrossRef]
- Felton, A.; Lindbladh, M.; Brunet, J.; Fritz, O. Replacing coniferous monocultures with mixed-species production stands: An assessment of the potential benefits for forest biodiversity in northern Europe. For. Ecol. Manag. 2010, 260, 939–947. [Google Scholar] [CrossRef]
- Yang, K.; Shi, W.; Zhu, J.J. The impact of secondary forests conversion into Larch plantations on soil chemical and microbiological properties. Plant Soil 2013, 368, 535–546. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, L.; He, Z.; Wang, R.; Wu, P.; Ma, X. Thinning increases understory diversity and biomass, and improves soil properties without decreasing growth of Chinese fir in southern China. Environ. Sci. Pollut. Res. 2016, 23, 24135–24150. [Google Scholar] [CrossRef] [PubMed]
- Holuša, J.; Lubojacký, J.; Čurn, V.; Tonka, T.; Lukášová, K.; Horák, J. Combined effects of drought stress and Armillaria infection on tree mortality in Norway spruce plantations. For. Ecol. Manag. 2018, 427, 434–445. [Google Scholar] [CrossRef]
- Mason, W.L.; Zhu, J.J. Silviculture of planted forests managed for multifunctional objectives: Lessons from Chinese and British experience. In Challenge and Opportunity for the World’s Forests in the 21st Century; Fenning, T., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 767–781. [Google Scholar]
- Yan, T.; Lü, X.T.; Yang, K.; Zhu, J.J. Leaf nutrient dynamics and nutrient resorption: A comparison between larch plantations and adjacent secondary forests in northeast China. J. Plant Ecol. 2016, 9, 165–173. [Google Scholar] [CrossRef]
- Mazza, G.; Agnelli, A.E.; Cantiani, P. Short-term effects of thinning on soil CO2, N2O and CH4 fluxes in Mediterranean forest ecosystems. Sci. Total Environ. 2019, 651, 713–724. [Google Scholar] [CrossRef]
- Zhu, J.J.; Yang, K.; Yan, Q.L.; Liu, Z.G.; Wang, H.X. The feasibility of implementing thinning in pure even-aged Larix olgensis plantations to establish uneven aged larch-broad leaved mixed forests. J. For. Res. 2010, 15, 71–80. [Google Scholar] [CrossRef]
- Carón, M.M.; De Frenne, P.; Brunet, J. Interacting effects of warming and drought on regeneration a-nd early growth of Acer pseudoplatanus and A. platanoides. Plant Biol. 2015, 17, 52–62. [Google Scholar] [CrossRef]
- Elemans, M. Light, nutrients and the growth of herbaceous forest species. Acta Oecol. 2004, 26, 197–202. [Google Scholar] [CrossRef]
- Ceccon, E.; Sánchez, S.; Campo, J. Tree seedling dynamics in two abandoned tropical dry forests of differing successional status in Yucatán, Mexico: A field experiment with N and P fertilization. Plant. Ecol. 2004, 170, 277–285. [Google Scholar] [CrossRef]
- Granhus, A.; Brække, F.H. Nutrient status of Norway spruce stands subjected to different levels of overstorey removal. Trees 2001, 15, 393–402. [Google Scholar] [CrossRef]
- Muraoka, H.; Tang, Y.; Koizumi, H.; Washitani, I. Effects of light and soil water availability on leaf photosynthesis and growth of Arisaema heterophyllum, a riparian forest understorey plant. J. Plant Res. 2002, 115, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Sterck, F.J.; Poorter, L.; Schieving, F. Leaf traits determine the growth-survival trade-off across rain forest tree species. Am. Nat. 2006, 167, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Dhubháin, A.N.; Ferguson, J.; Schmidt, O.; Dyckmans, J.; Osborne, B.; Black, K. Potential use of leaf carbon isotope discrimination for the selection of shade-tolerant species. For. Ecol. Manag. 2006, 237, 394–403. [Google Scholar] [CrossRef]
- González, A.L.; Kominoski, J.S.; Danger, M.; Ishida, S.; Iwai, N.; Rubach, A. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 2010, 119, 779–790. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2016, 85, 133–155. [Google Scholar] [CrossRef]
- Lambers, H.; Mougel, C.; Jaillard, B.; Hinsinger, P. Plant microbe-soil interactions in the rhizosphere: An evolutionary perspective. Plant Soil 2009, 321, 83–115. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.; Kerkhoff, A.; Swenson, N.; Enquist, B. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Bai, S.H.; Dempsey, R.; Reverchon, F.; Blumfield, T.J.; Ryan, S.; Cernusak, L.A. Effects of forest thinning on soil-plant carbon and nitrogen dynamics. Plant Soil 2017, 411, 437–449. [Google Scholar] [CrossRef]
- Ares, A.; Neill, A.R.; Puettmann, K.J. Understory abundance, species diversity and functional attribute response to thinning in coniferous stands. For. Ecol. Manag. 2010, 260, 1104–1113. [Google Scholar] [CrossRef]
- Willms, J.; Bartuszevige, A.; Schwilk, D.W.; Kennedy, P.L. The effects of thinning and burning on understory vegetation in North America: A meta-analysis. For. Ecol. Manag. 2017, 392, 184–194. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta-analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Haughian, S.R.; Frego, K.A. Short-term effects of three commercial thinning treatments on diversity of understory vascular plants in white spruce plantations of northern New Brunswick. For. Ecol. Manag. 2016, 370, 45–55. [Google Scholar] [CrossRef]
- Hale, S.E. The effect of thinning intensity on the below-canopy light environment in a Sitka spruce plantation. For. Ecol. Manag. 2003, 179, 341–349. [Google Scholar] [CrossRef]
- Ritter, E.; Dalsgaard, L.; Einhorn, K.S. Light, temperature and soil moisture regimes following gap formation in a semi-natural beech-dominated forest in Denmark. For. Ecol. Manag. 2005, 206, 15–23. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chiang, J.M.; Mcewan, R.W.; Lin, T.C. Decadal effects of thinning on understory light environments and plant community structure in a subtropical forest. Ecosphere 2018, 9, e02464. [Google Scholar] [CrossRef]
- Cogliastro, A.; Paquette, A. Thinning effect on light regime and growth of under planted red oak and black cherry in post-agricultural forests of south-eastern Canada. New For. 2012, 43, 941–954. [Google Scholar] [CrossRef]
- Bauhus, J.; Aubin, I.; Messier, C.; Connell, M. Composition, structure, light attenuation and nutrient content of the understorey vegetation in a Eucalyptus sieberi regrowth stand 6 years after thinning and fertilisation. For. Ecol. Manag. 2001, 144, 275–286. [Google Scholar] [CrossRef]
- Son, Y.; Lee, Y.Y.; Jun, Y.C.; Kim, Z.S. Light availability and understory vegetation four years after thinning in a Larix leptolepis plantation of central Korea. J. For. Res. 2004, 9, 133–139. [Google Scholar] [CrossRef]
- Tan, X.; Chang, S.X.; Comeau, P.G.; Wang, Y.H. Thinning effects on microbial biomass, N mineralization, and tree growth in a mid-rotation fire-origin lodge pole pine stand in the lower foothills of Alberta, Canada. For. Sci. 2008, 54, 465–474. [Google Scholar] [CrossRef]
- Jerabkova, L.; Prescott, C.E.; Titus, B.D.; Hope, G.D.; Walters, M.B. A meta-analysis of the effects of clearcut and variable-retention harvesting on soil nitrogen fluxes in boreal and temperate forests. Can. J. For. Res. 2011, 41, 1852–1870. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, S.Q.; Liu, S.; Oeding, J. A meta-analysis on the impacts of partial cutting on forest structure and carbon storage. Biogeosciences 2013, 10, 3691–3703. [Google Scholar] [CrossRef]
- Ganzlin, P.W.; Gundale, M.J.; Becknell, R.E.; Cleveland, C.C. Forest restoration treatments have subtle long-term effects on soil C and N cycling in mixed conifer forests. Ecol. Appl. 2016, 26, 1503–1516. [Google Scholar] [CrossRef]
- Hwang, J.; Son, Y. Short-term effects of thinning and liming on forest soils of pitch pine and Japanese larch plantations in central Korea. Ecol. Res. 2006, 21, 671–680. [Google Scholar] [CrossRef]
- Kuehne, C.; Puettmann, K.J. Natural regeneration in thinned Douglas-fir stands in Western Oregon. J. Sustain. Forest. 2008, 27, 246–274. [Google Scholar] [CrossRef]
- Dumais, D.; Marcel, P. Ecophysiology and growth of advance red spruce and balsam fir regeneration after partial cutting in yellow birch-conifer stands. Tree Physiol. 2008, 28, 1221–1229. [Google Scholar] [CrossRef]
- Ramovs, B.V.; Roberts, M.R. Response of plant functional groups within plantations and naturally regenerated forests in southern New Brunswick, Canada. Can. J. For. Res. 2005, 35, 1261–1276. [Google Scholar] [CrossRef]
- Renninger, H.J.; Meinzer, F.C.; Gartner, B.L. Hydraulic architecture and photosynthetic capacity as constraints on release from suppression in Douglas-fir and western hemlock. Tree Physiol. 2007, 27, 33–42. [Google Scholar] [CrossRef][Green Version]
- Yan, Q.L.; Zhu, J.J.; Gang, Q. Comparison of spatial patterns of soil seed banks between larch plantations and adjacent secondary forests in northeast China: Implication for spatial distribution of larch plantations. Trees 2013, 27, 1747–1754. [Google Scholar] [CrossRef]
- Bu, C.Q.; Hu, Z.B.; Yu, L.Z.; Yan, Q.L.; Zheng, X. Forest resources in Qingyuan of Liaoning, Northeast China: Their structure and optimal spatial allocation. Chin. J. Appl. Ecol. 2013, 24, 1070–1076. [Google Scholar]
- Liaoning Forestry Department. Regulation of Forest Tending of Liaoning Province; Liaoning Bureau of Quality and Technical Supervision: Shenyang, China, 2013.
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA): Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation; Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies: Millbrook, NY, USA, 1999. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Methods; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Giuggiola, A.; Jérôme, O.; Rigling, A.; Gessler, A.; Bugmann, H.; Treydte, K. Improvement of water and light availability after thinning at a xeric site: Which matters more? A dual isotope approach. New Phytol. 2016, 210, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Overby, S.T.; Hart, S.C. Short-term belowground responses to thinning and burning treatments in Southwestern ponderosa pine forests of the USA. Forests 2016, 7, 45. [Google Scholar] [CrossRef]
- Grady, K.C.; Hart, S.C. Influences of thinning, prescribed burning, and wildfire on soil processes and properties in southwestern ponderosa pine forests: A retrospective study. For. Ecol Manag. 2006, 234, 123–135. [Google Scholar] [CrossRef]
- Johnson, D.W.; Trettin, C.C.; Todd, D.E. Changes in forest floor and soil nutrients in a mixed oak forest 33 years after stem only and whole-tree harvest. For. Ecol. Manag. 2016, 361, 56–68. [Google Scholar] [CrossRef]
- Black, T.A.; Harden, J.W. Effect of timber harvest on soil carbon storage at Blodgett Experimental Forest, California. Can. J. For. Res. 1995, 25, 1385–1396. [Google Scholar] [CrossRef]
- Hu, B.; Yang, B.; Pang, X.; Bao, W.; Tian, G. Responses of soil phosphorus fractions to gap size in a reforested spruce forest. Geoderma 2016, 279, 61–69. [Google Scholar] [CrossRef]
- Wic-Baena, C.; Andrés-Abellán, M.; Lucas-Borja, M.E.; Martínez-García, E.; García-Morote, F.A.; Rubio, E.; López-Serrano, F.R. Thinning and recovery effects on soil properties in two sites of a Mediterranean forest, in Cuenca Mountain (South-eastern of Spain). For. Ecol. Manag. 2013, 308, 223–230. [Google Scholar] [CrossRef]
- Mitchell, A.K.; Barclay, H.J.; Brix, H.; Pollard, D.F.W.; Benton, R.; Dejong, R. Biomass and nutrient element dynamics in Douglas-fir: Effects of thinning and nitrogen fertilization over 18 years. Can. J. For. Res. 1996, 26, 376–388. [Google Scholar] [CrossRef]
- Kaye, J.P.; Hart, S.C.; Fulé, P.Z.; Covington, W.W.; Moore, M.M.; Kaye, M.W. Initial carbon, nitrogen, and phosphorus fluxes following ponderosa pine restoration treatments. Ecol. Appl. 2005, 15, 1581–1593. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.T.; Zhu, J.J.; Yang, K.; Yu, L.Z.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil 2018, 422, 385–396. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, D.X.; Chen, Z.X.; Li, H.; Deng, J.; Qiao, W.J.; Han, X.H.; Yang, G.H.; Zhong, F.Y.; Huang, J.Y. Substrate quality and soil environmental conditions predict litter decomposition and drive soil nutrient dynamics following afforestation on the Loess Plateau of China. Geoderma 2018, 325, 152–161. [Google Scholar] [CrossRef]
- Piatek, K.B.; Fajvan, M.A.; Turcotte, R.M. Thinning effects on foliar elements in eastern hemlock: Implications for managing the spread of the hemlock woolly adelgid. Can. J. For. Res. 2017, 47, 81–88. [Google Scholar] [CrossRef]
- Kranabetter, J.M.; Coates, K.D. Ten-year postharvest effects of silviculture systems on soil-resource availability and conifer nutrition in a northern temperate forest. Can. J. For. Res. 2004, 34, 800–809. [Google Scholar] [CrossRef]
- Li, M.C.; Zhu, J.J.; Zhang, M. Foliar carbon isotope discrimination and related traits along light gradients in two different functional-type tree species. Eur. J. For. Res. 2013, 132, 815–824. [Google Scholar] [CrossRef]
- Xie, H.; Yu, M.; Cheng, X. Leaf non-structural carbohydrate allocation and C:N:P stoichiometry in response to light acclimation in seedlings of two subtropical shade-tolerant tree species. Plant Physiol. Bioch. 2018, 124, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Iida, H.; Ito, Y.; Park, H.D.; Takahashi, K. Effects of light conditions on growth and defense compound contents of Datura inoxia and D. stramonium. J. Plant Res. 2019, 132, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Lilles, E.B.; Astrup, R.; Lefrançois, M.L.; David, C.K. Sapling leaf trait responses to light, tree height and soil nutrients for three conifer species of contrasting shade tolerance. Tree Physiol. 2014, 34, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Medhurst, J.L.; Beadle, C.L. Photosynthetic capacity and foliar nitrogen distribution in Eucalyptus nitens is altered by high-intensity thinning. Tree Physiol. 2005, 25, 981–991. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Dent, D.H.; Burslem, D.F.R.P. Leaf traits of dipterocarp species with contrasting distributions across a gradient of nutrient and light availability. Plant Ecol. Divers. 2016, 9, 521–533. [Google Scholar] [CrossRef]
- Portsmuth, A.; Niinemets, Ü. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 2007, 2, 61–77. [Google Scholar] [CrossRef]
- Nobles, M.M.; Dillon, W.; Mbila, M. Initial response of soil nutrient pools to prescribed burning and thinning in a managed forest ecosystem of northern Alabama. Soil Sci. Soc. Am. J. 2009, 73, 285–292. [Google Scholar] [CrossRef]
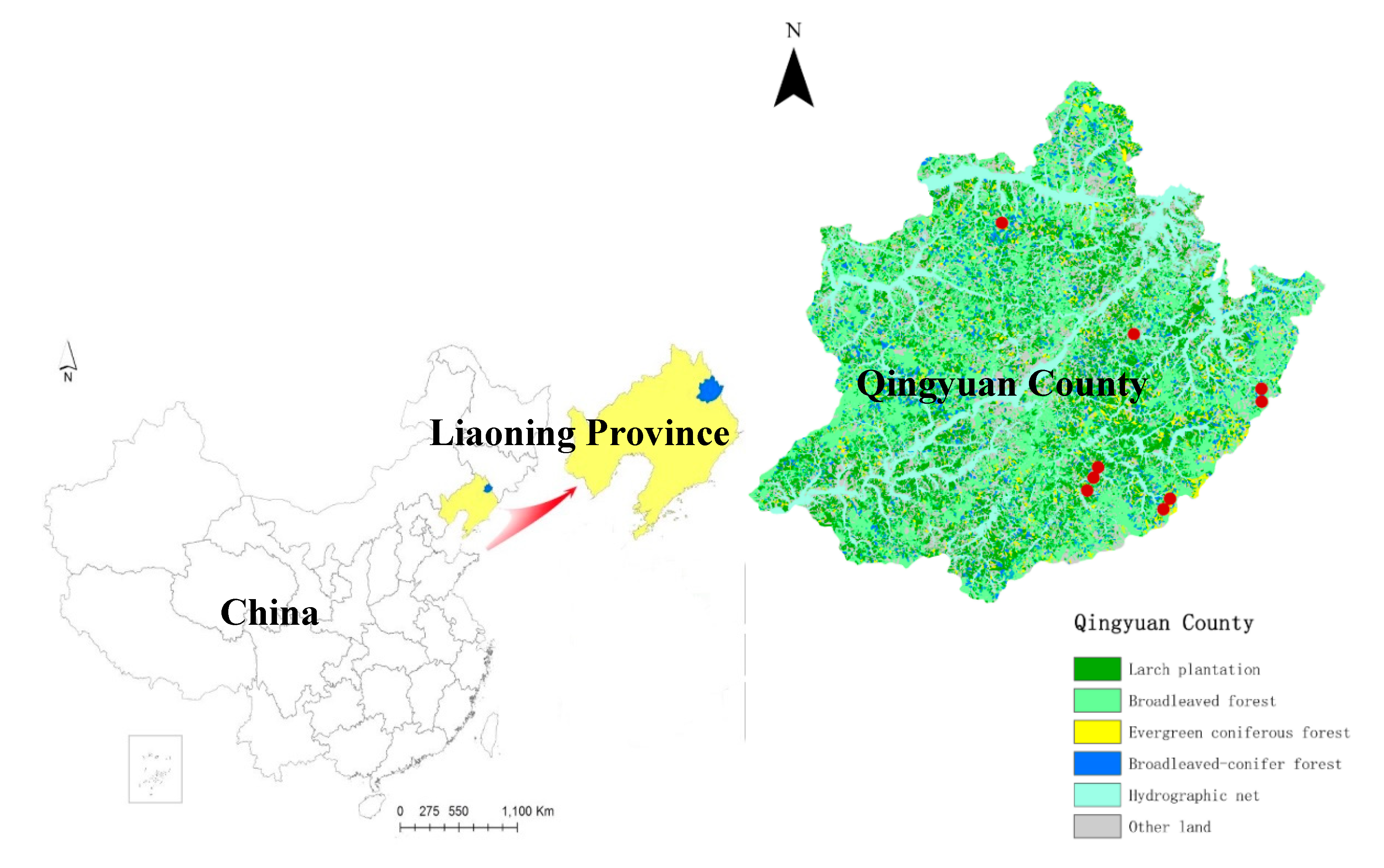
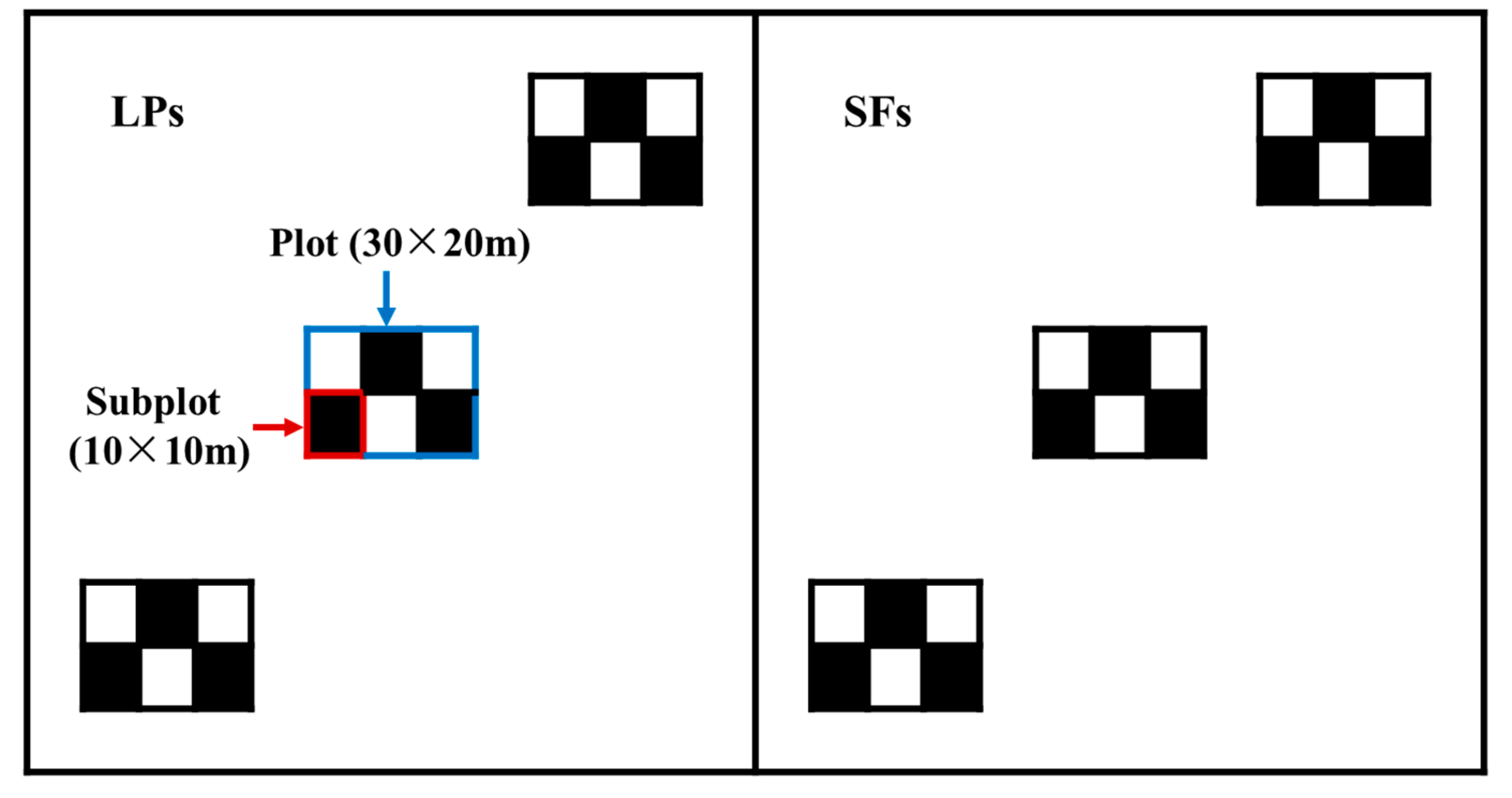
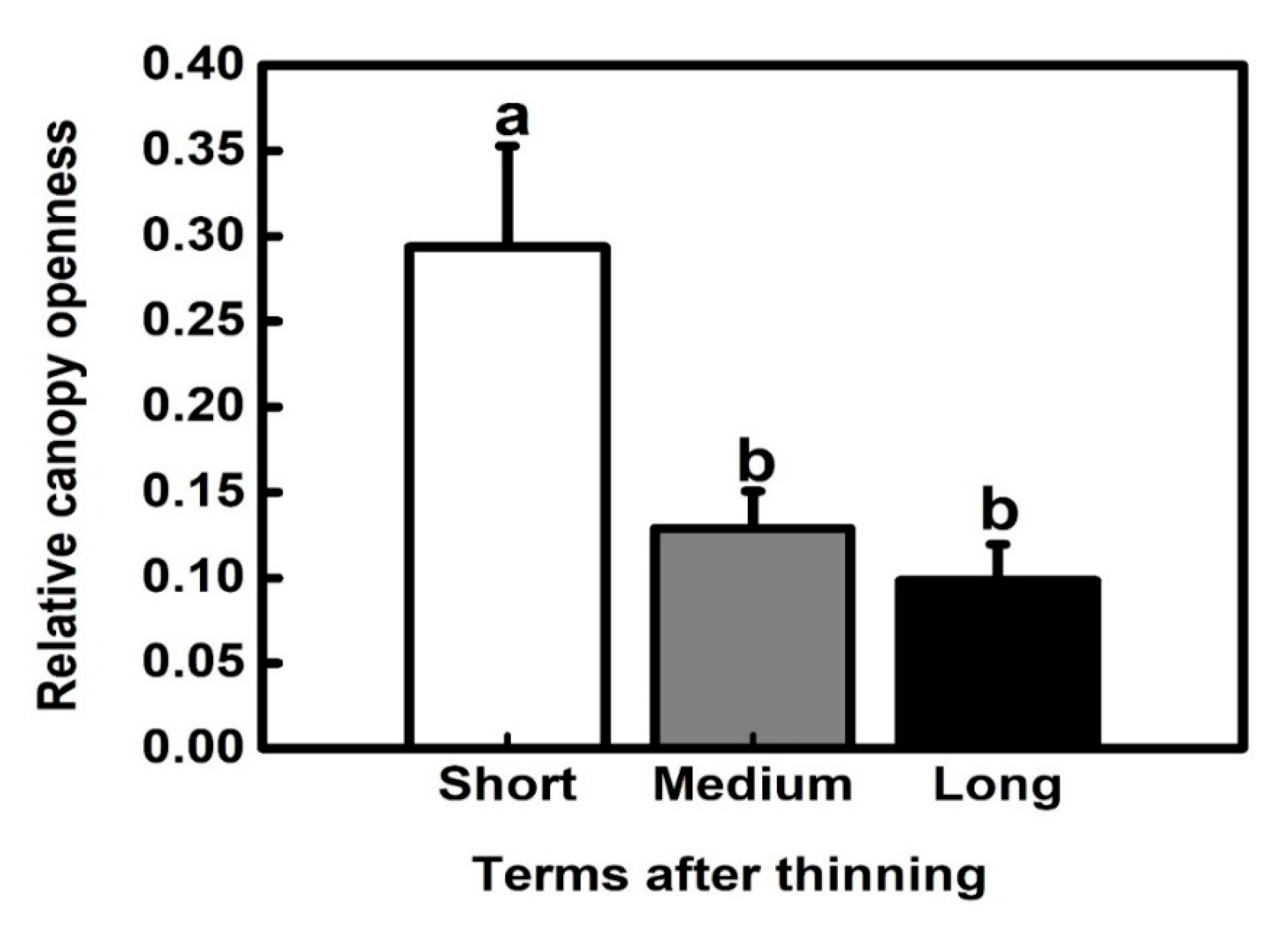
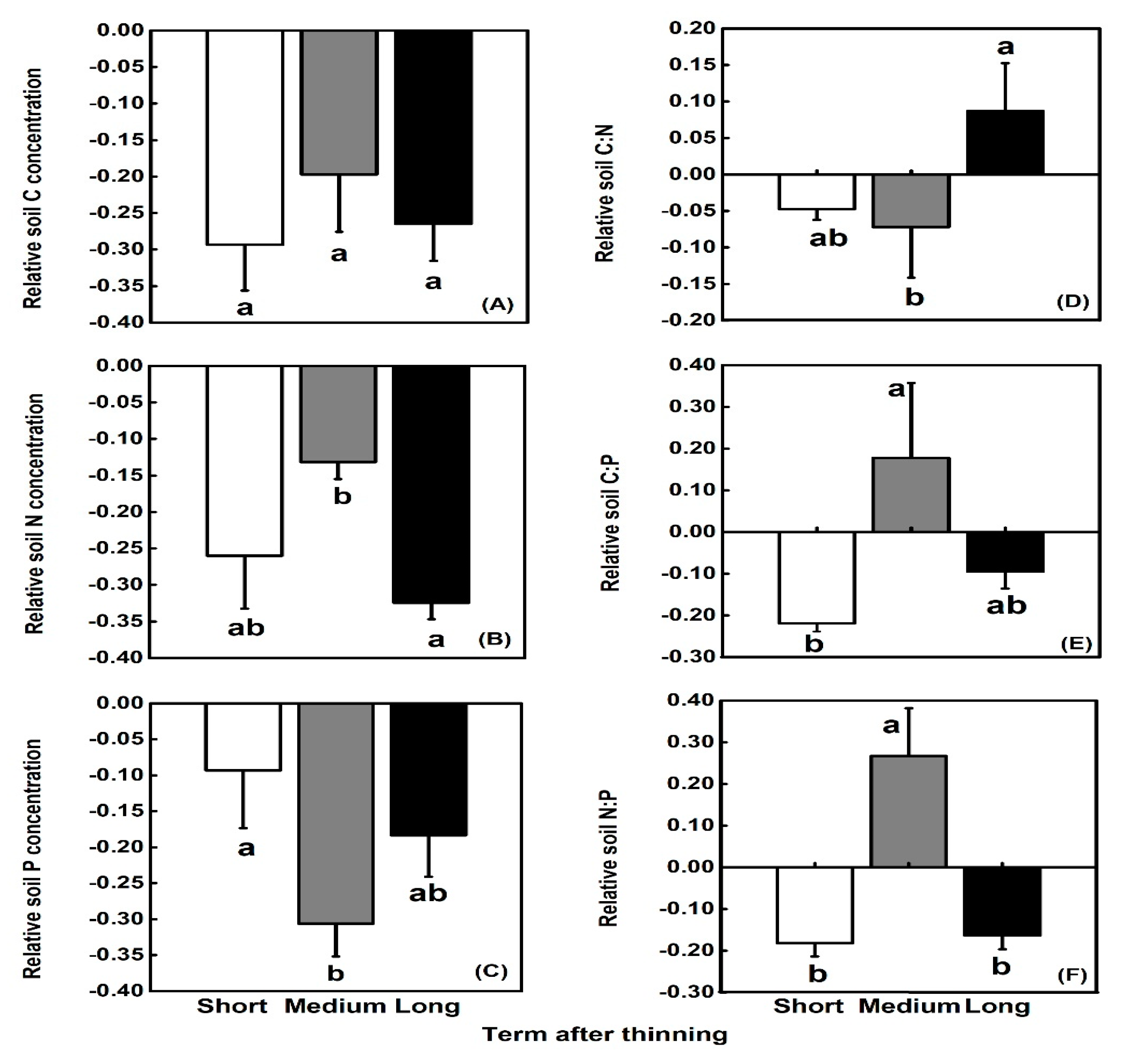
| Term after Thinning | Geographic Information | Slope (°) | LPs | SFs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 1 (yr) | DBH (cm) | Height (m) | YT (yr) | TI (%) | Age (yr) | DBH (cm) | Height (m) | Vegetation Composition of Overstory Trees (%) | |||
| Short | 125°0′23″ E, 42°3′15″ N | 20 | 18 | 16.3 | 18.7 | 1 | 13.0 | ~60 | 12.5 | 11.1 | Juglans mandshurica (27.8), Quercus mongolica (21.1), Ulmus pumila (15.6), Acer mono (9.4), Tilia tuan (7.2) |
| 125°19′18″ E, 41°59′40″ N | 17 | 17 | 15.1 | 13.6 | 2 | 13.5 | ~60 | 18.0 | 13.6 | Quercus mongolica (35.5), Ulmus pumila (20.8), Fraxinus rhynchophylla (15.8), Acer mono (7.7), Fraxinus mandshurica (4.4) | |
| 125°19′9″ E, 41°59′29″ N | 15 | 17 | 17.6 | 15.8 | 3 | 18.6 | ~60 | 13.7 | 10.9 | Fraxinus rhynchophylla (33.9), Juglans mandshurica (28.1), Ulmus pumila (11.3), Quercus mongolica (6.8), Fraxinus mandshurica (6.3) | |
| Medium | 125°7′57″ E, 42°41′31″ N | 18 | 17 | 15.0 | 15.6 | 5 | 14.3 | ~60 | 14.9 | 11.9 | Quercus mongolica (76.3), Acer mono (7.0), Acer pesudo-sieboldianum (6.5), Tilia tuan (5.7) |
| 124°52′26″ E, 42°17′45″ N | 25 | 18 | 18.7 | 19.1 | 5 | 16.5 | ~60 | 14.3 | 10.6 | Quercus mongolica (15.4), Sorbus alnifolia (12.4), Betula costata (11.8), Ulmus pumila (8.9), Fraxinus rhynchophylla (8.3) | |
| 125°0′13″ E, 41°51′36″ N | 25 | 16 | 18.9 | 18.4 | 9 | 16.3 | ~60 | 14.1 | 11.2 | Quercus mongolica (33.3), Acer pesudo-sieboldianum (24.8), Acer mono (7.9), Tilia tuan (6.1) Phellodendron amurense (5.5) | |
| Long | 124°53′32″ E, 41°55′38″ N | 16 | 16 | 18.3 | 17.6 | 11 | 10.1 | ~60 | 12.0 | 9.6 | Tilia tuan (23.3), Acer mono (21.6), Quercus mongolica (17.7), Fraxinus rhynchophylla (17.7), Ulmus pumila (3.9) |
| 124°53′43″ E, 41°55′26″ N | 15 | 15 | 17.8 | 16.7 | 11 | 13.7 | ~60 | 12.0 | 10.1 | Quercus mongolica (22.4), Tilia tuan (18.4), Acer mono (12.4), Ulmus pumila (12.8), Phellodendron amurense (8.4) | |
| 124°53′58″ E, 41°55′30″ N | 16 | 15 | 20.1 | 16.4 | 11 | 14.9 | ~60 | 12.2 | 9.8 | Quercus mongolica (24.3), Acer mono (15.7), Fraxinus rhynchophylla (14.9), Tilia tuan (11.0), Ulmus pumila (7.1) | |
| Plant | C | N | P | C:N | C:P | N:P | |
|---|---|---|---|---|---|---|---|
| Environment | |||||||
| Soil | C | −0.190 | 0.194 | 0.104 | −0.256 | −0.236 | 0.059 |
| N | −0.292 | 0.477 | 0.188 | −0.534 | −0.315 | 0.273 | |
| P | −0.180 | −0.260 | 0.131 | 0.168 | −0.195 | −0.378 | |
| C:N | 0.172 | −0.398 | −0.139 | 0.407 | 0.151 | −0.279 | |
| C:P | −0.014 | 0.319 | −0.035 | −0.300 | −0.008 | 0.328 | |
| N:P | −0.082 | 0.483 | 0.028 | −0.466 | −0.076 | 0.436 | |
| Light | CO | −0.399 | −0.083 | 0.066 | −0.024 | −0.141 | −0.115 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Yan, Q.; Yuan, J.; Li, R.; Lü, X.; Liu, S.; Zhu, J. Temporal Effects of Thinning on the Leaf C:N:P Stoichiometry of Regenerated Broadleaved Trees in Larch Plantations. Forests 2020, 11, 54. https://doi.org/10.3390/f11010054
Xie J, Yan Q, Yuan J, Li R, Lü X, Liu S, Zhu J. Temporal Effects of Thinning on the Leaf C:N:P Stoichiometry of Regenerated Broadleaved Trees in Larch Plantations. Forests. 2020; 11(1):54. https://doi.org/10.3390/f11010054
Chicago/Turabian StyleXie, Jin, Qiaoling Yan, Junfeng Yuan, Rong Li, Xiaotao Lü, Shengli Liu, and Jiaojun Zhu. 2020. "Temporal Effects of Thinning on the Leaf C:N:P Stoichiometry of Regenerated Broadleaved Trees in Larch Plantations" Forests 11, no. 1: 54. https://doi.org/10.3390/f11010054
APA StyleXie, J., Yan, Q., Yuan, J., Li, R., Lü, X., Liu, S., & Zhu, J. (2020). Temporal Effects of Thinning on the Leaf C:N:P Stoichiometry of Regenerated Broadleaved Trees in Larch Plantations. Forests, 11(1), 54. https://doi.org/10.3390/f11010054





