Assessing Forest Structure and Composition along the Altitudinal Gradient in the State of Sikkim, Eastern Himalayas, India
Abstract
1. Introduction
1.1. Background
1.2. Past Studies in the Eastern Himalayas
1.3. Aims of this Study
2. Materials and Methods
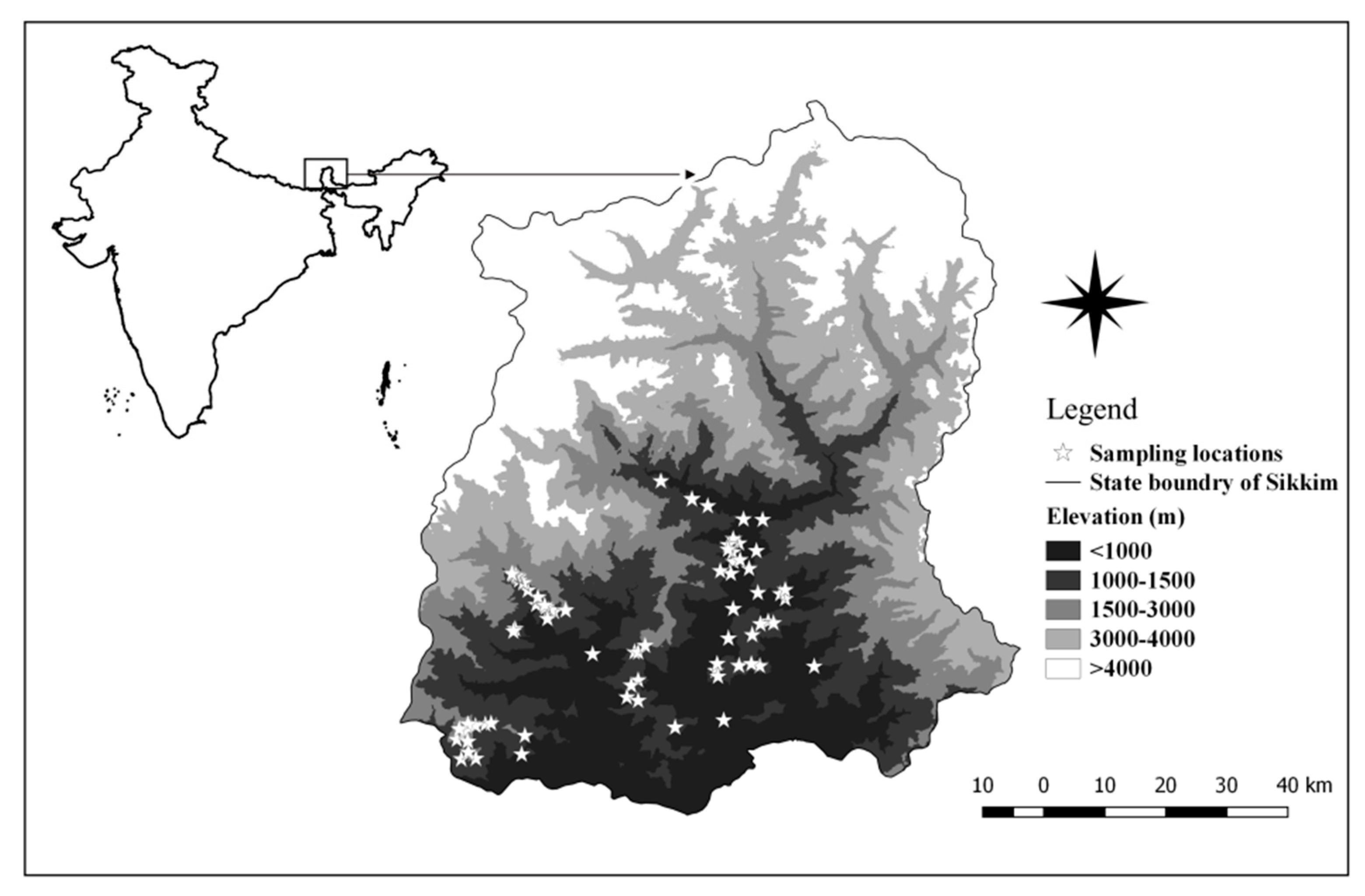
2.1. Study Area Description and Data Collection
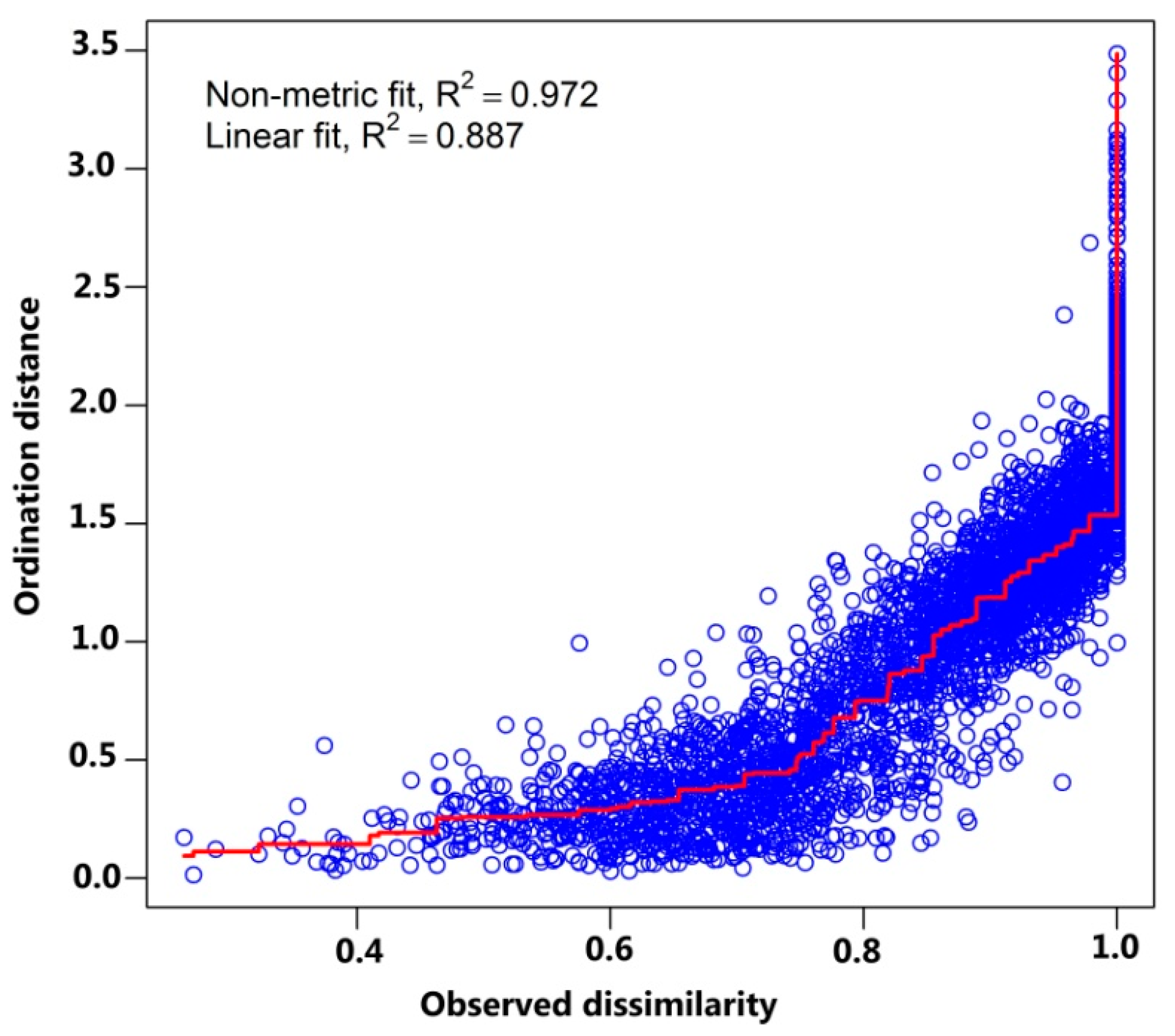
2.2. Data Analysis
3. Results
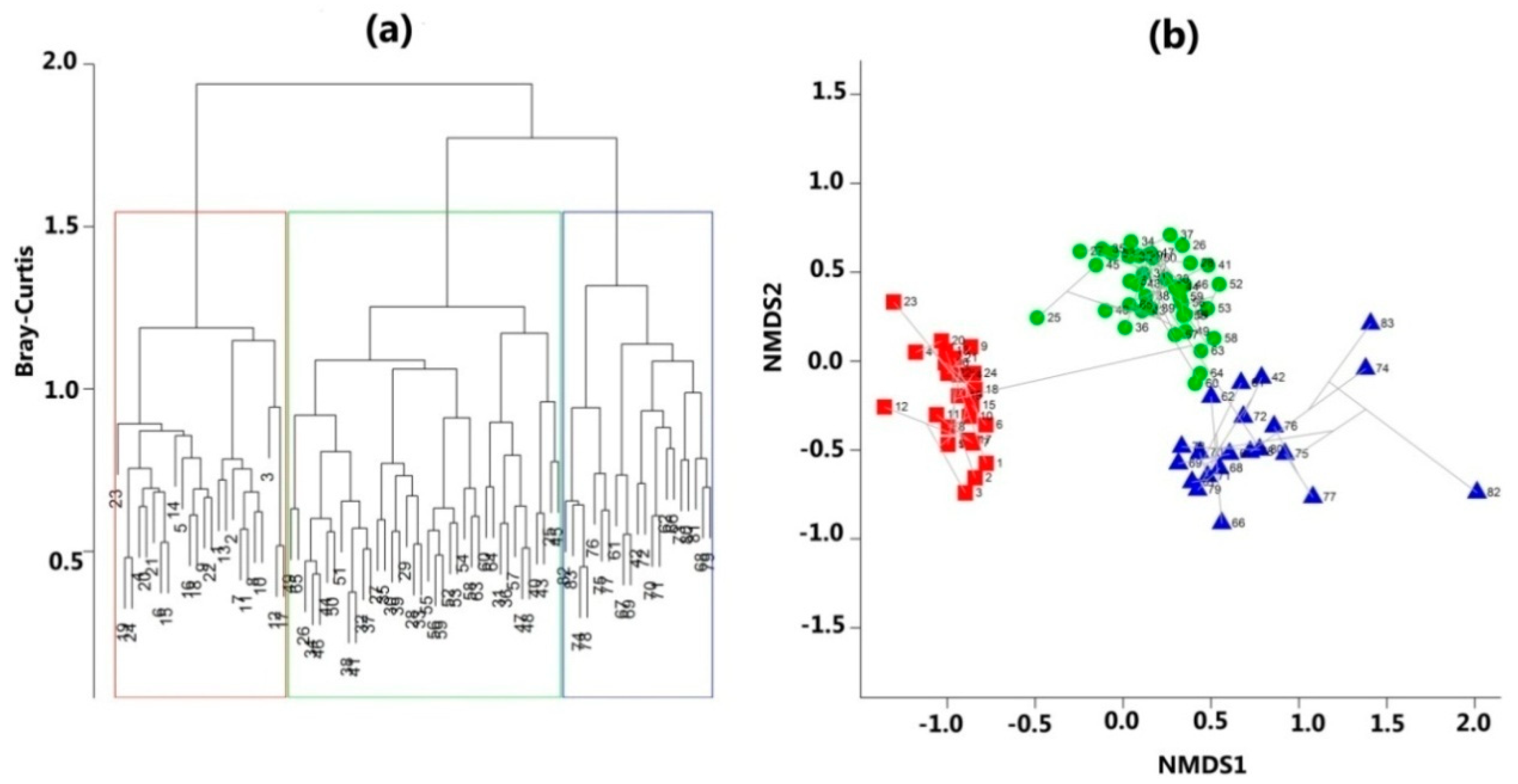
3.1. Distinct Forest Communities along the Altitude Gradient from 900 m to 3200 m
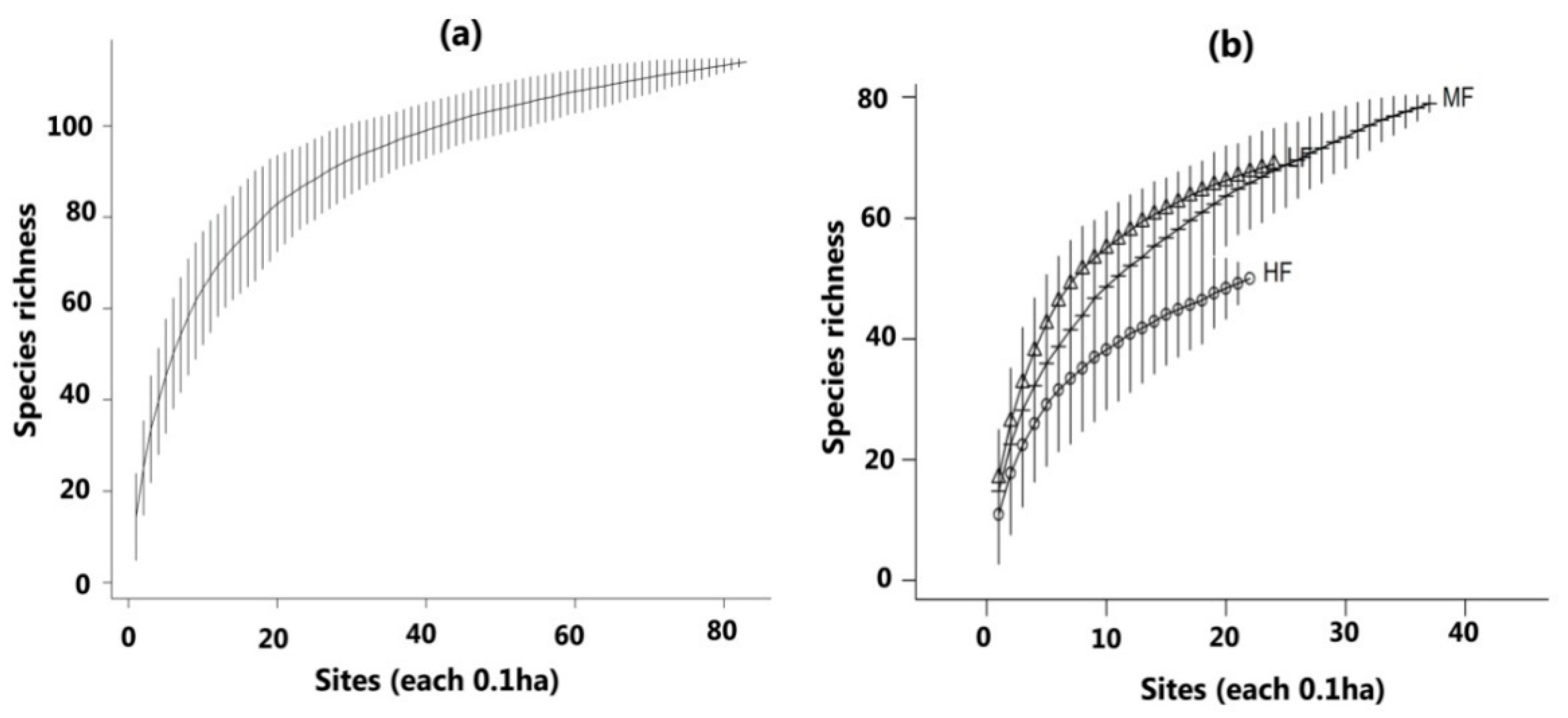
3.2. Diversity and Composition of Tree Species in Three Forest Communities
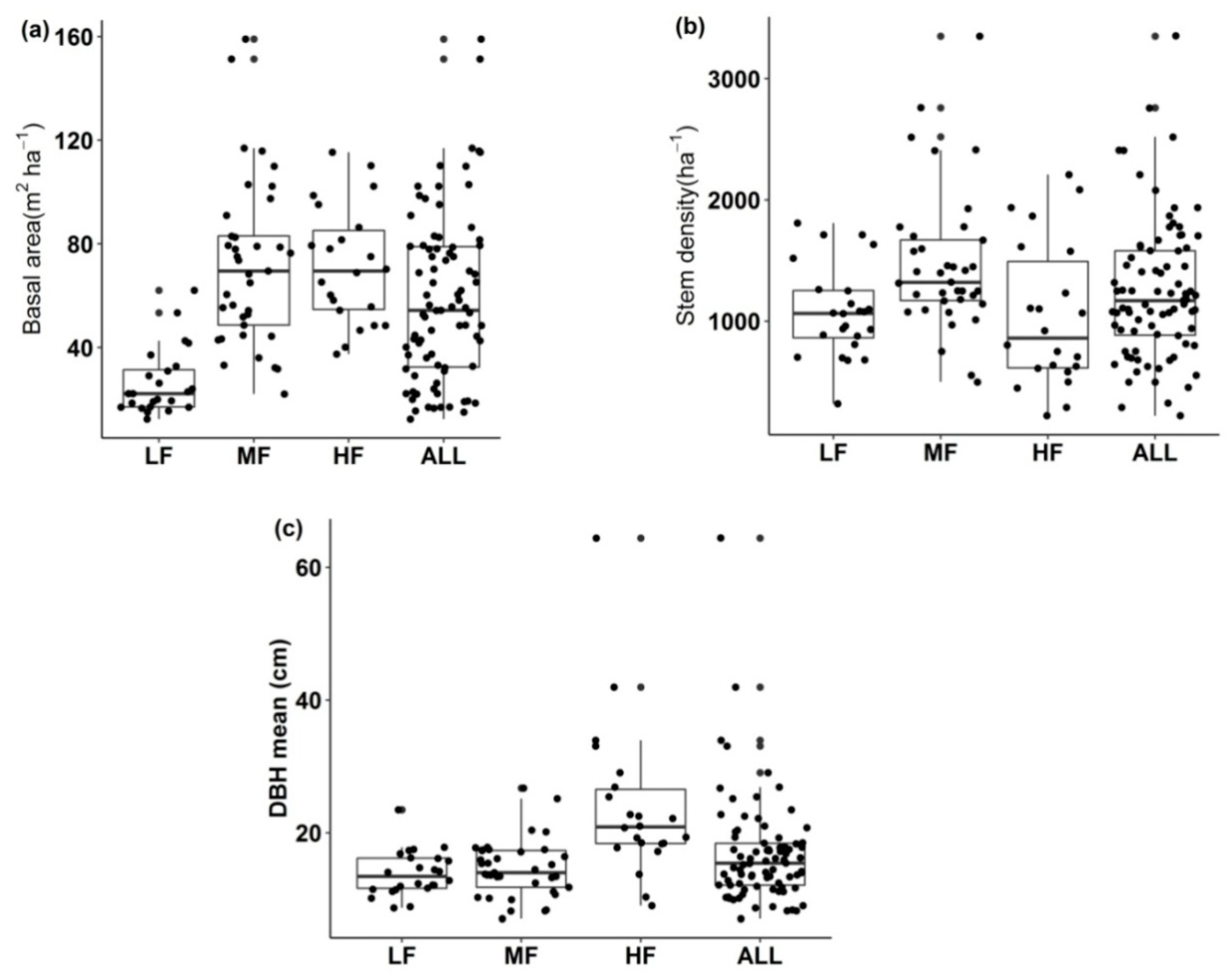
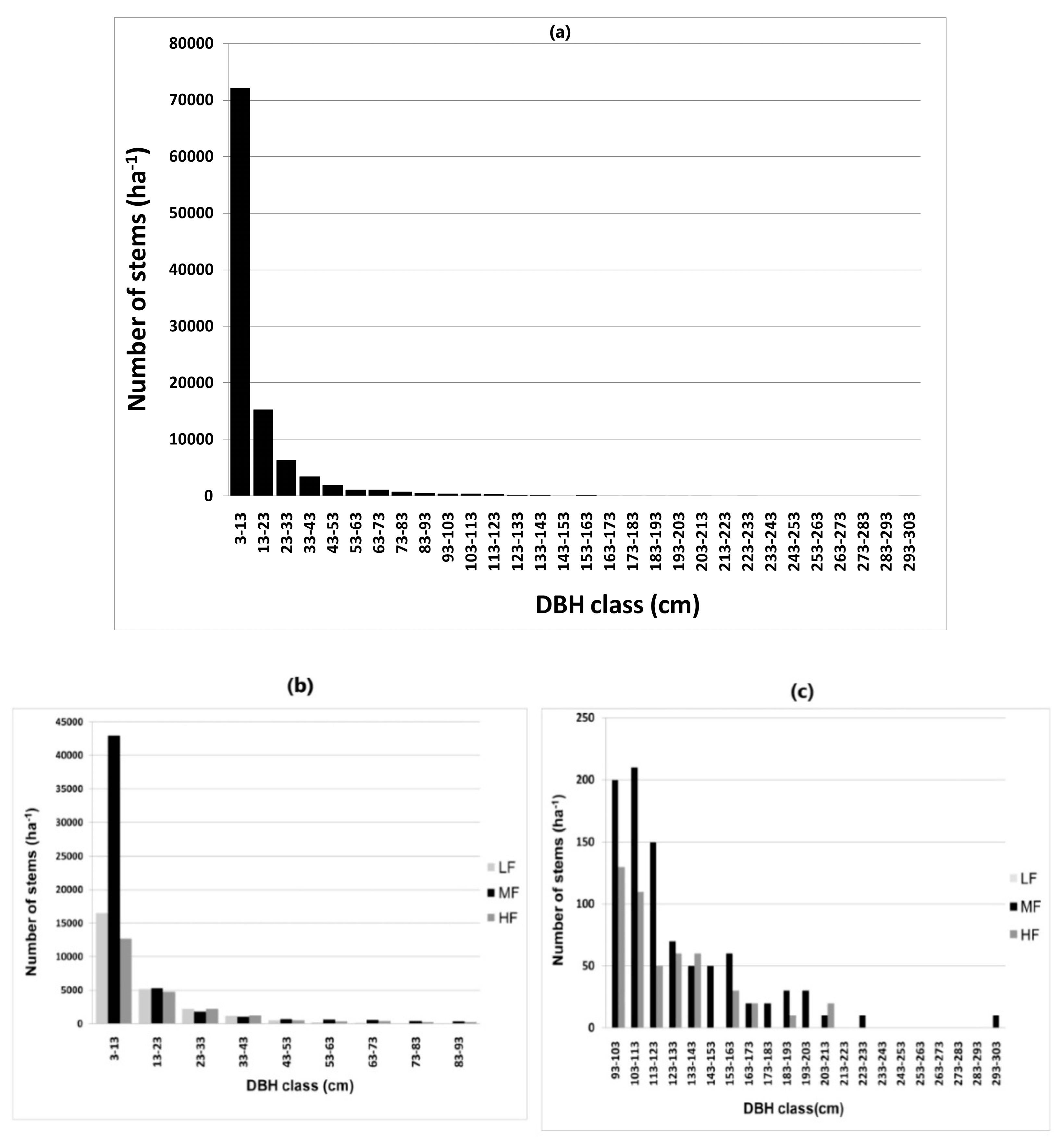
3.3. Forest Structure
4. Discussion
4.1. Forest Communities and Species Composition
4.2. Tree Species Richness and Diversity
4.3. Forest Structure: Stem Diameter, Stem Density, and Basal Area
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Condit, R.; Sukumar, R.; Hubbell, S.P.; Foster, R.B. Predicting population trends from size distributions: A direct test in a tropical tree community. Am. Nat. 1998, 152, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Tse-ring, K.; Chettri, N.; Shrestha, A.; Kathmandu, N. Biodiversity in the Himalayas–trends, perception and impacts of climate change. In Proceedings of the International Mountain Biodiversity Conference, Kathmandu, Nepal, 16–18 November 2008. [Google Scholar]
- Chaudhary, P.; Bawa, K.S. Local perceptions of climate change validated by scientific evidence in the Himalayas. Biol. Lett. 2011, 7, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Pandit, M.K. The Himalayas must be protected. Nat. News 2013, 501, 283. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.R.; Timilsina, G.R.; Acharya, K. Climate Change in the Himalayas: Current State of Knowledge; The World Bank: Washington, DC, USA, 2013. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Myers, N.; Mittermeier, C.G.; Robles, G. Hotspots: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; CEMEX, SA, Agrupación Sierra Madre, SC: Mexico City, Mexico, 1999. [Google Scholar]
- Upadhaya, K. Structure and floristic composition of subtropical broad-leaved humid forest of Cherapunjee in Meghalaya, Northeast India. J. Biodivers. Manag. For. 2015, 4, 2. [Google Scholar] [CrossRef]
- Brown, S.; Sathaye, J.; Cannell, M.; Kauppi, P.E. Mitigation of carbon emissions to the atmosphere by forest management. Commonw. For. Rev. 1996, 75, 80–91. [Google Scholar]
- Haripriya, G. Biomass carbon of truncated diameter classes in Indian forests. For. Ecol. Manag. 2002, 168, 1–13. [Google Scholar] [CrossRef]
- Saha, D.; Sundriyal, R.C. Utilization of non-timber forest products in humid tropics: Implications for management and livelihood. For. Policy Econ. 2012, 14, 28–40. [Google Scholar] [CrossRef]
- Diaz, H.F.; Grosjean, M.; Graumlich, L. Climate variability and change in high elevation regions: Past, present and future. Clim. Chang. 2003, 59, 1–4. [Google Scholar] [CrossRef]
- Williams, J.W.; Jackson, S.T.; Kutzbach, J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl. Acad. Sci. USA 2007, 104, 5738–5742. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Gautam, S.; Bawa, K.S. Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS ONE 2012, 7, e36741. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, J.; John, R.; Joseph, S. Consistent response of vegetation dynamics to recent climate change in tropical mountain regions. Glob. Chang. Biol. 2014, 20, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Saha, S.; Sachdeva, K.; Joshi, P.K. Vulnerability of forests in the Himalayan region to climate change impacts and anthropogenic disturbances: A systematic review. Reg. Environ. Chang. 2018, 18, 1783–1799. [Google Scholar] [CrossRef]
- Xu, J.; Grumbine, R.E.; Shrestha, A.; Eriksson, M.; Yang, X.; Wang, Y.; Wilkes, A. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conserv. Biol. 2009, 23, 520–530. [Google Scholar] [CrossRef]
- Khan, M.L.; Menon, S.; Bawa, K.S. Effectiveness of the protected area network in biodiversity conservation: A case-study of Meghalaya state. Biodivers. Conserv. 1997, 6, 853–868. [Google Scholar] [CrossRef]
- Shaheen, H.; Ullah, Z.; Khan, S.M.; Harper, D.M. Species composition and community structure of western Himalayan moist temperate forests in Kashmir. For. Ecol. Manag. 2012, 278, 138–145. [Google Scholar] [CrossRef]
- Peet, R.K. The measurement of species diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Khan, M.; Rai, J.; Tripathi, R. Population structure of some tree species in disturbed and protected subtropical forests of north-east India. Acta Oecolog. 1987, 8, 247–255. [Google Scholar]
- Brown, S. Measuring carbon in forests: Current status and future challenges. Environ. Pollut. 2002, 116, 363–372. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Forrester, D.I.; Tachauer, I.H.H.; Annighoefer, P.; Barbeito, I.; Pretzsch, H.; Ruiz-Peinado, R.; Stark, H.; Vacchiano, G.; Zlatanov, T.; Chakraborty, T.; et al. Generalized biomass and leaf area allometric equations for European tree species incorporating stand structure, tree age and climate. For. Ecol. Manag. 2017, 396, 160–175. [Google Scholar] [CrossRef]
- Pandey, K.P. Structure, Composition and Diversity of Forest along the Altitudinal Gradient in the Himalayas, Nepal. Appl. Ecol. Environ. Res. 2016, 14, 235–251. [Google Scholar] [CrossRef]
- Panthi, M.P.; Chaudhary, R.P.; Vetaas, O.R. Plant species richness and composition in a trans-Himalayan inner valley of Manang district, central Nepal. Himal. J. Sci. 2007, 4, 57–64. [Google Scholar] [CrossRef]
- Tenzin, J.; Hasenauer, H. Tree species composition and diversity in relation to anthropogenic disturbances in broad-leaved forests of Bhutan. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2016, 12, 274–290. [Google Scholar] [CrossRef][Green Version]
- Mukhia, P.K.; Wangyal, J.T.; Gurung, D. Floristic composition and species diversity of the chirpine forest ecosystem, Lobesa, Western Bhutan. For. Nepal 2011, 1–4. [Google Scholar]
- Wangda, P.; Ohsawa, M. Structure and regeneration dynamics of dominant tree species along altitudinal gradient in a dry valley slopes of the Bhutan Himalaya. For. Ecol. Manag. 2006, 230, 136–150. [Google Scholar] [CrossRef]
- Tang, C.Q.; Li, Y.-H.; Zhang, Z.-Y. Species Diversity Patterns in Natural Secondary Plant Communities and Man-made Forests in a Subtropical Mountainous Karst Area, Yunnan, SW China. Mt. Res. Dev. 2010, 30, 244–251. [Google Scholar] [CrossRef]
- Li, R.; Dao, Z.; Li, H. Seed Plant Species Diversity and Conservation in the Northern Gaoligong Mountains in Western Yunnan, China. Mt. Res. Dev. 2011, 31, 160–165. [Google Scholar] [CrossRef]
- Bharali, S.; Paul, A.; Khan, M.L.; Singha, L.B. Species diversity and community structure of a temperate mixed Rhododendron forest along an altitudinal gradient in West Siang District of Arunachal Pradesh, India. Nat. Sci. 2011, 9, 125–140. [Google Scholar]
- Nath, P.; Arunachalam, A.; Khan, M.; Arunachalam, K.; Barbhuiya, A. Vegetation analysis and tree population structure of tropical wet evergreen forests in and around Namdapha National Park, northeast India. Biodivers. Conserv. 2005, 14, 2109–2135. [Google Scholar] [CrossRef]
- Yam, G.; Tripathi, O.P. Tree diversity and community characteristics in Talle Wildlife Sanctuary, Arunachal Pradesh, Eastern Himalaya, India. J. Asia-Pac. Biodivers. 2016, 9, 160–165. [Google Scholar] [CrossRef][Green Version]
- Tripathi, O.; Pandey, H.; Tripathi, R. Distribution, community characteristics and tree population structure of subtropical pine forest of Meghalaya, northeast India. Int. J. Ecol. Environ. Sci. 2004, 29, 207–214. [Google Scholar]
- Tripathi, O.P.; Tripathi, R.S. Community composition, structure and management of subtropical vegetation of forests in Meghalaya State, northeast India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2011, 6, 157–163. [Google Scholar] [CrossRef]
- Sinha, S.; Badola, H.K.; Chhetri, B.; Gaira, K.S.; Lepcha, J.; Dhyani, P.P. Effect of altitude and climate in shaping the forest compositions of Singalila National Park in Khangchendzonga Landscape, Eastern Himalaya, India. J. Asia-Pac. Biodivers. 2018, 11, 267–275. [Google Scholar] [CrossRef]
- Gogoi, A.; Sahoo, U.K. Impact of anthropogenic disturbance on species diversity and vegetation structure of a lowland tropical rainforest of eastern Himalaya, India. J. Mt. Sci. 2018, 15, 2453–2465. [Google Scholar] [CrossRef]
- Dutta, G.; Devi, A. Plant diversity, population structure, and regeneration status in disturbed tropical forests in Assam, northeast India. J. For. Res. 2013, 24, 715–720. [Google Scholar] [CrossRef]
- Sundriyal, R.C.; Sharma, E.; Rai, L.K.; Rao, S.C. Tree structure, regeneration and woody biomass removal in a subtropical forest of Mamlay watershed in the Sikkim Himalaya. Vegetatio 1994, 113, 53–63. [Google Scholar] [CrossRef]
- Singh, H.B.; Sundriyal, R.C. Composition, Economic Use, and Nutrient Contents of Alpine Vegetation in the Khangchendzonga Biosphere Reserve, Sikkim Himalaya, India. Arct. Antarct. Alp. Res. 2005, 37, 591–601. [Google Scholar] [CrossRef][Green Version]
- Tambe, S.; Rawat, G.S. The Alpine Vegetation of the Khangchendzonga Landscape, Sikkim Himalaya. Mt. Res. Dev. 2010, 30, 266–274. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, S.; Kumar, D. Changes in vegetation attributes along an elevation gradient towards timberline in Khangchendzonga National Park, Sikkim. Trop. Ecol. 2018, 59, 259–271. [Google Scholar]
- Chettri, N.; Sharma, E.; Deb, D.C.; Sundriyal, R.C. Impact of Firewood Extraction on Tree Structure, Regeneration and Woody Biomass Productivity in a Trekking Corridor of the Sikkim Himalaya. Mt. Res. Dev. 2002, 22, 150–158. [Google Scholar] [CrossRef]
- Acharya, B.K.; Chettri, B.; Vijayan, L. Distribution pattern of trees along an elevation gradient of Eastern Himalaya, India. Acta Oecolog. 2011, 37, 329–336. [Google Scholar] [CrossRef]
- Sharma, N.; Behera, M.D.; Das, A.P.; Panda, R.M. Plant richness pattern in an elevation gradient in the Eastern Himalaya. Biodivers. Conserv. 2019, 28, 2085–2104. [Google Scholar] [CrossRef]
- Tambe, S.; Arrawatia, M.L.; Sharma, N. Assessing the Priorities for Sustainable Forest Management in the Sikkim Himalaya, India: A Remote Sensing Based Approach. J. Indian Soc. Remote Sens. 2011, 39, 555–564. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L.F. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24. [Google Scholar]
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. Biol. Divers. Front. Meas. Assess. 2011, 12, 39–54. [Google Scholar]
- Keel, S.; Gentry, A.H.; Spinzi, L. Using vegetation analysis to facilitate the selection of conservation sites in eastern Paraguay. Conserv. Biol. 1993, 7, 66–75. [Google Scholar] [CrossRef]
- Ganesh, T.; Ganesan, R.; Devy, M.S.; Davidar, P.; Bawa, K.S. Assessment of plant biodiversity at a mid-elevation evergreen forest of Kalakad-Mundanthurai Tiger Reserve, Western Ghats, India. Curr. Sci. 1996, 71, 379–392. [Google Scholar]
- Korner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Grierson, A.J.; Long, D.G. Flora of Bhutan, Including a Record of Plants from Sikkim; Royal Botanic Garden: Edinburgh, UK, 1983. [Google Scholar]
- Manish, K.; Telwala, Y.; Nautiyal, D.C.; Pandit, M.K. Modelling the impacts of future climate change on plant communities in the Himalaya: A case study from Eastern Himalaya, India. Model. Earth Syst. Environ. 2016, 2, 92. [Google Scholar] [CrossRef]
- Kharkwal, G.; Mehrotra, P.; Rawat, Y.S.; Pangtey, Y.P.S. Phytodiversity and growth form in relation to altitudinal gradient in the Central Himalayan (Kumaun) region of India. Curr. Sci. 2005, 89, 873–878. [Google Scholar]
- Mishra, B.; Tripathi, O.; Tripathi, R.; Pandey, H. Effects of anthropogenic disturbance on plant diversity and community structure of a sacred grove in Meghalaya, northeast India. Biodivers. Conserv. 2004, 13, 421–436. [Google Scholar] [CrossRef]
- Saxena, A.K.; Singh, J.S. Tree population-structure of certain Himalayan forest associations and implications concerning their future composition. Vegetatio 1984, 58, 61–69. [Google Scholar] [CrossRef]
- Veblen, T.T. Structure and dynamics of nothofagus forests near timberline in south-central Chile. Ecology 1979, 60, 937–945. [Google Scholar]
- Vetaas, O.R. The effect of environmental factors on the regeneration of Quercus semecarpifolia Sm. in Central Himalaya, Nepal. Plant Ecol. 2000, 146, 137–144. [Google Scholar] [CrossRef]
- Kanade, R.; John, R. Topographical influence on recent deforestation and degradation in the Sikkim Himalaya in India; Implications for conservation of East Himalayan broadleaf forest. Appl. Geogr. 2018, 92, 85–93. [Google Scholar] [CrossRef]
- Gudade, B.; Chhetri, P.; Gupta, U.; Deka, T.; Vijayan, A. Traditional practices of large cardamom cultivation in Sikkim and Darjeeling. Life Sci. Leafl. 2013, 9, 62–68. [Google Scholar]


| Parameters | ALL | LF | MF | HF |
|---|---|---|---|---|
| Altitude (m) | 900–3200 | 900–1700 | >1700–2500 | >2500–3200 |
| Total sampled area (ha) | 8.3 | 2.4 | 3.7 | 2.2 |
| Total species richness | 114 | 68 | 77 | 51 |
| Total genus richness | 75 | 52 | 54 | 37 |
| Total family richness | 42 | 33 | 35 | 25 |
| Median of species’ richness ± SE | 14 ± 4.34 | 16 ± 4.43 | 14 ± 3.04 | 10 ± 4.50 |
| Median of genus’ richness ± SE | 12 ± 3.96 | 15.50 ± 4.06 | 12 ± 2.72 | 10 ± 3.51 |
| Median of families’ richness ± SE | 10 ± 2.89 | 13 ± 2.94 | 9 ± 1.95 | 9 ± 2.81 |
| Total individual counted | 10344 | 2591 | 5463 | 2290 |
| Shannon index (H’) ± SD | 1.87 ± 0.44 | 2.10 ± 0.35 | 1.71 ± 0.40 | 1.78 ± 0.48 |
| Fisher’s alpha richness | 4.44 ± 1.84 | 5.68 ± 2.00 | 4.14 ± 1.35 | 3.51 ± 1.64 |
| Pielou’s evenness (J) | 0.39 ± 0.39 | 0.46 ± 0.7 | 0.35 ± 0.10 | 0.38 ± 0.13 |
| Dominant family | Fagaceae | Fagaceae | Fagaceae | Fagaceae |
| Dominant species | Lithocarpus pachyphyllus | Schima wallichii | Symplocos ramosissima | Lithocarpus pachyphyllus |
| Species | Family | Stem Density (ha−1) | Stem Density% | Basal Area (m2 ha−1) | Basal Area% | SIVI% |
| Lithocarpus pachyphyllus | Fagaceae | 51.20 | 4.11 | 12.31 | 21.07 | 9.30 |
| Symplocos ramosissima | Symplocaceae | 248.07 | 19.19 | 1.61 | 2.76 | 8.72 |
| Castanopsis hystrix | Fagaceae | 62.05 | 4.98 | 9.91 | 16.97 | 8.23 |
| Castanopsis tribuloides | Fagaceae | 62.41 | 5.01 | 4.22 | 7.23 | 4.99 |
| Schima wallichii | Theaceae | 78.19 | 6.27 | 2.40 | 4.11 | 4.46 |
| Rhododendron arboreum | Ericaceae | 85.54 | 6.86 | 2.54 | 4.35 | 4.33 |
| Viburnum erubescens | Adoxaceae | 103.98 | 8.34 | 0.38 | 0.64 | 4.16 |
| Eurya acuminata DC. | Pentaphylacaceae | 60.96 | 4.89 | 0.81 | 1.39 | 3.91 |
| Quercus lamellosa | Fagaceae | 9.88 | 0.79 | 4.95 | 8.49 | 3.86 |
| Symplocos heishanensis | Symplocaceae | 49.40 | 3.96 | 0.38 | 0.65 | 2.93 |
| Families | Richness | Stem Density (ha−1) | Stem Density% | Basal Area (m2 ha−1) | Basal Area% | FIVI% |
| Fagaceae | 9 | 20.88 | 16.75 | 33.61 | 57.19 | 27.86 |
| Symplocaceae | 4 | 33.55 | 26.92 | 2.18 | 3.71 | 12.26 |
| Theaceae | 2 | 13.90 | 11.16 | 3.20 | 5.45 | 8.04 |
| Ericaceae | 7 | 14.76 | 11.84 | 3.81 | 6.48 | 7.70 |
| Lauraceae | 13 | 4.63 | 3.71 | 4.97 | 8.45 | 6.62 |
| Adoxaceae | 2 | 10.41 | 8.35 | 0.38 | 0.64 | 4.64 |
| Magnoliaceae | 4 | 1.12 | 0.90 | 2.09 | 3.64 | 2.70 |
| Betulaceae | 4 | 1.93 | 1.55 | 0.99 | 1.69 | 2.19 |
| Primulaceae | 3 | 2.28 | 1.83 | 0.41 | 0.23 | 2.10 |
| Sapindaceae | 1 | 0.69 | 0.55 | 1.23 | 2.10 | 1.89 |
| Species | Family | Stem Density (ha−1) | Stem Density% | Basal Area (m2 ha−1) | Basal Area% | SIVI% |
| LF (900 m to 1700 m a.s.l.) | ||||||
| Schima wallichii | Theaceae | 259.17 | 24.01 | 7.08 | 26.73 | 18.90 |
| Castanopsis tribuloides | Fagaceae | 202.50 | 18.76 | 7.68 | 29.00 | 17.91 |
| Engelhardtia spicata | Juglandaceae | 60.42 | 5.60 | 2.35 | 8.86 | 6.72 |
| Gynocardia odorata | Achariaceae | 92.50 | 8.57 | 1.23 | 4.63 | 6.05 |
| Euryaa cuminata | Pentaphylacaceae | 66.67 | 8.57 | 0.76 | 2.86 | 4.83 |
| Betula alnoides Buch.-Ham.ex.D.Don | Betulaceae | 21.25 | 6.18 | 0.99 | 3.75 | 2.57 |
| Macaranga denticulata Blume | Euphorbiaceae | 18.08 | 1.97 | 0.39 | 1.48 | 2.43 |
| Albizia lebbeck (L.) Benth. | Leguminosae | 17.08 | 1.58 | 1.23 | 4.64 | 2.31 |
| Alnus nepalensis D.Don | Betulaceae | 12.08 | 0.66 | 1.00 | 3.77 | 2.21 |
| Brassaiopsis hispida Seem. | Araliaceae | 36.25 | 3.36 | 0.19 | 0.53 | 1.87 |
| Families | Richness | Stem density (ha−1) | Stem density% | Basal area (m2 ha−1) | Basal area% | FIVI% |
| Theaceae | 2 | 9.42 | 30.18 | 7.84 | 29.60 | 22.54 |
| Fagaceae | 6 | 6.73 | 21.57 | 8.23 | 31.09 | 20.17 |
| Juglandaceae | 3 | 1.80 | 5.75 | 2.38 | 8.99 | 7.42 |
| Achariaceae | 1 | 2.67 | 8.57 | 1.23 | 4.63 | 6.58 |
| Betulaceae | 3 | 1.04 | 3.32 | 2.09 | 7.89 | 5.26 |
| Euphorbiaceae | 5 | 1.23 | 3.94 | 0.59 | 2.22 | 4.34 |
| Araliaceae | 3 | 1.57 | 5.02 | 0.17 | 0.66 | 3.53 |
| Leguminosae | 3 | 0.30 | 0.96 | 1.27 | 4.80 | 2.90 |
| Anacardiaceae | 3 | 0.59 | 1.89 | 0.48 | 1.81 | 2.76 |
| Lauraceae | 6 | 0.63 | 2.01 | 0.09 | 0.35 | 2.64 |
| Species | Family | Stem Density (ha−1) | Stem Density% | Basal Area (m2 ha−1) | Basal Area% | SIVI% |
| MF (>1700 m to 2500 m a.s.l.) | ||||||
| Symplocos ramosissima | Symplocaceae | 501.89 | 33.99 | 3.36 | 4.67 | 15.09 |
| Castanopsis hystrix | Fagaceae | 132.43 | 8.97 | 21.72 | 30.15 | 14.99 |
| Viburnum erubescens | Adoxaceae | 211.89 | 14.35 | 0.75 | 1.05 | 7.09 |
| Quercus lamellosa | Fagaceae | 20.54 | 1.39 | 10.67 | 14.81 | 6.85 |
| Eurya acuminata | Pentaphylacaceae | 83.78 | 5.67 | 1.23 | 1.71 | 4.42 |
| Lithocarpus pachyphyllus | Fagaceae | 48.92 | 3.31 | 5.17 | 7.18 | 4.32 |
| Symplocos heishanensis | Symplocaceae | 73.78 | 5.00 | 0.56 | 0.78 | 4.07 |
| Castanopsis tribuloides | Fagaceae | 8.65 | 0.59 | 4.49 | 6.23 | 2.78 |
| Quercus glauca | Fagaceae | 14.32 | 0.97 | 3.09 | 4.29 | 2.70 |
| Elaeocarpus sikkimensis Mast. | Elaeocarpaceae | 10.54 | 0.71 | 2.21 | 3.07 | 2.52 |
| Families | Richness | Stem Density (ha−1) | Stem Density% | Basal Area (m2 ha−1) | Basal Area% | FIVI% |
| Fagaceae | 7 | 10.82 | 16.44 | 45.70 | 45.70 | 30.12 |
| Symplocaceae | 4 | 28.90 | 43.91 | 4.33 | 4.33 | 20.14 |
| Lauraceae | 11 | 3.08 | 4.69 | 9.60 | 9.60 | 9.22 |
| Adoxaceae | 2 | 9.46 | 14.37 | 0.75 | 0.75 | 8.07 |
| Theaceae | 2 | 3.95 | 6.00 | 1.71 | 1.71 | 5.73 |
| Elaeocarpaceae | 2 | 0.48 | 0.73 | 2.23 | 2.23 | 3.17 |
| Ericaceae | 5 | 2.49 | 3.79 | 0.49 | 0.49 | 2.72 |
| Sapindaceae | 1 | 0.40 | 0.60 | 1.50 | 1.50 | 2.60 |
| Primulaceae | 3 | 1.24 | 1.89 | 0.16 | 0.16 | 2.60 |
| Magnoliaceae | 4 | 0.59 | 0.90 | 1.75 | 1.75 | 2.43 |
| Species | Family | Stem Density (ha−1) | Stem Density% | Basal Area (m2 ha−1) | Basal Area% | SIVI% |
| HF (2500 m to 3200 m a.s.l.) | ||||||
| Lithocarpus pachyphyllus | Fagaceae | 110.91 | 10.66 | 7.88 | 54.56 | 24.37 |
| Rhododendron arboreum | Ericaceae | 310.00 | 29.78 | 7.47 | 13.08 | 16.78 |
| Magnolia campbellii | Magnoliaceae | 16.82 | 1.62 | 5.39 | 6.62 | 4.54 |
| Symplocos heishanensis | Symplocaceae | 61.82 | 5.94 | 5.81 | 0.69 | 4.14 |
| Rhododendron hodgsonii Hook.f. | Ericaceae | 54.55 | 5.24 | 2.90 | 3.15 | 3.76 |
| Corylus ferox Wall. | Betulaceae | 26.82 | 2.58 | 4.15 | 1.33 | 2.68 |
| Viburnum erubescens Wall. | Adoxaceae | 35.91 | 3.45 | 4.15 | 0.21 | 2.60 |
| Acer campbellii | Sapindaceae | 10.91 | 1.05 | 3.73 | 2.98 | 2.59 |
| Rhododendron falconeri Hook.f. | Ericaceae | 39.55 | 3.80 | 2.07 | 1.86 | 2.58 |
| Families | Richness | Stem Density (ha−1) | Stem Density% | Basal Area (m2 ha−1) | Basal Area% | FIVI% |
| Fagaceae | 4 | 3.33 | 90.11 | 40.96 | 57.16 | 79.21 |
| Ericaceae | 7 | 11.82 | 29.63 | 13.47 | 18.79 | 72.16 |
| Symplocaceae | 3 | 4.64 | 2.07 | 0.94 | 1.31 | 26.02 |
| Magnoliaceae | 2 | 0.48 | 10.90 | 4.95 | 6.91 | 16.03 |
| Lauraceae | 6 | 0.92 | 5.51 | 2.51 | 3.50 | 15.24 |
| Betulaceae | 2 | 0.76 | 2.16 | 0.98 | 1.37 | 9.91 |
| Theaceae | 2 | 0.53 | 1.46 | 0.67 | 0.93 | 9.69 |
| Berberidaceae | 2 | 1.39 | 0.37 | 0.17 | 0.24 | 9.47 |
| Adoxaceae | 1 | 0.95 | 0.33 | 0.15 | 0.21 | 9.92 |
| Sapindaceae | 1 | 0.29 | 4.70 | 2.14 | 2.98 | 8.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhutia, Y.; Gudasalamani, R.; Ganesan, R.; Saha, S. Assessing Forest Structure and Composition along the Altitudinal Gradient in the State of Sikkim, Eastern Himalayas, India. Forests 2019, 10, 633. https://doi.org/10.3390/f10080633
Bhutia Y, Gudasalamani R, Ganesan R, Saha S. Assessing Forest Structure and Composition along the Altitudinal Gradient in the State of Sikkim, Eastern Himalayas, India. Forests. 2019; 10(8):633. https://doi.org/10.3390/f10080633
Chicago/Turabian StyleBhutia, Yangchenla, Ravikanth Gudasalamani, Rengaian Ganesan, and Somidh Saha. 2019. "Assessing Forest Structure and Composition along the Altitudinal Gradient in the State of Sikkim, Eastern Himalayas, India" Forests 10, no. 8: 633. https://doi.org/10.3390/f10080633
APA StyleBhutia, Y., Gudasalamani, R., Ganesan, R., & Saha, S. (2019). Assessing Forest Structure and Composition along the Altitudinal Gradient in the State of Sikkim, Eastern Himalayas, India. Forests, 10(8), 633. https://doi.org/10.3390/f10080633






