Prolonging Rotation of Chinese Fir to over 25 Years Could Maintain a Better Soil Status in Subtropical China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sampling
2.3. Soil Physicochemical Properties Analysis
2.4. Phospholipid Fatty Acid (PLFA) Analysis
2.5. Enzyme Activity Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Microbial PLFA Biomass and Structure
3.3. Soil Enzyme Activity
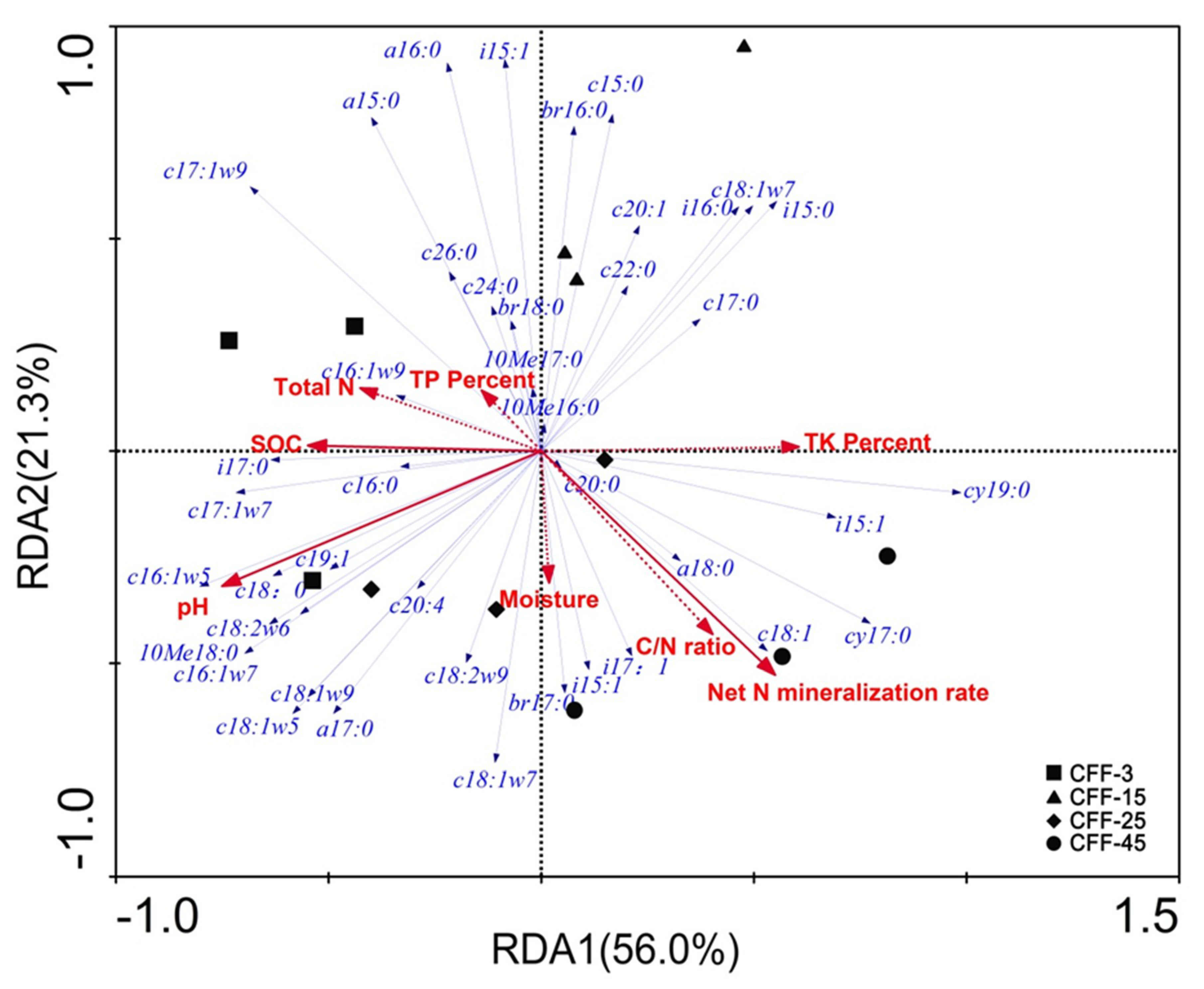
3.4. Relationship between Soil Physicochemical and Biological Indicators
4. Discussion
4.1. Effect of Stand Age on Soil Physicochemical Properties
4.2. Effect of Stand Age on Microbial Communities and Enzyme Activities
4.3. Relationships Between Physicochemical Properties and Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.L.; Chen, H.H.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q.W. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef]
- Yan, W.D.; Deng, X.W.; Chen, X.Y.; Tian, D.L.; Xiang, W.H.; Peng, Y.Y. Long-term variations of rainfall interception in different growth stages of Chinese fir plantations. Hydrol. Sci. J. 2015, 60, 2178–2188. [Google Scholar] [CrossRef]
- Zhao, M.F.; Xiang, W.H.; Tian, D.L.; Deng, X.W.; Huang, Z.H.; Zhou, X.L.; Peng, C.H. Effects of increased nitrogen deposition and rotation length on long-term productivity of Cunninghamia lanceolata plantation in Southern China. PLoS ONE 2013, 8, e55376. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.J.; Guo, F.T.; Ma, X.Q. Influence of long–term successive rotations and stand age of Chinese fir (Cunninghamia lanceolata) plantations on soil properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Tian, D.L.; Xiang, W.H.; Chen, X.Y.; Yan, W.D.; Fang, X.; Kang, W.X.; Dan, X.W.; Peng, C.H.; Peng, Y.Y. A long-term evaluation of biomass production in first and second rotations of Chinese fir plantations at the same site. Forestry 2011, 84, 411–418. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Kirschbaum, M.U.F.; Hou, Z.H.; Guo, Z.H. Carbon stock changes in successive rotations of Chinese fir (Cunninghamia lanceolata (lamb) hook) plantations. For. Ecol. Manag. 2004, 202, 131–147. [Google Scholar] [CrossRef]
- Wei, X.H.; Blanco, J.A. Significant increase in ecosystem C can be achieved with sustainable forest management in subtropical plantation forests. PLoS ONE 2014, 9, e89688. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, A.; Funaro, D.; Noellemeyer, E.; Peinemann, N. Barley yield response to soil organic matter and texture in the Pampas of Argentina. Soil Tillage Res. 2006, 90, 63–68. [Google Scholar] [CrossRef]
- Nakane, K. Soil carbon cycling in a Japanese cedar (Cryptomeria japonica) plantation. For. Ecol. Manag. 1995, 72, 185–197. [Google Scholar] [CrossRef]
- Reeves, D.W. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Wang, C.Q.; Xue, L.; Dong, Y.H.; Hou, L.Y.; Wei, Y.H.; Chen, J.Q.; Jiao, R.Z. Contrasting effects of Chinese fir plantations of different stand ages on soil enzyme activities and microbial communities. Forests 2019, 10, 11. [Google Scholar] [CrossRef]
- Yu, X.; Yang, L.; Fei, S.X.; Ma, Z.T.; Hao, R.Q.; Zhao, Z. Effect of soil layer and plant-soil interaction on soil microbial diversity and function after canopy gap disturbance. Forests 2018, 9, 680. [Google Scholar] [CrossRef]
- Harris, J.A. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Biol. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Smith, A.P.; Marin-Spiotta, E.; Balser, T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Glob. Chang. Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.B.; Wang, D.; Dai, W.; Li, P. Soil carbon budget in different-aged Chinese fir plantations in south China. J. For. Res. 2014, 25, 621–626. [Google Scholar] [CrossRef]
- Li, P.; Zheng, A.B.; Ruan, H.H.; Li, L.; Shen, Y.J.; Zhao, Q.Q.; Xie, T. Variation of soil labile organic carbon in different age Chinese fir plantations in south Jiangsu. Chin. J. Ecol. 2011, 30, 778–783. [Google Scholar]
- Lan, S.A.; Du, H.; Zeng, F.P.; Song, T.Q.; Peng, W.X.; Han, C.; Chen, L.; Su, L. Carbon storage and allocation in Cunninghamia lanceolata plantations with different stand ages. Chin. J. Appl. Ecol. 2016, 27, 1125–1134. [Google Scholar]
- Yu, Y.C.; Yang, J.Y.; Zeng, S.C.; Wu, D.M.; Jacobs, D.F.; Sloan, J.L. Soil pH, organic matter, and nutrient content change with the continuous cropping of Cunninghamia lanceolata plantations in South China. J. Soils Sediments 2017, 17, 2230–2238. [Google Scholar] [CrossRef]
- Li, H.T.; Zhang, Y.; Wei, Z.C.; Jia, D.D.; Liu, Y.H.; Liu, A.Q. Evaluation on soil fertility of Chinese fir plantations in different development stages. For. Res. 2017, 30, 322–328. [Google Scholar]
- Jiao, R.Z.; Yang, C.D.; Tu, X.N.; Sheng, W.T. The change of undergrowth, soil microorganism, enzyme activity and nutrient in different developing stage of the Chinese fir plantation. For. Res. 1997, 4, 34–40. [Google Scholar]
- Wang, D.; Dai, W.; Wang, B.; Ping, L.I.; Deng, Z.F. Changes of soil properties at different developmental stages of Chinese fir plantations. J. Beijing For. Univ. 2010, 32, 59–63. [Google Scholar]
- Zhou, L.L.; Shalom, A.D.D.; Wu, P.F.; He, Z.M.; Liu, C.H.; Ma, X.Q. Biomass production, nutrient cycling and distribution in age-sequence Chinese fir (Cunninghamia lanceolate) plantations in subtropical China. J. For. Res. 2016, 27, 357–368. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Haack, S.E.; Lin, W.X.; Li, B.L.; Wu, L.K.; Fang, C.X.; Zhang, Z.X. Soil microbial community structure and metabolic activity of pinus elliottii plantations across different stand ages in a subtropical area. PLoS ONE 2015, 10, e0135354. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, Z.H.; Guo, X.M.; Sheng, K.Y.; Zhu, X.C.; Wen, W.H.; Zhang, W.Y. Soil microbial community structure characteristics of Chenshan Red-heart Chinese fir of different stand ages. Chin. J. Appl. Ecol. 2016, 22, 510–517. [Google Scholar]
- Sheng, W.T.; Yang, C.D.; Fan, S.H. Variation of soil properties of Chinese fir plantation. For. Res. 2003, 16, 377–385. [Google Scholar]
- Ma, X.Q.; Heal, K.V.; Liu, A.Q.; Jarvis, P.G. Nutrient cycling and distribution in different-aged plantations of Chinese fir in southern China. For. Ecol. Manag. 2007, 243, 61–74. [Google Scholar] [CrossRef]
- Pei, Y.M.; Lei, P.F.; Xiang, W.H.; Ouyang, S.; Xu, Y.Y. Effect of stand age on fine root biomass, production and morphology in Chinese fir plantations in Subtropical China. Sustainability 2018, 10, 2280. [Google Scholar] [CrossRef]
- Kang, H.Z.; Liu, C.J.; Yu, W.J.; Wu, L.L.; Chen, D.M.; Xiao, S.; Ma, X.P.; Hu, H.B.; Zhu, X.L. Variation in foliar δ15N among oriental oak (Quercus variabilis) stands over eastern China: Patterns and interactions. J. Geochem. Explor. 2011, 110, 8–14. [Google Scholar] [CrossRef]
- Kang, H.Z.; Gao, H.H.; Yu, W.J.; Yi, Y.; Wang, Y.; Ning, M.L. Changes in soil microbial community structure and function after afforestation depend on species and age: Case study in a subtropical alluvial island. Sci. Total Environ. 2018, 625, 1423–1432. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Hobbs, P.J.; Frostegard, A. Changes in soil fungal-bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. For. Ecol. Manag. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Cao, Y.S.; Fu, S.L.; Zou, X.M.; Cao, H.L.; Shao, Y.H.; Zhou, L.X. Soil microbial community composition under Eucalyptus plantations of different age in subtropical China. Eur. J. Soil Biol. 2010, 46, 128–135. [Google Scholar] [CrossRef]
- Wei, Z.C.; Huang, J.; Liu, Y.H.; Jia, D.D.; Li, H.T.; Wu, P.F.; Liu, A.Q. Community characteristics of soil bacteria of Cunninghamia lanceolata plantations at different developmental stages. J. Southwest For. Univ. 2017, 37, 122–129. [Google Scholar]
- Chen, G.S.; Yang, Z.J.; Gao, R.; Xie, J.S.; Guo, J.F.; Huang, Z.Q.; Yang, Y.S. Carbon storage in a chronosequence of Chinese fir plantations in southern China. For. Ecol. Manag. 2013, 300, 68–76. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.J.; Fang, H.Y.; Zhou, G.M.; Xu, X.J.; Zhou, Y.F.; Tao, J.X.; Ji, B.Y.; Xu, J.; Li, C.; et al. Vegetation carbon stocks driven by canopy density and forest age in subtropical forest ecosystems. Sci. Total Environ. 2018, 631–632, 619–626. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.C.; Li, H.T.; Guo, F.T.; Wu, P.F.; Zhou, L.L.; Ma, X.Q. Biochemical quality and accumulation of soil organic matter in an age sequence of Cunninghamia lanceolata plantations in southern China. J. Soils Sediments 2017, 17, 2218–2229. [Google Scholar] [CrossRef]
- Jia, Y.Y.; Zhou, L.L.; Wu, P.F.; Liu, Y.H.; Ma, X.Q. Characterization of understory community of Cunninghamia lanceolata plantations at different developmental stages. J. For. Environ. 2016, 36, 36–41. [Google Scholar]
- Lin, K.M.; Ma, X.Q.; Fan, S.H.; Zhang, X.Q.; Zhuo, S.A.; Jin, C.X. The laws of understory plant growth in Chinese fir plantations. J. Fujian Coll. For. 2000, 3, 231–234. [Google Scholar]
- Yang, C.; Tian, D.L.; Hu, Y.L.; Yan, W.D.; Fang, X.; Liang, X.C. Dynamics of understory vegetation biomass in successive rotations of Chinese fir (Cunninghamia lanceolata) plantations. Acta Ecol. Sin. 2011, 31, 2737–2747. [Google Scholar]
- Selvalakshmi, S.; Vasu, D.; Huang, Z.J.; Guo, F.; Ma, X.Q. Soil nutrients dynamics in broadleaved forest and Chinese fir plantations in subtropical forests. J. Trop. For. Sci. 2018, 30, 242–251. [Google Scholar]
- Chen, L.C.; Wang, H.; Yu, X.; Zhang, W.D.; Lu, X.T.; Wang, S.L. Recovery time of soil carbon pools of conversional Chinese fir plantations from broadleaved forests in subtropical regions, China. Sci. Total Environ. 2017, 587, 296–304. [Google Scholar] [CrossRef]
- Pietikainen, J.; Tikka, P.J.; Valkonen, S.; Isomaki, A.; Fritze, H. Is the soil microbial community related to the basal area of trees in a Scots pine stand? Soil Biol. Biochem. 2007, 39, 1832–1834. [Google Scholar] [CrossRef]
- Chen, F.L.; Zheng, H.; Zhang, K.; Ouyang, Z.Y.; Li, H.L.; Wu, B.; Shi, Q. Soil microbial community structure and function responses to successive planting of Eucalyptus. J. Environ. Sci. 2013, 25, 2102–2111. [Google Scholar] [CrossRef]
- Mackay, J.E.; Cunningham, S.C.; Cavagnaro, T.R. Riparian reforestation: Are there changes in soil carbon and soil microbial communities? Sci. Total Environ. 2016, 566, 960–967. [Google Scholar] [CrossRef]
- Yang, Q.; Lei, A.P.; Li, F.L.; Liu, L.A.; Zan, Q.J.; Shin, P.K.S.; Cheung, S.G.; Tam, N.F.Y. Structure and function of soil microbial community in artificially planted Sonneratia apetala and S. caseolaris forests at different stand ages in Shenzhen Bay, China. Mar. Pollut. Bull. 2014, 85, 754–763. [Google Scholar] [CrossRef]
- Yang, M.; Yang, D.; Yu, X. Soil microbial communities and enzyme activities in sea-buckthorn (Hippophae rhamnoides) plantation at different ages. PLoS ONE 2018, 13, e0190959. [Google Scholar] [CrossRef]
- Luo, D.; Liu, S.; Shi, Z.M.; Feng, Q.H.; Liu, Q.L.; Zhang, L.; Huang, Q.; He, J.S. Soil microbial community structure in Picea asperata plantations with different ages in subalpine of western Sichuan, Southwest China. Chin. J. Appl. Ecol. 2017, 28, 519–527. [Google Scholar]
- Liu, Z.K.; Tian, S.; Tang, M. Spatial distribution of arbuscular Mycorrhizal fungi and its relationship with soil factors in the Rhizosphere of Robinia pseudoacacia at different ages. Scientia Silvae Sinicae 2013, 49, 89–95. [Google Scholar]
- Baddeley, J.A.; Watson, C.A. Influences of root diameter, tree age, soil depth and season on fine root survivorship in Prunus avium. Plant Soil 2005, 276, 15–22. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Cunningham, S.C.; Fitzpatrick, S. Pastures to woodlands: Changes in soil microbial communities and carbon following reforestation. Appl. Soil Ecol. 2016, 107, 24–32. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, J.J.; Zheng, J.; Liu, J.F.; Liu, S.Y.; Lin, W.X.; Wu, C.Z. Soil microbial community structure and catabolic activity are significantly degenerated in successive rotations of Chinese fir plantations. Sci. Rep. 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Corneo, P.E.; Pellegrini, A.; Cappellin, L.; Roncador, M.; Chierici, M.; Gessler, C.; Pertot, I. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. For. Ecol. Manag. 2013, 84, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Grayston, S.J.; Campbell, C.D.; Bardgett, R.D.; Mawdsley, J.L.; Clegg, C.D.; Ritz, K.; Griffiths, B.S.; Rodwell, J.S.; Edwards, S.J.; Davies, W.J.; et al. Assessing shifts in microbial community structure across a range of grasslands of differing management intensity using CLPP, PLFA and community DNA techniques. Appl. Soil Ecol. 2004, 25, 63–84. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Wan, X.H.; He, Z.M.; Yu, Z.P.; Wang, M.H.; Hu, Z.H.; Yang, Y.S. Soil microbial biomass, community composition and soil nitrogen cycling in relation to tree species in subtropical China. Soil Biol. Biochem. 2013, 62, 68–75. [Google Scholar] [CrossRef]
- Puri, G.; Ashman, M.R. Relationship between soil microbial biomass and gross N mineralisation. Soil Biol. Biochem. 1998, 30, 251–256. [Google Scholar] [CrossRef]
- Wan, X.H.; Huang, Z.Q.; He, Z.M.; Yu, Z.P.; Wang, M.H.; Davis, M.R.; Yang, Y.S. Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
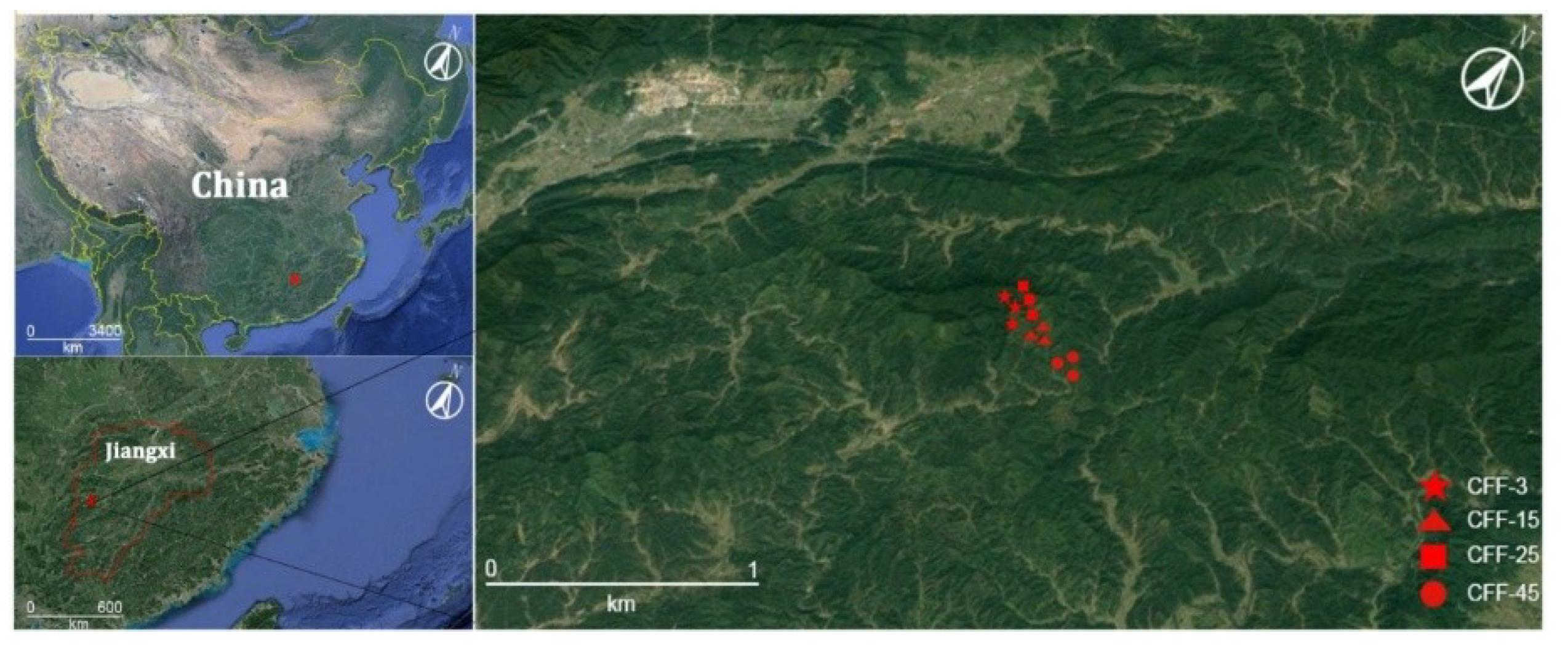
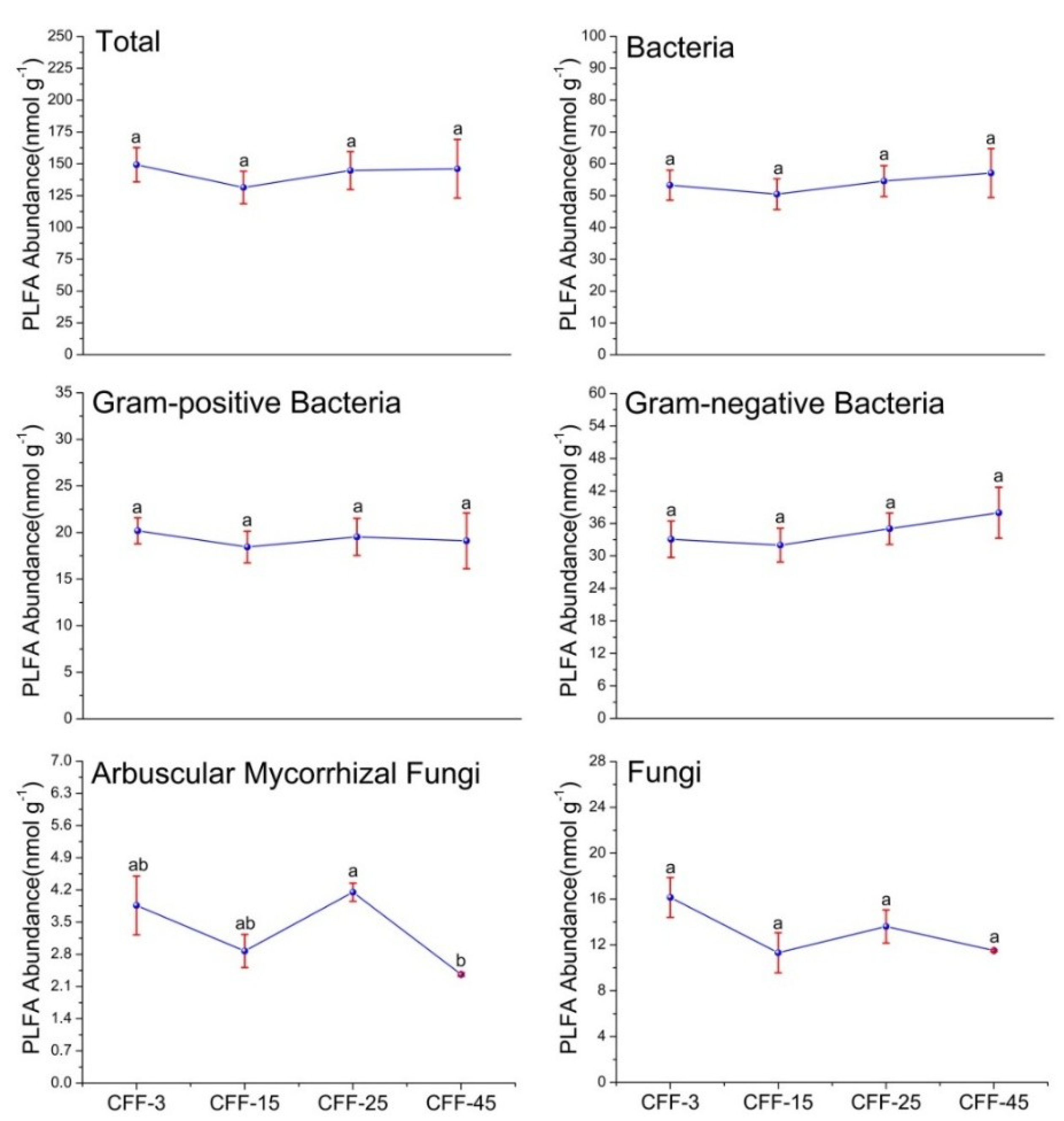
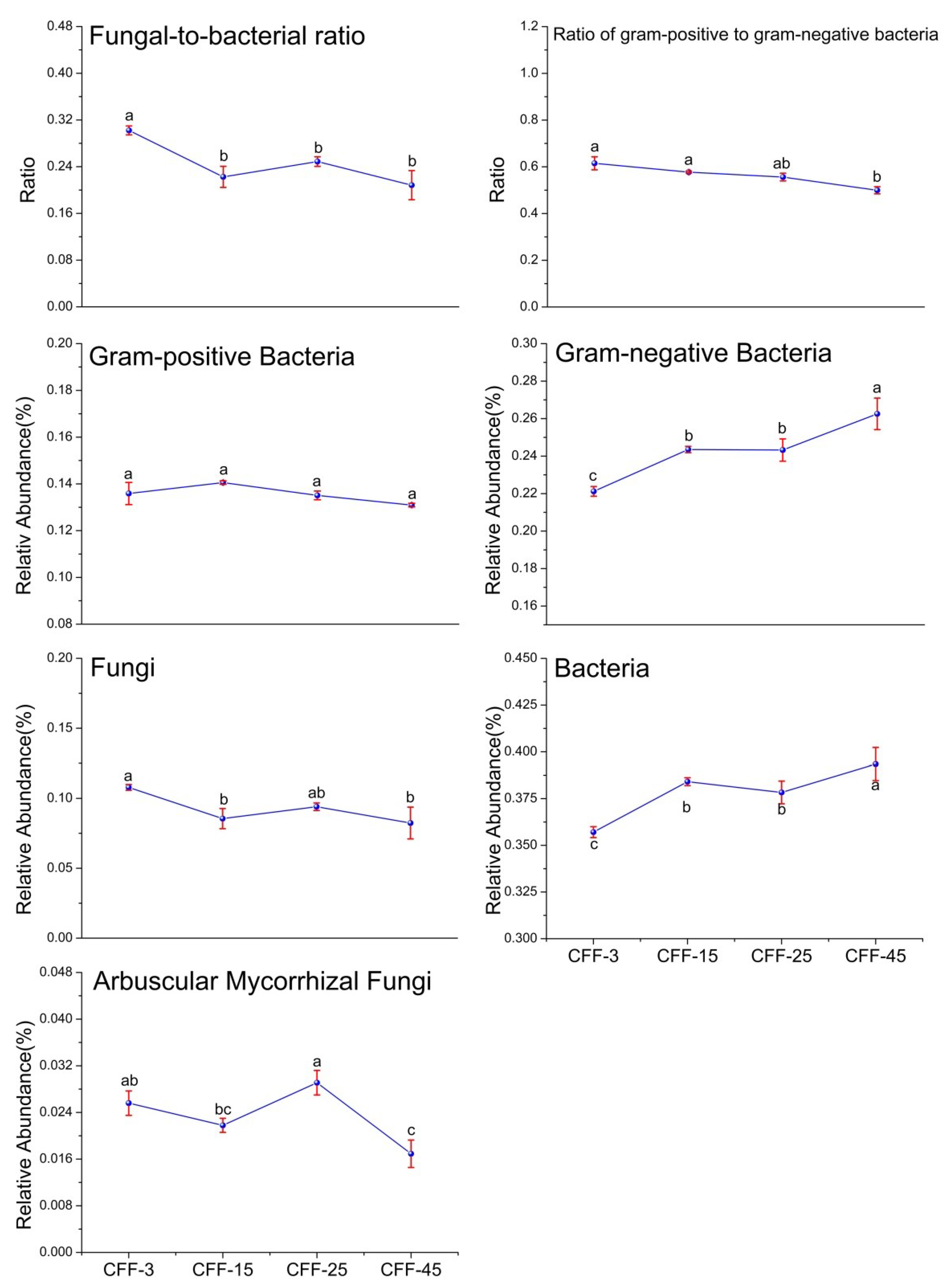
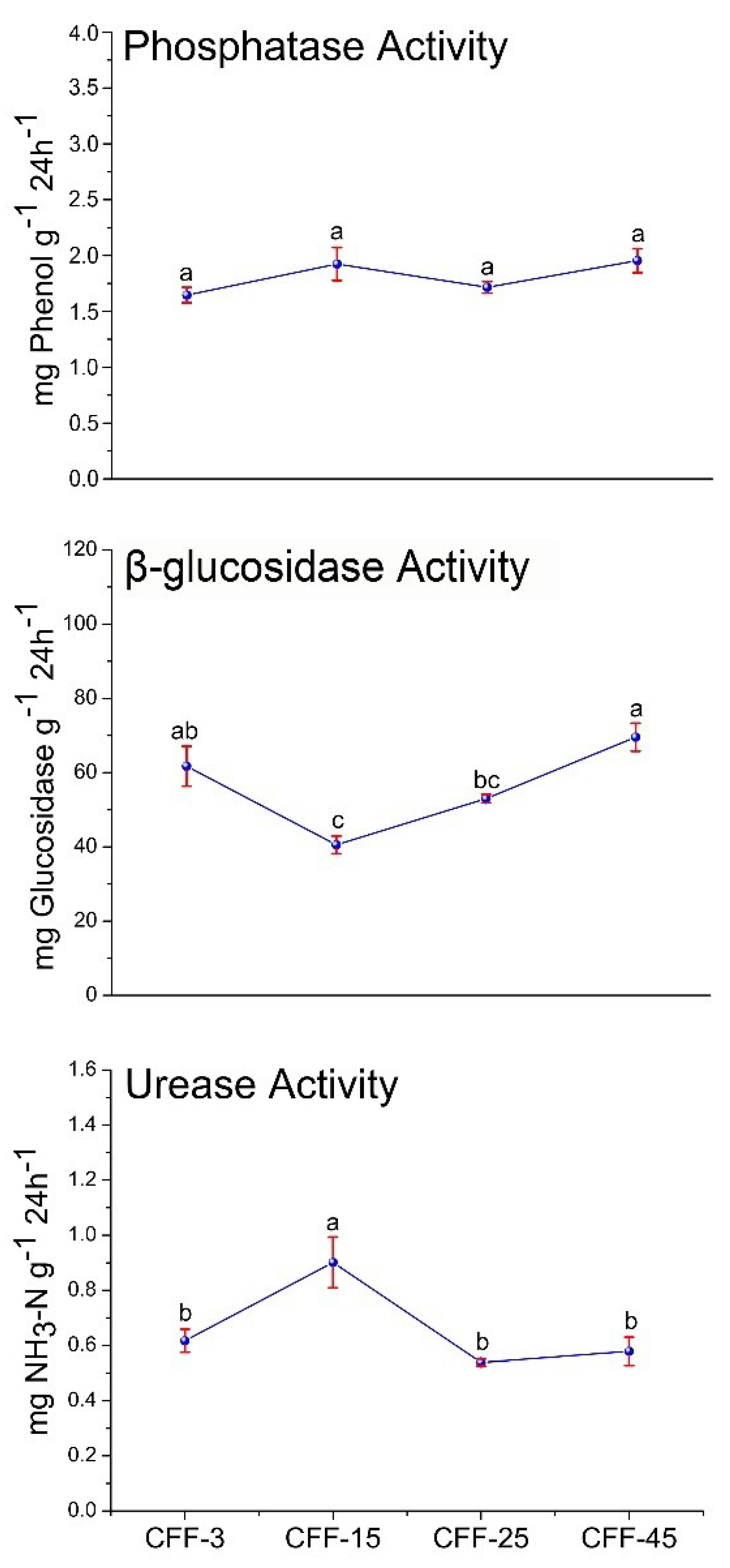
| Characteristics | CFF-3 | CFF-15 | CFF-25 | CFF-45 |
|---|---|---|---|---|
| Stand age (year) | 3 | 15 | 25 | 45 |
| DBH (cm) | 1.3 | 10.2 | 18.3 | 40.5 |
| Canopy height (m) | 1.5 | 8.5 | 11.2 | 25.4 |
| Tree density (trees ha−1) | 4430 | 3713 | 1325 | 583 |
| Elevation (m) | 315 | 327 | 322 | 306 |
| Slope (°) | 21 | 19 | 20 | 23 |
| Aspect | South | South | South | South |
| pH (in water) | 4.47 ± 0.06ab | 4.24 ± 0.05b | 4.49 ± 0.08a | 4.30 ± 0.08ab |
| Moisture (%) | 0.43 ± 0.01ab | 0.40 ± 0.00b | 0.40 ± 0.00b | 0.45 ± 0.02a |
| SOC (g/kg) | 37.21 ± 0.11a | 33.09 ± 0.34ab | 29.17 ± 0.08b | 32.02 ± 0.27ab |
| TN (g/kg) | 3.04 ± 0.04a | 2.76 ± 0.02a | 2.44 ± 0.02a | 2.54 ± 0.02a |
| C/N | 12.62 ± 1.48a | 11.94 ± 0.55a | 12.01 ± 0.55a | 12.57 ± 0.15a |
| Net N mineralization (μg g−1 d−1) | 0.61 ± 0.01b | 0.58 ± 0.03bc | 0.55 ± 0.01c | 0.89 ± 0.01a |
| TK (g/kg) | 8.97 ± 0.02c | 16.94 ± 0.01a | 17.08 ± 0.04a | 15.20 ± 0.07b |
| TP (g/kg) | 0.40 ± 0.000a | 0.44 ± 0.00a | 0.44 ± 0.00a | 0.40 ± 0.01a |
| Microbial Group | PLFA Biomarkers | Reference |
|---|---|---|
| Saprotrophic fungi | 18: 2ω6, 9 and 18: 1ω9 | [31] |
| Gram-positive bacteria | i15: 0, a15: 0, i16: 0, i17: 0, a17: 0 | [32,33,34] |
| Gram-negative bacteria | 16: 1ω9, 16: 1ω7, cy17: 0, cy19: 0 | [32,33,34] |
| Bacteria | i15: 0, a15: 0, i16: 0, i17: 0, a17: 0,16: 1ω9, 16: 1ω7, cy17: 0, cy19: 0 | [32,33,34] |
| Arbuscular mycorrhizal fungi | C16: 1ω5 | [35] |
| β-glucosidase | Urease | Phosphatase | |
|---|---|---|---|
| Substrate | sucrose | urea | disodium phenyl phosphate |
| Incubation temperature (°C) | 37 | 37 | 37 |
| Incubation time (h) | 24 | 24 | 24 |
| Absorbance (nm) | 508 | 578 | 570 |
| Product (mg g−1 24 h−1) | glucose | NH4+ | phenol |
| Microbial Communities | Soil Physicochemical Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | Moisture | SOC | TN | C/N | TK | TP | Net N Mineralization | |
| Total PLFAs | 0.34 | 0.36 | 0.61 * | 0.45 | 0.06 | −0.22 | 0.169 | 0.07 |
| Bacterial PLFAs | 0.20 | 0.46 | 0.52 | 0.33 | 0.11 | −0.05 | 0.219 | 0.26 |
| G+ bacterial PLFAs | 0.36 | 0.37 | 0.64 * | 0.43 | 0.14 | −0.19 | 0.312 | 0.01 |
| G− bacterial PLFAs | 0.10 | 0.48 | 0.44 | 0.26 | 0.08 | 0.04 | 0.158 | 0.38 |
| Fungal PLFAs | 0.37 | −0.04 | 0.58 | 0.46 | 0.08 | −0.58 * | 0.033 | −0.19 |
| AM Fungal PLFAs | 0.45 | −0.42 | 0.24 | 0.12 | 0.12 | −0.20 | 0.113 | −0.57 |
| F/B ratio | 0.34 | −0.26 | 0.31 | 0.31 | 0.04 | −0.68 * | −0.132 | −0.36 |
| G+/G− ratio | 0.52 | −0.17 | 0.39 | 0.29 | 0.15 | −0.47 | 0.294 | −0.68 * |
| G+ bacteria% | 0.11 | −0.05 | 0.14 | −0.12 | 0.35 | 0.10 | 0.639 * | −0.36 |
| G− bacteria% | −0.58 | 0.21 | −0.41 | −0.45 | 0.04 | 0.59 * | −0.033 | 0.71 ** |
| Fungi% | 0.23 | −0.29 | 0.23 | 0.20 | 0.09 | −0.59 * | −1.00 | −0.25 |
| Bacteria% | −0.57 | 0.20 | −0.39 | −0.50 | 0.15 | 0.64 * | 0.153 | 0.63 * |
| AM Fungi% | 0.33 | −0.66 * | −0.11 | −0.18 | 0.16 | −0.05 | 0.016 | −0.72 ** |
| β−glucosidase activity | 0.18 | 0.68 * | 0.18 | 0.19 | −0.02 | −0.41 | −0.47 | 0.66 * |
| Urease activity | −0.34 | −0.12 | 0.04 | 0.20 | −0.26 | 0.11 | 0.223 | −0.28 |
| Phosphatase activity | −0.42 | −0.16 | −0.66 * | −0.51 | −0.14 | 0.40 | 0.015 | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Q.; Yu, W.; Kang, H.; Wang, J. Prolonging Rotation of Chinese Fir to over 25 Years Could Maintain a Better Soil Status in Subtropical China. Forests 2019, 10, 629. https://doi.org/10.3390/f10080629
Miao Q, Yu W, Kang H, Wang J. Prolonging Rotation of Chinese Fir to over 25 Years Could Maintain a Better Soil Status in Subtropical China. Forests. 2019; 10(8):629. https://doi.org/10.3390/f10080629
Chicago/Turabian StyleMiao, Quanxin, Wenjuan Yu, Hongzhang Kang, and Jiaojiao Wang. 2019. "Prolonging Rotation of Chinese Fir to over 25 Years Could Maintain a Better Soil Status in Subtropical China" Forests 10, no. 8: 629. https://doi.org/10.3390/f10080629
APA StyleMiao, Q., Yu, W., Kang, H., & Wang, J. (2019). Prolonging Rotation of Chinese Fir to over 25 Years Could Maintain a Better Soil Status in Subtropical China. Forests, 10(8), 629. https://doi.org/10.3390/f10080629





