Biochar Fertilization Significantly Increases Nutrient Levels in Plants and Soil but Has No Effect on Biomass of Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook Saplings During the First Growing Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Collection
2.2. Plant Material and Biochar Treatments
2.3. Growth and Root Morphology Measurements
2.4. Tissue and Soil Chemical Analysis
2.5. Statistical Analysis
3. Results
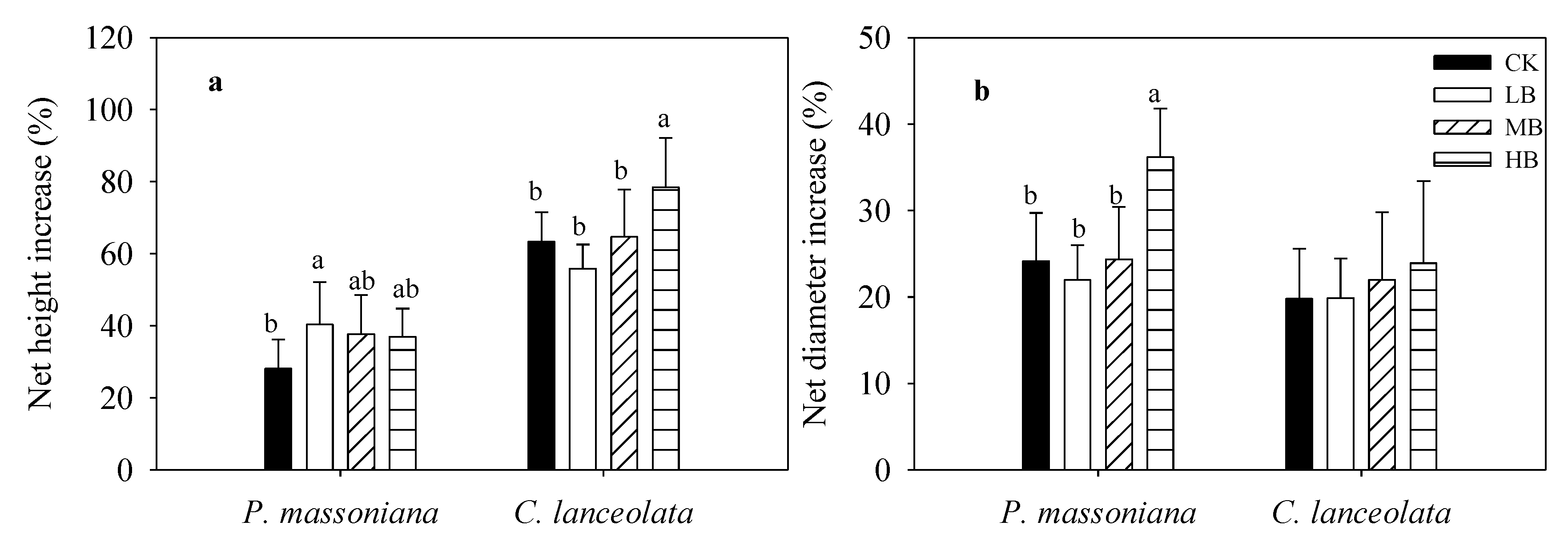
3.1. Effects of Biochar Fertilization on Sapling Growth
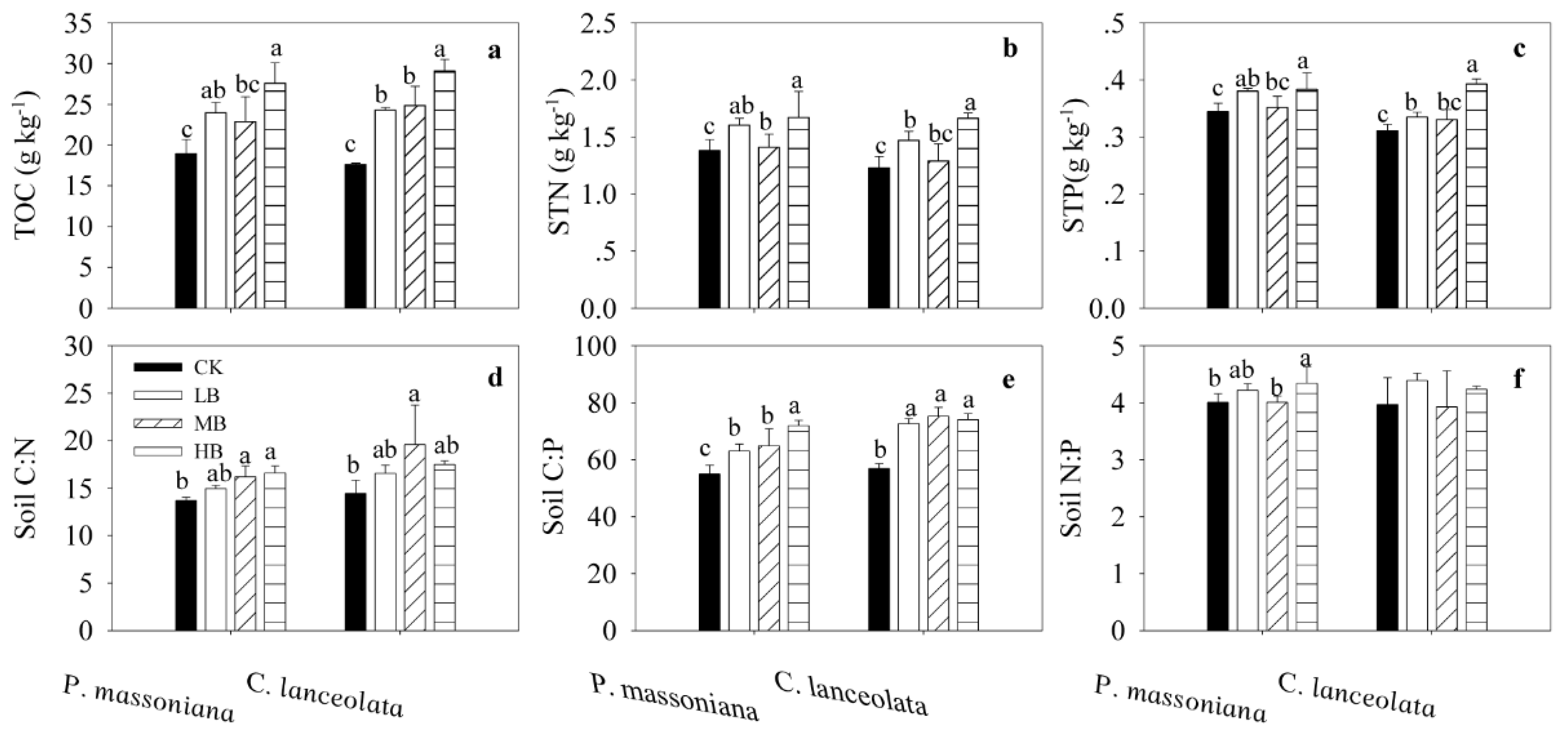
3.2. Effects of Biochar Fertilization on SOC, STN, STP, and Stoichiometry
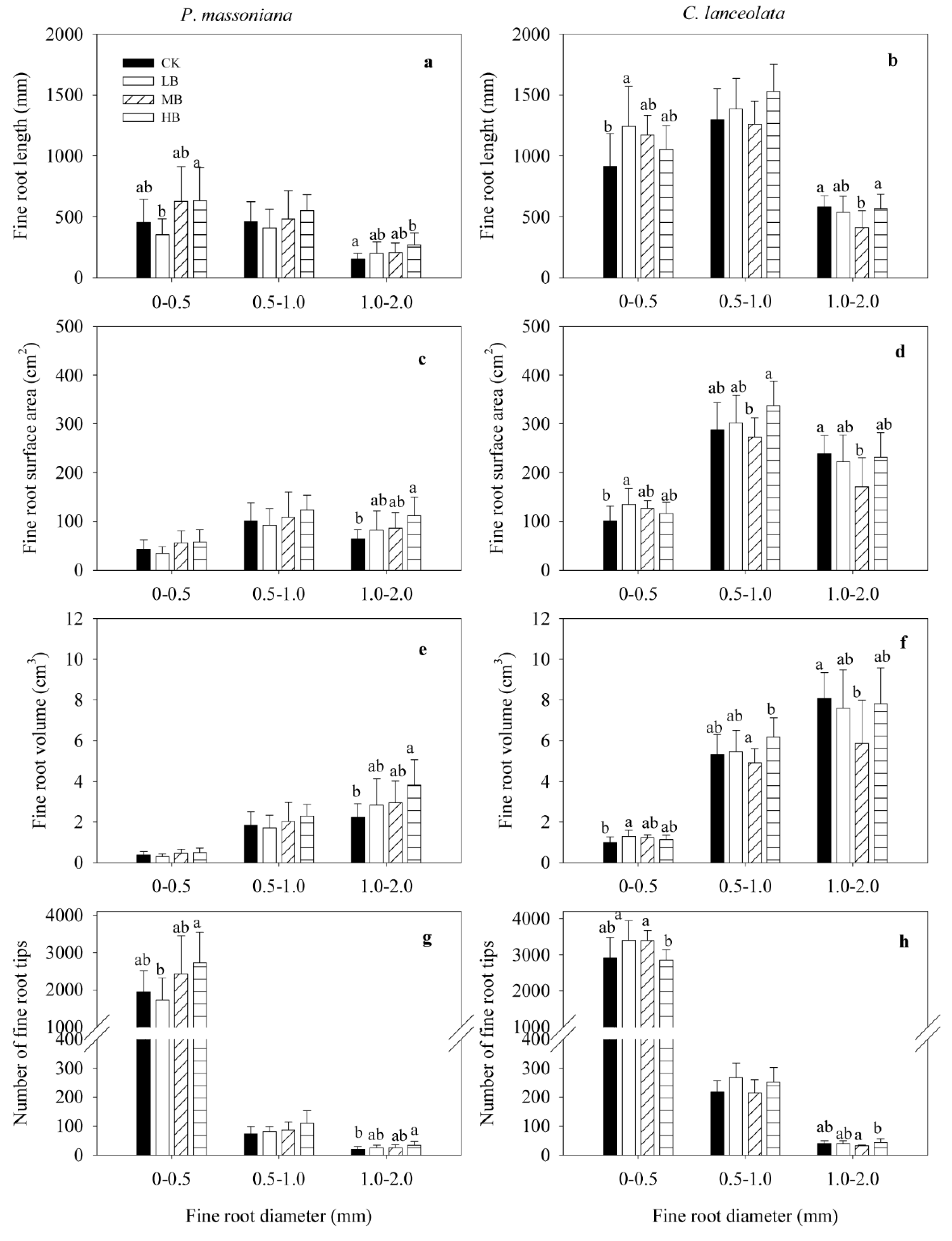
3.3. Effect of Biochar Fertilization on Fine Root Morphology
3.4. Effects of Biochar Fertilization on Plant Nutrients and Stoichiometry
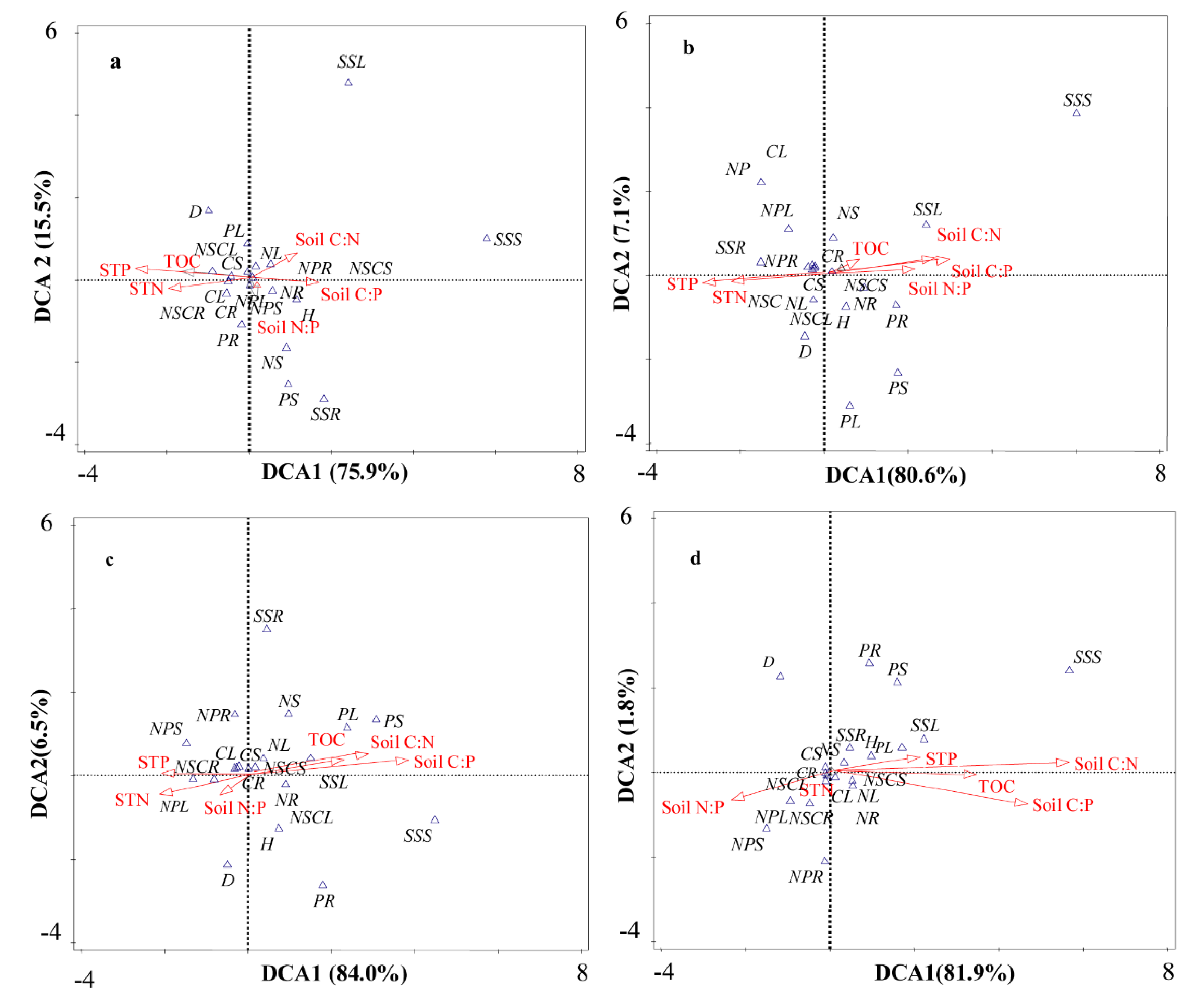
3.5. Relationships of Stoichiometry between Plants and Soil under Biochar Fertilization
4. Discussion
4.1. Effects of Biochar Fertilization on Plant Growth and Fine Root Morphology
4.2. Effects of Biochar Fertilization on Plant Nutrients in Relation to Soil Nutrients
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Singh, N.; Abiven, S.; Maestrini, B.; Bird, J.A.; Torn, M.S.; Schmidt, M.W.I. Transformation and stabilization of pyrogenic organic matter in a temperate forest field experiment. Glob. Chang. Biol. 2014, 20, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. In Advances in Agronomy; Donald, D.L., Ed.; Academic Press: Burlington, MA, USA, 2010. [Google Scholar]
- Amendola, C.; Montagnoli, A.; Terzaghi, M.; Trupiano, D.; Oliva, F.; Baronti, S.; Miglietta, F.; Chiatante, D.; Scippa, G.S. Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric. Ecosyst. Environ. 2017, 239, 236–245. [Google Scholar] [CrossRef]
- Makoto, K.; Tamai, Y.; Kim, Y.S.; Koike, T. Buried charcoal layer and ectomycorrhizae cooperatively promote the growth of Larix gmelinii seedlings. Plant Soil 2010, 327, 143–152. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Allah, E.F.A.; Berg, G. Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Front. Microbiol. 2016, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, S.; Bezemer, T.M.; Cornelissen, G.; Kuyper, T.W.; Lehmann, J.; Mommer, L.; Sohi, S.P.; van de Voorde, T.F.J.; Wardle, D.A.; van Groenigen, J.W. The way forward in biochar research: Targeting trade-offs between the potential wins. GCB Bioenergy 2015, 7, 1–13. [Google Scholar] [CrossRef]
- Makoto, K.; Choi, D.; Hashidoko, Y.; Koike, T. The growth of Larix gmelinii seedlings as affected by charcoal produced at two different temperatures. Biol. Fertil. Soils 2011, 47, 467–472. [Google Scholar] [CrossRef]
- McElligott, K.M. Biochar Amendments to Forest Soils: Effects on Soil Properties and Tree Growth. Master’s Thesis, University of Idaho, Moscow, ID, USA, 2011. [Google Scholar]
- Hagner, M.; Kemppainen, R.; Jauhiainen, L.; Tiilikkala, K.; Setälä, H. The effects of birch (Betula spp.) biochar and pyrolysis temperature on soil properties and plant growth. Soil Tillage Res. 2016, 163, 224–234. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar absorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Sackett, T.E.; Basiliko, N.; Noyce, G.L.; Winsborough, C.; Schurman, J.; Ikeda, C.; Thomas, S.C. Soil and greenhouse gas responses to biochar additions in a temperature hardwood forest. GCB Bioenergy 2015, 7, 1062–1074. [Google Scholar] [CrossRef]
- Gale, N.V.; Thomas, S.C. Dose-dependence of growth and ecophysiological responses of plants to biochar. Sci. Total Environ. 2019, 658, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Razaq, M.; Salahuddin; Shen, H.L.; Sher, H.; Zhang, P. Influence of biochar and nitrogen of fine root morphology, physiology, and chemistry of Acer mono. Sci. Rep. 2018, 7, 5367. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ugalde, O.; Gartzia-Bengoetxea, N.; Arostegi, J.; Moragues, L.; Arias-González, A. Storage and stability of biochar-derived carbon and total organic carbon in relation to minerals in an acid forest soil of the Spanish Atlantic area. Sci. Total Environ. 2017, 587–588, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Simpson, A.J.; Soong, R.; Simpson, M.J. Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol. Biochem. 2015, 81, 244–254. [Google Scholar] [CrossRef]
- Birge, H.E.; Conant, R.T.; Follett, R.F.; Haddix, M.L.; Morris, S.J.; Snapp, S.S.; Wallenstein, M.D.; Paul, E.A. Soil respiration is not limited by reductions in microbial biomass during long-term soil incubations. Soil Biol. Biochem. 2015, 81, 304–310. [Google Scholar] [CrossRef]
- Makoto, K.; Shibata, H.; Kim, Y.S.; Satomura, T.; Takagi, K.; Nomura, M.; Satoh, F.; Koike, T. Contribution of charcoal to short-term nutrient dynamics after surface fire in the humus layer of a dwarf bamboo-dominated forest. Biol. Fertil. Soils 2012, 48, 569–577. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.P.; Liu, W.F.; Yuan, Y.H.; Hu, L.; Cai, Q.K. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Robertson, S.J.; Rutherford, P.M.; López-Gutiérrez, J.C.; Massicotte, H.B. Biochar enhances seedling growth and alters root symbioses and properties of sub-boreal forest soils. Can. J. Soil Sci. 2012, 92, 329–340. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Y.F.; Chang, S.X.; Yang, Y.F.; Fu, S.L.; Jiang, P.K.; Luo, Y.; Yang, M.; Chen, Z.H.; Hu, S.D.; et al. Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Palviainen, M.; Berninger, F.; Bruckman, V.J.; Köster, K.; Ribeiro, C.; De Assumpção, M.; Aaltonen, H.; Makita, N.; Mishra, A.; Kulmala, L.; et al. Effects of biochar on carbon and nitrogen fluxes in boreal forest soil. Plant Soil 2018, 425, 71–85. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Xie, J.S.; Lyu, M.K.; Xiong, D.C.; Wang, J.; Chen, Y.M.; Li, Y.Q.; Wang, M.K.; Yang, Y.S. Short-term effects of soil warming and nitrogen addition on the N:P stoichiometry of Cunninghamia lanceolata in subtropical regions. Plant Soil 2017, 411, 395–407. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2015: How are the World’s Forests Changing? Food and Agricultural organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Ge, X.G.; Xiao, W.F.; Zeng, L.X.; Huang, Z.L.; Lei, J.P.; Li, M.H. The link between litterfall, substrate quality, decomposition rate and soil nutrient supply in 30-year-old Pinus massoniana forests in the Three Gorges Reservoir Area, China. Soil Sci. 2013, 178, 442–451. [Google Scholar] [CrossRef]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Short-term effects of biochar on soil properties and wheat yield formation with meat bone meal and inorganic fertiliser on a boreal loamy sand. Agric. Ecosyst. Environ. 2014, 191, 108–116. [Google Scholar] [CrossRef]
- Miller, R.H.; Keeney, D.R. Methods of Soil Analysis, 2nd ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Yang, Y.S.; Guo, J.F.; Chen, G.S.; Xie, J.S.; Cai, L.P.; Lin, P. Litterfall, nutrient return, and leaf-litter decomposition in four plantations compared with a natural forest in subtropical China. Ann. For. Sci. 2004, 61, 465–476. [Google Scholar] [CrossRef]
- Li, M.H.; Xiao, W.F.; Shi, P.L.; Wang, S.G.; Zhong, Y.D.; Liu, X.L.; Wang, X.D.; Cai, X.H.; Shi, Z.M. Nitrogen and carbon source-sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant Cell Environ. 2008, 31, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Hol, W.H.G.; Vestergård, M.; ten Hooven, F.; Duyts, H.; van de Voorde, T.F.J.; Bezemer, T.M. Transient negative biochar effects on plant growth are strongest after microbial species loss. Soil Biol. Biochem. 2017, 115, 442–451. [Google Scholar] [CrossRef]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar] [CrossRef]
- Rees, F.; Sterckeman, T.; Morel, J.L. Root development of non-accumulating and hyperaccumulating plants in metal-contaminated soils amended with biochar. Chemosphere 2016, 142, 48–55. [Google Scholar] [CrossRef]
- Wu, P.F.; Wang, G.Y.; Farooq, T.H.; Li, Q.; Zou, X.H.; Ma, X.Q. Low phosphorus and competition affect Chinese fir cutting growth and root organic acid content: Does neighboring root activity aggravate P nutrient deficiency ? J. Soils Sediments 2017, 17, 2775–2785. [Google Scholar] [CrossRef]
- Obia, A.; Mulder, J.; Martinsen, V.; Cornelissen, G.; Børresen, T. In situ effects of biochar on aggregation, water retention and porosity in light-textured tropical soils. Soil Tillage Res. 2016, 155, 35–44. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Al-Soud, W.A.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Backer, R.G.M.; Saeed, W.; Seguin, P.; Smith, D.L. Root traits and nitrogen fertilizer recovery efficiency of corn grown in biochar-amended soil under greenhouse condition. Plant Soil 2017, 415, 465–477. [Google Scholar] [CrossRef]
- Ventura, M.; Zhang, C.; Baldi, E.; Fornasier, F.; Sorrenti, G.; Panzacchi, P.; Tonon, G. Effect of biochar addition on soil respiration partitioning and root dynamics in an apple orchard. Eur. J. Soil Sci. 2014, 65, 186–195. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Marschner, B.; Itanna, F.; Gebrekidan, H. Effect of biochar derived from faecal matter on yield and nutrient content of lettuce (Lactuca sativa) in two contrasting soils. Environ. Syst. Res. 2017, 6, 2. [Google Scholar] [CrossRef]
- Castellanos, A.E.; Llano-Sotelo, J.M.; Machado-Encinas, L.I.; López-Piña, J.E.; Romo-Leon, J.R.; Sardans, J.; Peñuelas, J. Folia C, N, and P stoichiometry characterize successful plant ecological strategies in the Sonoran Desert. Plant Ecol. 2018, 219, 775–788. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Deng, Q.; Duan, H.L.; Guo, Y. Effects of biochar application on root traits: A meta-analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Ǻgren, G.I. The C:N:P stoichiometry of autotrophs-theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Y.X.; Lin, J.H.; Hu, Y.Y.; Hänninen, H.; Wu, J.S. Biochar application alleviates unbalanced nutrient uptake caused by N deposition in Torreya grandis trees and seedlings. For. Ecol. Manag. 2019, 432, 319–326. [Google Scholar] [CrossRef]
- Li, N.; He, N.P.; Yu, G.R.; Wang, Q.F.; Sun, J. Leaf non-structural carbohydrates regulated by plant functional groups and climate: Evidences from a tropical to cold-temperate forest transect. Ecol. Indic. 2016, 62, 22–31. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial red field-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Gregory, S.J.; Anderson, C.W.N.; Arbestain, M.C.; McManus, M.T. Response of plant and soil microbes to biochar amendment of an arsenic-contaminated soil. Agric. Ecosyst. Environ. 2014, 191, 133–141. [Google Scholar] [CrossRef]
- Reverchon, F.; Flicker, R.C.; Yang, H.; Yan, G.J.; Xu, Z.H.; Chen, C.R.; Bai, S.H.; Zhang, D.K. Changes in δ15N in a soil–plant system under different biocharfeedstocks and application rates. Biol. Fertil. Soils 2014, 50, 275–283. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, L.M.; Farrell, M.; Van Zwieten, L.; Krull, E.S. Plant growth responses to biochar addition: An Australian soils perspective. Biol. Fertil. Soils 2014, 50, 1035–1045. [Google Scholar] [CrossRef]
- Bell, C.; Carrillo, Y.; Boot, C.M.; Rocca, J.D.; Pendall, E.; Wallenstein, M.D. Rhizosphere stoichiometry: Are C:N:P ratios of plants, soils, and enzymes conserved at the plant species-level? New Phytol. 2014, 201, 505–517. [Google Scholar] [CrossRef]
- Richardson, S.J.; Allen, R.B.; Doherty, J.E. Shifts in leaf N:P ratio during resorption reflect soil P in temperate rainforest. Funct. Ecol. 2008, 22, 738–745. [Google Scholar] [CrossRef]


| Variable | Species (S) | Biochar (B) | S × B | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Leaves biomass | 16.37 | 0.000 | 0.76 | 0.526 | 0.76 | 0.526 |
| Shoots biomass | 5.97 | 0.019 | 1.34 | 0.276 | 1.45 | 0.243 |
| Roots biomass | 6.05 | 0.018 | 1.45 | 0.242 | 2.08 | 0.118 |
| Total biomass | 5.29 | 0.027 | 1.14 | 0.344 | 0.62 | 0.604 |
| Initial height | 108.37 | 0.000 | 0.63 | 0.601 | 1.34 | 0.277 |
| Initial diameter | 0.29 | 0.596 | 2.55 | 0.069 | 2.17 | 0.107 |
| Final height | 39.16 | 0.000 | 1.43 | 0.248 | 3.61 | 0.021 |
| Final diameter | 0.53 | 0.472 | 2.10 | 0.115 | 2.65 | 0.062 |
| Soil organic C | 0.69 | 0.419 | 29.27 | 0.000 | 0.92 | 0.453 |
| Soil total N | 4.28 | 0.055 | 11.38 | 0.000 | 0.44 | 0.731 |
| Soil total P | 12.17 | 0.003 | 15.99 | 0.000 | 3.25 | 0.049 |
| Soil C:N | 5.96 | 0.027 | 5.93 | 0.006 | 0.80 | 0.513 |
| Soil C:P | 22.89 | 0.000 | 35.72 | 0.000 | 3.34 | 0.046 |
| Soil N:P | 0.01 | 0.918 | 2.12 | 0.137 | 0.26 | 0.854 |
| Variable | Fine Root Length | Fine Root Surface Area | Fine Root Volume | Numbers of Fine Root Tips | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Total fine roots | ||||||||
| Species (S) | 201.47 | 0.000 | 210.32 | 0.000 | 168.77 | 0.000 | 34.69 | 0.000 |
| Biochar (B) | 1.50 | 0.230 | 1.70 | 0.183 | 1.82 | 0.024 | 1.46 | 0.241 |
| Diameter class (D) | 113.64 | 0.000 | 115.50 | 0.000 | 240.97 | 0.159 | 801.94 | 0.000 |
| S × B | 1.32 | 0.283 | 1.04 | 0.387 | 1.30 | 0.288 | 3.01 | 0.042 |
| 0–0.5 mm roots | ||||||||
| Species (S) | 71.06 | 0.000 | 109.31 | 0.000 | 141.52 | 0.000 | 26.59 | 0.000 |
| Biochar (B) | 1.75 | 0.172 | 1.48 | 0.235 | 1.33 | 0.278 | 1.47 | 0.237 |
| S × B | 2.40 | 0.082 | 2.04 | 0.124 | 1.84 | 0.155 | 2.99 | 0.042 |
| 0.5–1.0 mm roots | ||||||||
| Species (S) | 229.18 | 0.000 | 219.51 | 0.000 | 208.74 | 0.000 | 175.43 | 0.000 |
| Biochar (B) | 1.84 | 0.155 | 2.00 | 0.130 | 2.12 | 0.113 | 2.20 | 0.104 |
| S × B | 0.72 | 0.545 | 0.77 | 0.517 | 0.85 | 0.473 | 1.27 | 0.297 |
| 1.0–2.0 mm roots | ||||||||
| Species (S) | 112.99 | 0.000 | 108.69 | 0.000 | 104.30 | 0.000 | 20.10 | 0.000 |
| Biochar (B) | 2.20 | 0.103 | 2.00 | 0.129 | 1.81 | 0.162 | 2.66 | 0.041 |
| S × B | 2.46 | 0.047 | 2.28 | 0.094 | 2.10 | 0.116 | 0.99 | 0.406 |
| Variables | C | N | P | NSC | N to P Ratio | Sugar to Starch Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| Leaves | ||||||||||||
| Species (S) | 8.56 | 0.010 | 17.04 | 0.001 | 43.68 | 0.000 | 76.73 | 0.000 | 17.16 | 0.001 | 65.20 | 0.000 |
| Biochar (B) | 0.94 | 0.046 | 10.06 | 0.001 | 8.49 | 0.001 | 13.92 | 0.000 | 4.67 | 0.016 | 1.13 | 0.367 |
| S × B | 0.32 | 0.089 | 3.45 | 0.042 | 8.86 | 0.001 | 1.078 | 0.387 | 6.44 | 0.005 | 0.93 | 0.450 |
| Shoots | ||||||||||||
| Species (S) | 14.06 | 0.002 | 29.88 | 0.000 | 94.44 | 0.000 | 23.44 | 0.000 | 29.12 | 0.000 | 81.48 | 0.000 |
| Biochar (B) | 3.33 | 0.046 | 2.28 | 0.119 | 8.53 | 0.001 | 5.10 | 0.011 | 9.80 | 0.001 | 1.48 | 0.259 |
| S × B | 1.77 | 0.193 | 3.51 | 0.040 | 6.04 | 0.006 | 2.07 | 0.145 | 11.51 | 0.000 | 2.09 | 0.141 |
| Roots | ||||||||||||
| Species (S) | 16.65 | 0.001 | 184.71 | 0.000 | 25.21 | 0.000 | 95.17 | 0.000 | 6.92 | 0.353 | 3.81 | 0.069 |
| Biochar (B) | 0.19 | 0.905 | 7.74 | 0.002 | 1.49 | 0.254 | 9.54 | 0.001 | 2.14 | 0.135 | 5.87 | 0.007 |
| S × B | 0.60 | 0.622 | 1.14 | 0.279 | 2.23 | 0.124 | 5.88 | 0.007 | 2.42 | 0.104 | 4.81 | 0.014 |
| Variables | Leaves | Stems | Roots | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | P | NSC | RNP | RSS | C | N | P | NSC | RNP | RSS | C | N | P | NSC | RNP | RSS | |
| SOC | 0.26 | 0.35 | 0.47 * | 0.05 | −0.39 | 0.22 | −0.31 | 0.10 | 0.46 * | −0.36 | −0.55 ** | 0.13 | −0.10 | 0.00 | 0.26 | 0.16 | −0.34 | 0.43 * |
| STN | 0.42 * | 0.02 | 0.10 | 0.01 | −0.12 | −0.12 | −0.14 | −0.24 | 0.00 | −0.51 * | −0.22 | −0.12 | 0.32 | −0.38 | −0.01 | 0.36 | −0.44 * | −0.46 * |
| STP | 0.58 ** | 0.14 | 0.15 | 0.02 | −0.18 | 0.77 ** | 0.00 | −0.24 | 0.01 | −0.65 ** | −0.20 | −0.19 | 0.21 | −0.42 * | −0.04 | 0.38 | −0.44 * | −0.52 |
| Soil C:N | −0.05 | 0.43 * | 0.52 ** | 0.36 | −0.40 | 0.41 * | −0.25 | 0.39 | 0.62 ** | 0.05 | −0.51 * | 0.26 | −0.47 * | 0.39 | 0.36 | −0.16 | −0.01 | −0.06 |
| Soil C:P | −0.07 | 0.36 | 0.57 ** | 0.40 | −0.53 ** | 0.45 * | −0.44 * | 0.29 | 0.65 ** | −0.04 | −0.66 ** | 0.30 | −0.30 | 0.29 | 0.40 | −0.04 | −0.19 | −0.23 |
| Soil N:P | 0.02 | −0.14 | 0.01 | 0.05 | −0.14 | 0.02 | −0.24 | −0.13 | 0.01 | −0.09 | −0.17 | 0.03 | 0.30 | −0.17 | 0.04 | 0.17 | −0.27 | −0.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, X.; Yang, Z.; Zhou, B.; Cao, Y.; Xiao, W.; Wang, X.; Li, M.-H. Biochar Fertilization Significantly Increases Nutrient Levels in Plants and Soil but Has No Effect on Biomass of Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook Saplings During the First Growing Season. Forests 2019, 10, 612. https://doi.org/10.3390/f10080612
Ge X, Yang Z, Zhou B, Cao Y, Xiao W, Wang X, Li M-H. Biochar Fertilization Significantly Increases Nutrient Levels in Plants and Soil but Has No Effect on Biomass of Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook Saplings During the First Growing Season. Forests. 2019; 10(8):612. https://doi.org/10.3390/f10080612
Chicago/Turabian StyleGe, Xiaogai, Zhenya Yang, Benzhi Zhou, Yonghui Cao, Wenfa Xiao, Xiaoming Wang, and Mai-He Li. 2019. "Biochar Fertilization Significantly Increases Nutrient Levels in Plants and Soil but Has No Effect on Biomass of Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook Saplings During the First Growing Season" Forests 10, no. 8: 612. https://doi.org/10.3390/f10080612
APA StyleGe, X., Yang, Z., Zhou, B., Cao, Y., Xiao, W., Wang, X., & Li, M.-H. (2019). Biochar Fertilization Significantly Increases Nutrient Levels in Plants and Soil but Has No Effect on Biomass of Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook Saplings During the First Growing Season. Forests, 10(8), 612. https://doi.org/10.3390/f10080612





