Divergent Last Century Tree Growth along An Altitudinal Gradient in A Pinus sylvestris L. Dry-edge Population
Abstract
1. Introduction
2. Materials and Methods
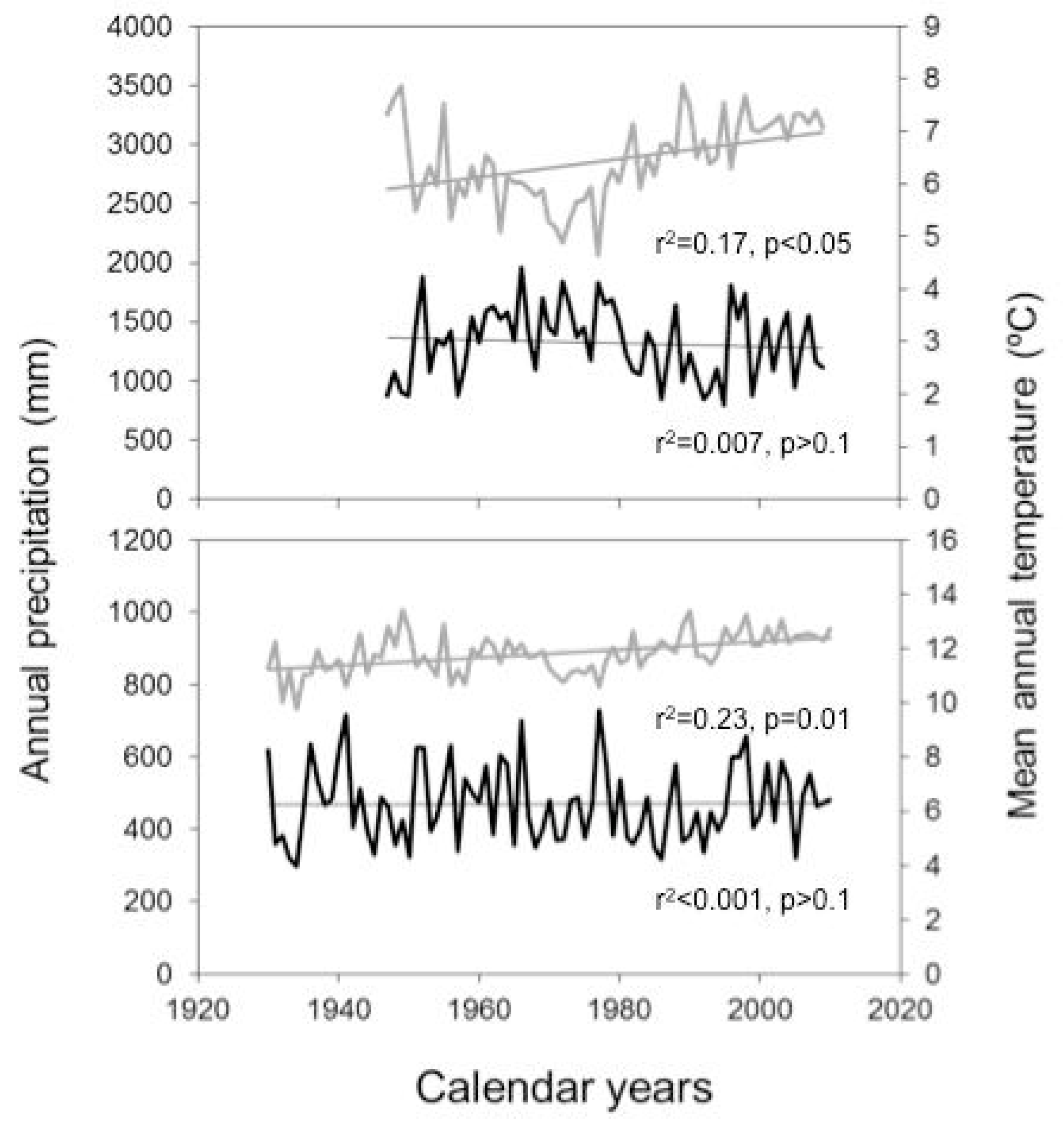
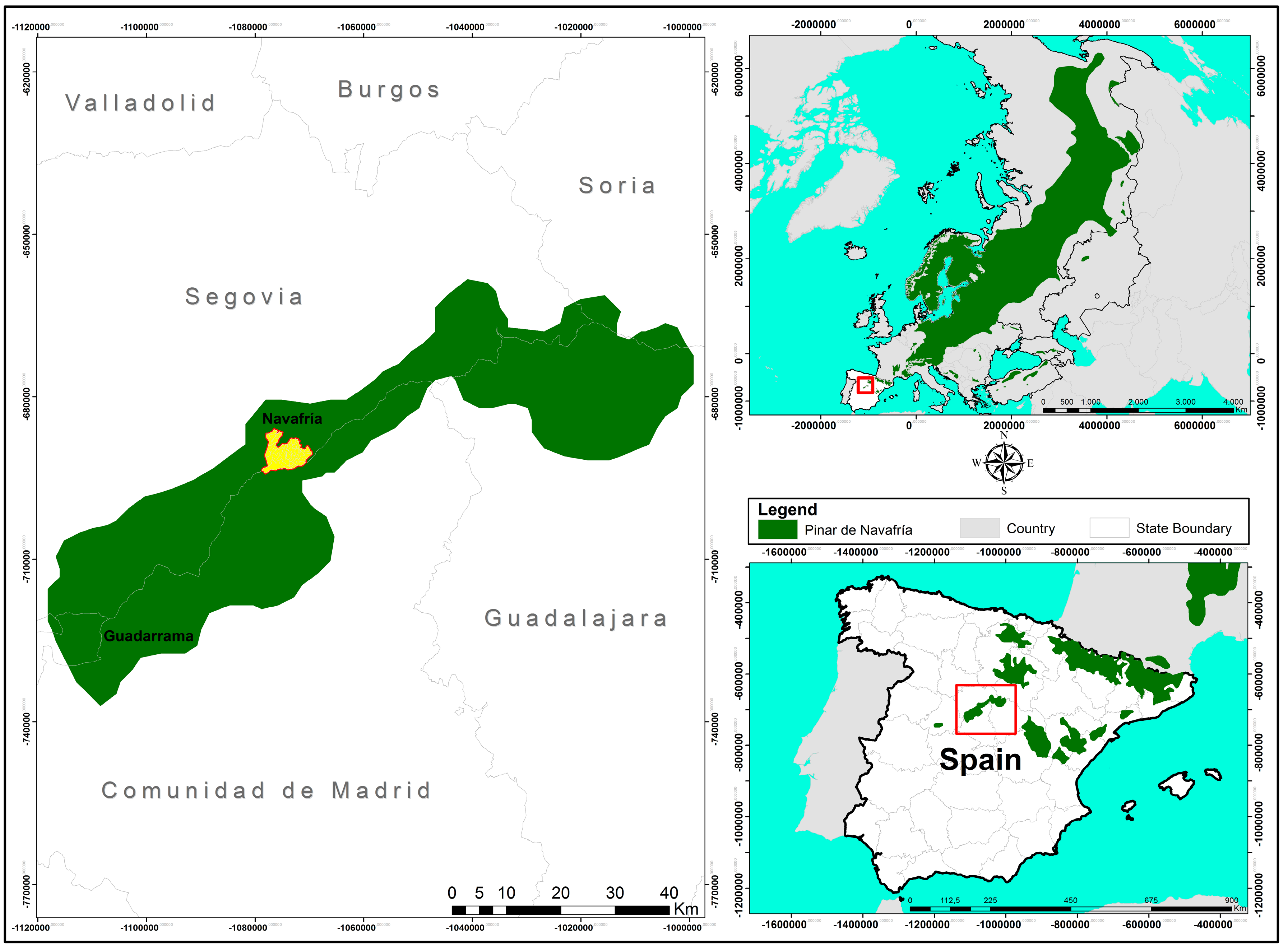
2.1. Study Area
2.2. Tree Size and Growth Measurements
2.3. Data Analysis
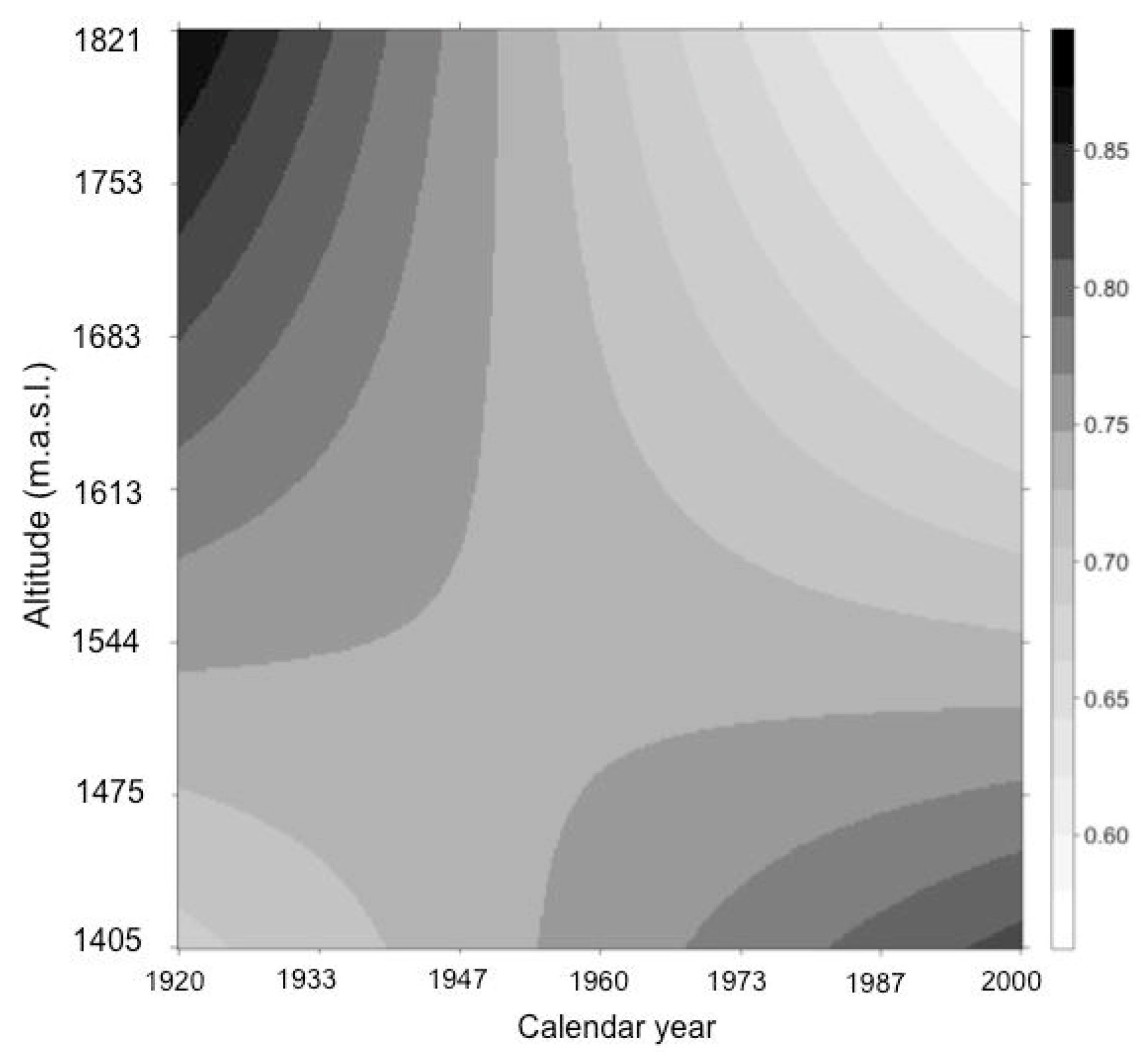
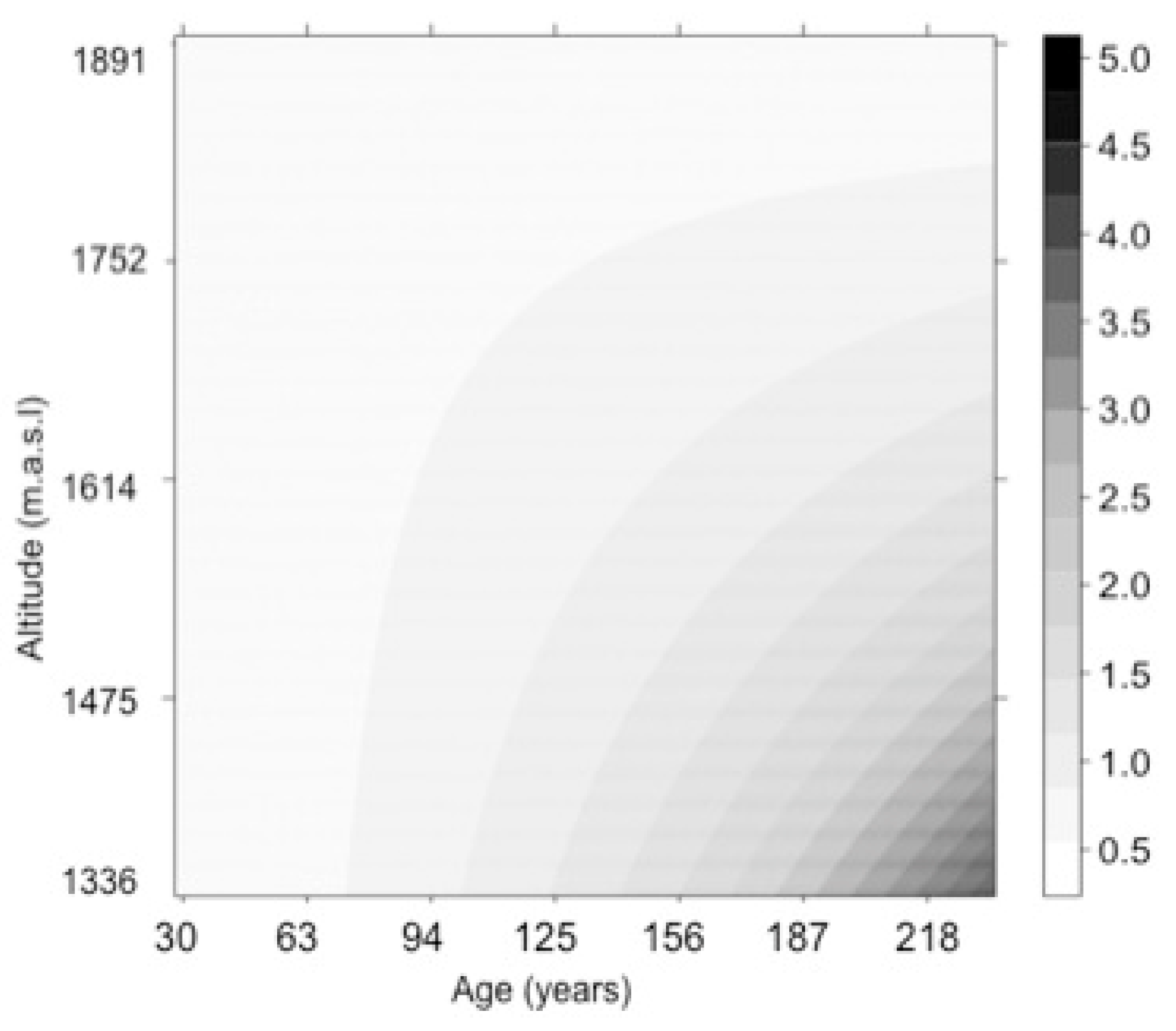
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Elevation (m a.s.l.) | ||||
|---|---|---|---|---|
| Section | Area (ha) | Min | Max | Mean |
| I | 1051 | 1320 | 2010 | 1620 |
| II | 904 | 1270 | 1990 | 1554 |
| III | 758 | 1330 | 2070 | 1700 |
References
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; García-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Smith, M. An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. J. Ecol. 2011, 99, 656–663. [Google Scholar] [CrossRef]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; Van der Velde, M.; Vicca, S.; Babst, F.; et al. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Chang. Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.; Wullschleger, S.; Gunderson, C.; Johnson, D.W.; Ceulemans, R. Tree responses to rising CO2 in field experiments: Implications for the future forest. Plant Cell Environ. 1999, 22, 683–714. [Google Scholar] [CrossRef]
- Ainsworth, E.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.; DeLucia, E.; Gielen, B.; Calfapietra, C.; Giardina, C.P.; King, J.S.; Ledford, J.; McCarthy, H.R.; Moore, D.J.P.; Ceulemans, R.; et al. Forest response to elevated CO2 is conserved across a broad range of productivity. Proc. Natl. Acad. Sci. USA 2005, 102, 18052–18056. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, V.; Graybill, D.; Fritts, H.; Rose, M. Increasing atmospheric carbon dioxide: Tree ring evidence for growth enhancement in natural vegetation. Science 1984, 225, 1019–1021. [Google Scholar] [CrossRef]
- Bolker, B.; Pacala, S.; Bazzaz, F.; Canhams, C.D.; Levin, S.A. Species diversity and ecosystem response to carbon dioxide fertilization: Conclusions from a temperate forest model. Glob. Chang. Biol. 1995, 1, 373–381. [Google Scholar] [CrossRef]
- Resco De Dios, V.; Fischer, C.; Colinas, C. Climate change effects on mediterranean forests and preventive measures. New For. 2007, 33, 29–40. [Google Scholar] [CrossRef]
- Wallace, Z.; Lovett, G.; Hart, J.; Machona, B. Effects of nitrogen saturation on tree growth and death in a mixed-oak forest. For. Ecol. Manag. 2007, 243, 210–218. [Google Scholar] [CrossRef]
- Camarero, J.; Gazol, A.; Tardif, J.; Conciatori, F. Attributing forest responses to global-change drivers: Limited evidence of a CO2-fertilization effect in Iberian pine growth. J. Biogeogr. 2015, 42, 2220–2233. [Google Scholar] [CrossRef]
- Keenan, T.; Sabaté, S.; Gracia, C. The importance of mesophyll conductance in regulating forest ecosystem productivity during drought periods. Glob. Chang. Biol. 2010, 16, 1019–1034. [Google Scholar] [CrossRef]
- Vayreda, J.; Martinez-Vilalta, J.; Gracia, M.; Retana, J. Recent climate changes interact with stand structure and management to determine changes in tree carbon stocks in Spanish forests. Glob. Chang. Biol. 2012, 18, 1028–1041. [Google Scholar] [CrossRef]
- Madrigal-González, J.; Hantson, S.; Yue, C.; Poulter, B.; Ciais, P.; Zavala, M.A. Long-term wood production in water-limited forests: Evaluating potential CO2 fertilization along with historical confounding factors. Ecosystems 2015, 18, 1043–1055. [Google Scholar] [CrossRef]
- Peñuelas, J.; Canadell, J.; Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2011, 20, 597–608. [Google Scholar] [CrossRef]
- Linares, J.; Camarero, J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Chang. Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Koutavas, A. Late 20th century growth acceleration in greek firs (Abies cephalonica) from Cephalonia Island, Greece: A CO2 fertilization effect? Dendrochronologia 2008, 26, 13–19. [Google Scholar] [CrossRef]
- Granda, E.; Rossatto, D.; Camarero, J.; Voltas, J.; Valladares, F. Growth and carbon isotopes of Mediterranean trees reveal contrasting responses to increased carbon dioxide and drought. Oecologia 2014, 174, 307–317. [Google Scholar] [CrossRef] [PubMed]
- García-Valdés, R.; Zavala, M.A.; Araújo, M.; Purves, D. Chasing a moving target: Projecting climate change-induced shifts in non-equilibrial tree species distributions. J. Ecol. 2013, 101, 441–453. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. (Eds.) Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Moreno, J.M. Principales Conclusiones de la Evaluación Preliminar de los Impactos en España por Efecto del Cambio Climático; Ministerio de Medio Ambiente: Madrid, Spain, 2005.
- Keller, T.; Guiot, J.; Tessier, L. Climatic effect of atmospheric CO2 doubling on radial tree growth in south eastern France. J. Biogeogr. 1997, 24, 857–864. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Martínez-Vilalta, J.; Retana, J. Variation in reproduction and growth in declining Scots pine populations. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 111–120. [Google Scholar] [CrossRef]
- Martinez del Castillo, E.; Longares, L.; Gričar, J.; Prislan, P.; Gil-Peregrín, E.; Čufar, K.; de Luis, M. Living on the edge: Contrasted wood-formation dynamics in Fagus sylvatica and Pinus sylvestris under mediterranean conditions. Front. Plant Sci. 2016, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.; Norton, D. Climate and growth of Pinus sylvestris at its upper altitudinal limit in Scotland: Evidence from tree growth-rings. J. Ecol. 1990, 78, 601–610. [Google Scholar] [CrossRef]
- Rigling, A.; Bräker, O.; Schneiter, G.; Schweingruber, F. Intra-annual tree-ring parameters indicating differences in drought stress of Pinus sylvestris forests within the Erico-Pinion in the Valais (Switzerland). Plant Ecol. 2002, 163, 105–121. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Hevia, A.; Madrigal-González, J.; Linares, J.C.; Ballesteros-Canovas, J.A.; Sánchez-Miranda, A.; Alfaro-Sánchez, R.; Sangüesa-Barreda, G.; Diego, G.A.; et al. What drives growth of Scots pine in continental Mediterranean climates: Drought, low temperatures or both? Agric. For. Meteorol. 2015, 206, 151–162. [Google Scholar] [CrossRef]
- Martínez-García, F. Los bosques de Pinus sylvestris L. del Sistema Central Español. Distribución, Historia, Composición Florística y Tipología. Ph.D. Thesis, INIA. Departamento de Biología Vegetal I, Facultad de Ciencias Biológicas, Universidad Complutense de Madrid, Madrid, Spain, 1999. [Google Scholar]
- Vaganov, E.; Hughes, M.; Shashkin, A. Growth dynamics of conifer tree rings. In Image of Past and Future Environments; Springer: Berlin, Germany, 2006. [Google Scholar]
- Díaz, B.L.; Prieto, R.A. Modelos de planificación forestal basados en la programación lineal. aplicación al monte “Pinar de Navafría” (Segovia). Investig. Agr. Sist. Recur. For. 1999, 8, 63–92. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear Mixed-Effects Models Using Eigen and S4. Available online: https://www.researchgate.net/publication/279236477_Package_Lme4_Linear_Mixed-Effects_Models_Using_Eigen_and_S4 (accessed on 25 August 2015).
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Savaliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Körner, C. A re-assessment of high elevation treeline positions and their explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Ecological impacts of atmospheric CO2 enrichment on terrestrial ecosystems. Philos. Trans. R. Soc. Lond. 2003, 361, 2023–2041. [Google Scholar] [CrossRef] [PubMed]
- Larcher, W. Climatic constraints drive the evolution of low temperature resistance in woody plants. J. Agric. Meteorol. 2005, 61, 189–202. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Alleviation of summer drought boosts establishment success of Pinus sylvestris in a Mediterranean mountain: An experimental approach. Plant Ecol. 2004, 181, 191–202. [Google Scholar] [CrossRef]
- Beniston, M. Climatic change in mountain regions: A review of possible impacts. Clim. Chang. 2003, 59, 5–31. [Google Scholar] [CrossRef]
- García-Romero, A.; Muñoz, J.; Andrés, N.; Palacios, D. Relationship between climate change and vegetation distribution in the Mediterranean mountains: Manzanares Head valley, Sierra de Guadarrama (Central Spain). Clim. Chang. 2010, 100, 645–666. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Grabherr, G. Effects of climate change on the alpine and nival vegetation of the Alps. J. Meteorol. Ecol. 2003, 7, 9–12. [Google Scholar] [CrossRef]
- Salzer, M.; Hughes, M.; Bunn, A.; Kipfmueller, K. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Nat. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand dynamics in Central Europe has accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef] [PubMed]
- Briffa, K.; Osborn, T.; Schweingruber, F. Large-scale temperature inferences from tree rings: A review. Glob. Planet. Chang. 2004, 40, 11–26. [Google Scholar] [CrossRef]
- Wilmking, M.; Juday, G.; Barber, V.; Zald, H. Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Glob. Chang. Biol. 2004, 10, 1724–1736. [Google Scholar] [CrossRef]
- Muñoz, J.; García, R. Modificaciones climáticas y evolución de la cubierta vegetal en las áreas culminantes de la Sierra de Guadarrama (Sistema Central español) durante la segunda mitad del XX: Las altas cuencas del Ventisquero de La Condesa y de Valdemartín. Cuad. Investig. Geog. 2004, 30, 117–146. [Google Scholar]
- Cannell, M.; Thornley, J.; Mobbs, D.; Friend, A. UK conifer forests may be growing faster in response to increased N deposition, atmospheric CO2 and temperature. Forestry 1998, 71, 277–296. [Google Scholar] [CrossRef][Green Version]
- Emmett, B.; Boxman, D.; Bredemeier, M.; Gundersen, P.; Kjønaas, O.J.; Modan, F.; Schleppi, P.; Tietema, A.; Wright, R.F. Predicting the effects of atmospheric nitrogen deposition in conifer stands: Evidence from the NITREX ecosystem-scale experiments. Ecosystems 1998, 1, 352–360. [Google Scholar] [CrossRef]
- Nissinen, A.; Hari, P. Effects of nitrogen deposition on tree growth and soil nutrients in boreal Scots pine stands. Environ. Pollut. 1998, 102, 61–68. [Google Scholar] [CrossRef]
- Reynolds, B.; Wilson, E.; Emmett, B. Evaluating critical loads of nutrient nitrogen and acidity for terrestrial systems using ecosystem-scale experiments (NITREX). For. Ecol. Manag. 1998, 101, 81–94. [Google Scholar] [CrossRef]
- García-Gómez, H.; Garrido, J.; Vivanco, M.; Lassaletta, L.; Rábago, I.; Ávila, A.; Tsyro, S.; Sánchez, G.; González, O.A.; González-Fernández, I.; et al. Nitrogen deposition in Spain: Modeled patterns and threatened habitats within the Natura 2000 network. Sci. Total. Environ. 2014, 485–486, 450–486. [Google Scholar] [CrossRef]
- Saurer, M.; Siegwolf, R.; Schweingruber, F. Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Chang. Biol. 2004, 10, 2109–2120. [Google Scholar] [CrossRef]
- Charan, G.; Bharti, V.K.; Jadhav, S.E.; Kumar, S.; Acharya, S.; Kumar, P.; Gogoi, D.; Srivastava, R.B. Altitudinal variations in soil physico-chemical properties at cold desert high altitude. J. Soil Sci. Plant Nutr. 2013, 13, 267–277. [Google Scholar] [CrossRef]
- Pachepsky, Y.A.; Timlin, D.J.; Rawls, W.J. Soil and water management and conservation. Soil Sci. Soc. Am. J. 2001, 65, 1787–1795. [Google Scholar] [CrossRef]
- Allen, C.; Breshears, D. Drought-induced shift of a forest-woodland ecotone: Rapid landscape response to climate variation. Proc. Nat. Acad. Sci. USA 1998, 95, 14839–14842. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Rathgeber, C.B.K.; Ulrich, E. Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba, Picea abies and Pinus sylvestris). J. Veg. Sci. 2010, 21, 364–376. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Linares, J.; Camarero, J.J.; Madrigal-González, J.; Hevia, A.; Sánchez-Miranda, A.; Ballesteros-Canovas, J.A.; Alfaro-Sánchez, R.; García-Cervigón, A.I.; Bigler, C.; et al. Disentangling the effects of competition and climate on individual tree growth: A retrospective and dynamic approach in Scots pine. For. Ecol. Manag. 2015, 358, 12–25. [Google Scholar] [CrossRef]
- Fournier, N.; Rigling, A.; Dobbertin, M.; Gugerli, F. Faible différenciation génétique, à partir d’amplification aléatoire d’ADN polymorphe (RAPD), entre les types de pin sylvestre (Pinus sylvestris L.) d’altitude et de plaine dans les Alpes à climat continental. Ann. For. Sci. 2006, 63, 431–439. [Google Scholar] [CrossRef]
- Matías, L.; González-Díaz, P.; Jump, A.S. Larger investment in roots in southern range-edge populations of Scots pine is associated with increased growth and seedling resistance to extreme drought in response to simulated climate change. Environ. Exp. Bot. 2014, 105, 32–38. [Google Scholar] [CrossRef]
- Kubiske, M.; Abrams, M. Photosynthesis, water relations, and leaf morphology of xeric versus mesic Quercus rubra ecotypes in central Pennsylvania in relation to moisture stress. Can. J. For. Res. 1992, 22, 1402–1407. [Google Scholar] [CrossRef]
- Reich, P.; Oleksyn, J.; Tjoelker, M. Needle respiration and nitrogen concentration in Scots Pine populations from a broad latitudinal range: A common garden test with field-grown trees. Funct. Ecol. 1996, 10, 768–776. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Bresson, C.; Michalet, R.; Kremer, A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res. 2009, 39, 1259–1269. [Google Scholar] [CrossRef]
- Vitasse, Y.; Hoch, G.; Randin, C.; Lenz, A.; Kollas, C.; Scheepens, J.F.; Körner, C. Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 2013, 171, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Matías, L.; Linares, J.C.; Sánchez-Miranda, Á.; Jump, A.S. Contrasting growth forecasts across the geographical range of Scots pine due to altitudinal and latitudinal differences in climatic sensitivity. Glob. Chang. Biol. 2017, 23, 4106–4116. [Google Scholar] [CrossRef] [PubMed]
- Fisichelli, N.; Vor, T.; Ammer, C. Broadleaf seedling responses to warmer temperatures "chilled" by late frost that favors conifers. Eur. J. Forest. Res. 2014, 133, 587–596. [Google Scholar] [CrossRef]
- Bigras, F.J.; Ryyppo, A.; Lindström, A.; Stattin, E. Cold acclimation and deacclimation of shoots and roots of conifer seedlings. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 57–88. [Google Scholar]
- Ögren, E.; Nilsson, T.; Sundblad, L.G. Relationship between respiratory depletion of sugars and loss of cold hardiness in coniferous seedlings over-wintering at raised temperatures: Indications of different sensitivities of spruce and pine. Plant Cell Environ. 1997, 20, 247–253. [Google Scholar] [CrossRef]
- Morin, X.; Améglio, T.; Ahas, R.; Kurz-Besson, C.; Lanta, V.; Lebourgeois, F.; Miglietta, F.; Chuine, I. Variation in cold hardiness and carbohydrate concentration from dormancy induction to bud burst among provenances of three European oak species. Tree Physiol. 2007, 27, 817–825. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Man, R.; Kayahara, G.J.; Dang, Q.L.; Rice, A. A case of severe frost damage prior to budbreak in young conifers in Northestearn Ontario: Consequence of climate change? Fores. Chron. 2009, 85, 453–462. [Google Scholar] [CrossRef]
- Hufkens, K.; Friedl, M.A.; Keenan, T.F.; Sonnentag, O.; Bailey, A.; O’Keefe, J.; Richardson, A.D. Ecological impacts of a widespread frost event following early spring leaf-out. Glob. Chang. Biol. 2012, 18, 2365–2377. [Google Scholar] [CrossRef]
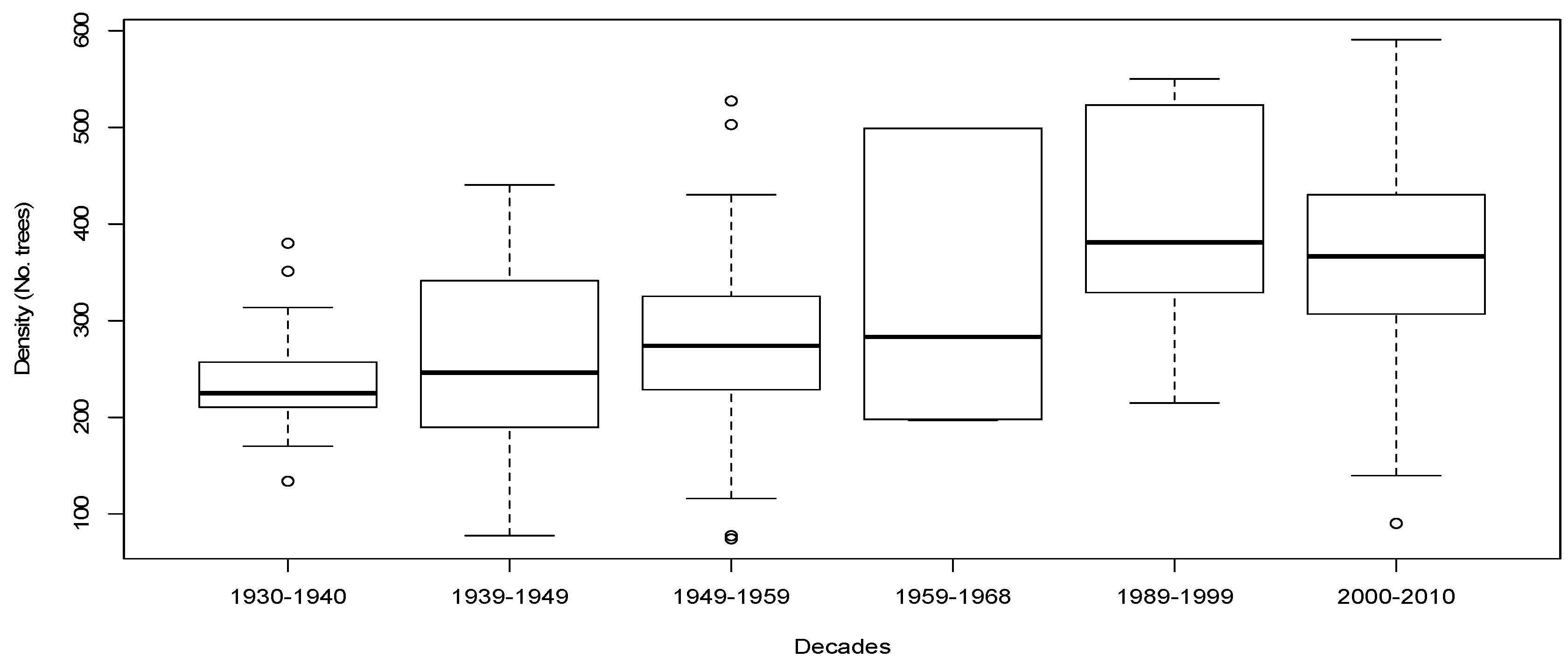
| Year | No. Trees | DBH | Average Height | Average Age | Min Age | Max Age | Density (no. ind/ha) |
|---|---|---|---|---|---|---|---|
| 1930 | 109 | 39.53 (13.57) | 14.08 | 92 | 32 | 156 | 178.26 (64.7) |
| 1940 | 132 | 39.65 (13.32) | 13.68 | 95 | 31 | 235 | 258.6 (101.34) |
| 1950 | 96 | 37.28 (14.43) | 12.82 | 89 | 40 | 172 | 302.21 (128.1) |
| 1959 | 73 | 42.61 (12.86) | 12.53 | 101 | 40 | 235 | 398.41 (140.87) |
| 1990 | 83 | 40.35 (12.65) | 12.38 | 96 | 39 | 181 | 405.55 (86.7) |
| 2000 | 116 | 36.17 (11.66) | 14.44 | 94 | 30 | 235 | 369.08 (89.67) |
| Total | 609 |
| Variables | Year | Altitude | Stand Density | Trunk Diameter | Tree Age |
|---|---|---|---|---|---|
| Altitude | −0.04 | 1 | − | − | − |
| Stand density | 0.39 | −0.37 | 1 | − | − |
| Trunk diameter | −0.06 | −0.01 | −0.04 | 1 | − |
| Tree age | −0.02 | 0.07 | −0.04 | 0.70 | 1 |
| Step | Model | Fixed Effects | Param | AICc | Δ AICC |
|---|---|---|---|---|---|
| 1# | Y × Alt × Ag | Full model | 8 | 1238.042 | 0 |
| Y × Alt + Ag × Y + Alt × Ag | Removed Y × Alt × Ag | 7 | 1232.405 | 5.637 | |
| 2# | Y × Alt + Ag × Y + Alt × Ag | Removed Y × Alt × Ag | 7 | 1232.405 | 0 |
| Y × Alt + Ag × Y | Removed Ag × Alt | 6 | 1234.160 | –1.75 | |
| Ag × Alt + Ag × Y | Removed Y × Alt | 6 | 1225.914 | 6.491 | |
| Ag × Alt + Y × Alt | Removed Ag × Y | 6 | 1234.757 | –2.352 | |
| 3# | Null | intercept | 1 | 1700.557 | 0 |
| Supported | Ag × Alt + Alt × Y | 6 | 1225.914 | 474.643 |
| Model Parameters | Parameter Value | Std.Error | t-Value | P Value |
|---|---|---|---|---|
| Intercept | −0.148 | 0.024 | −6.09 | <0.0001 |
| Age | 0.156 | 0.024 | 6.37 | <0.0001 |
| Altitude | −0.115 | 0.025 | −4.63 | <0.0001 |
| Year | −0.042 | 0.024 | −1.88 | 0.082 |
| Age × Altitude | −0.089 | 0.025 | −3.60 | 0.0004 |
| Altitude × Year | −0.063 | 0.025 | −2.49 | 0.012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Pérez, L.; Zavala, M.A.; Villar -Salvador, P.; Madrigal-González, J. Divergent Last Century Tree Growth along An Altitudinal Gradient in A Pinus sylvestris L. Dry-edge Population. Forests 2019, 10, 532. https://doi.org/10.3390/f10070532
Fernández-Pérez L, Zavala MA, Villar -Salvador P, Madrigal-González J. Divergent Last Century Tree Growth along An Altitudinal Gradient in A Pinus sylvestris L. Dry-edge Population. Forests. 2019; 10(7):532. https://doi.org/10.3390/f10070532
Chicago/Turabian StyleFernández-Pérez, Laura, Miguel A. Zavala, Pedro Villar -Salvador, and Jaime Madrigal-González. 2019. "Divergent Last Century Tree Growth along An Altitudinal Gradient in A Pinus sylvestris L. Dry-edge Population" Forests 10, no. 7: 532. https://doi.org/10.3390/f10070532
APA StyleFernández-Pérez, L., Zavala, M. A., Villar -Salvador, P., & Madrigal-González, J. (2019). Divergent Last Century Tree Growth along An Altitudinal Gradient in A Pinus sylvestris L. Dry-edge Population. Forests, 10(7), 532. https://doi.org/10.3390/f10070532





