Effect of Deadwood on Ectomycorrhizal Colonisation of Old-Growth Oak Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Deadwood Measurements
2.3. Root Sampling and Morphotyping of Mycorrhizae
2.4. Molecular Identification of Ectomycorrhizal Root Tips
2.5. Chemical Analysis
2.6. Data Analysis
3. Results
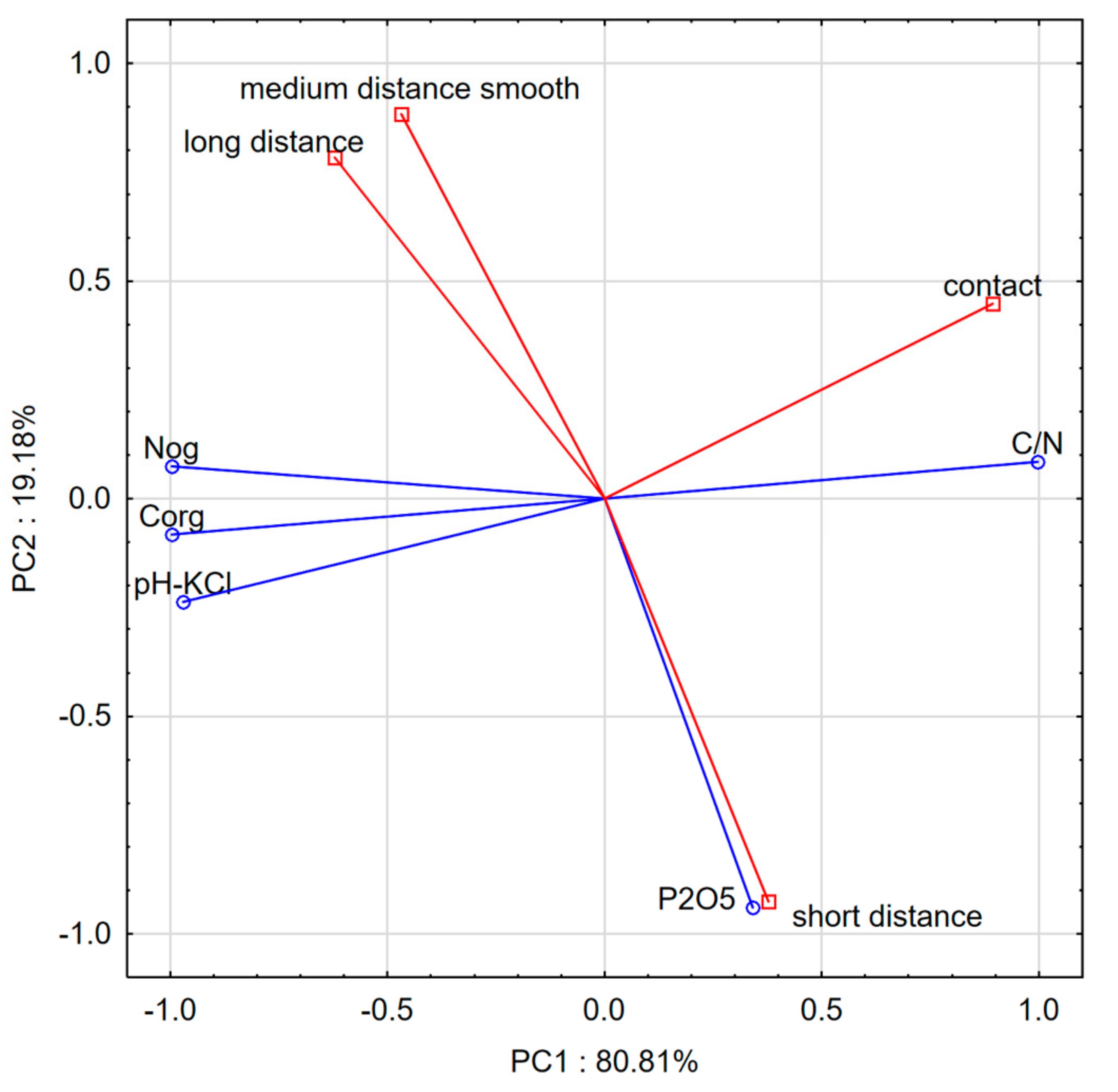
3.1. Soil Substrate Properties
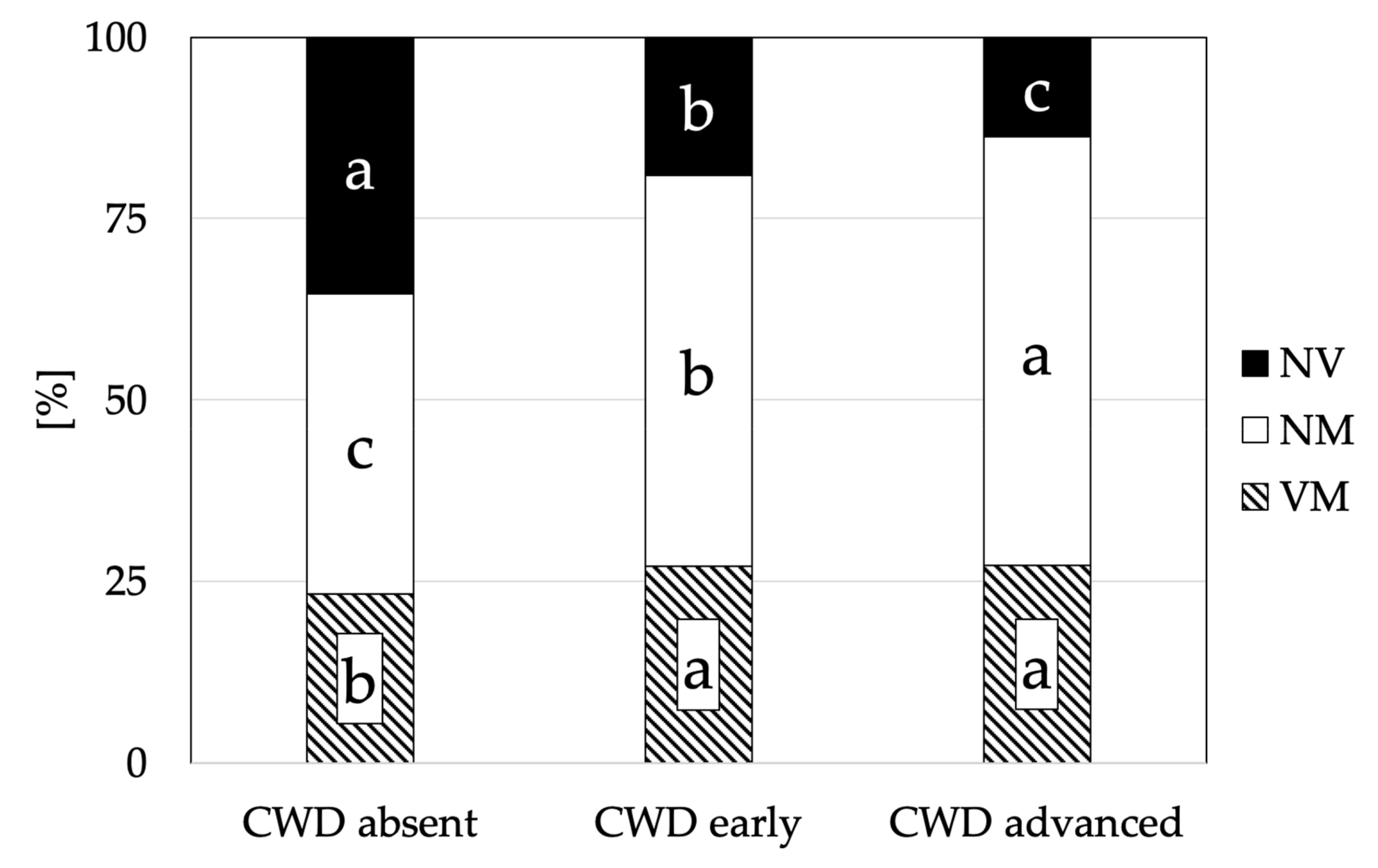
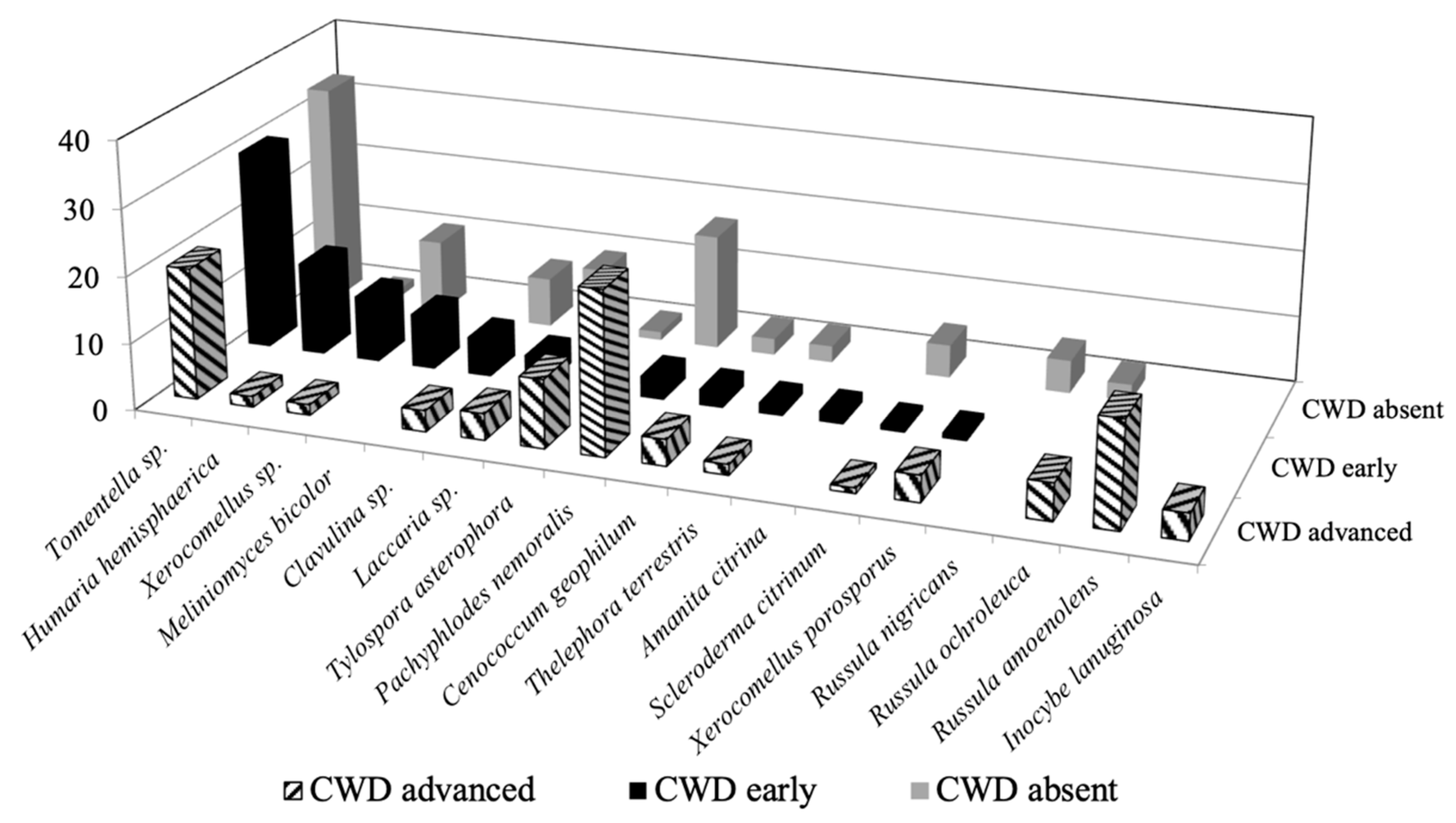
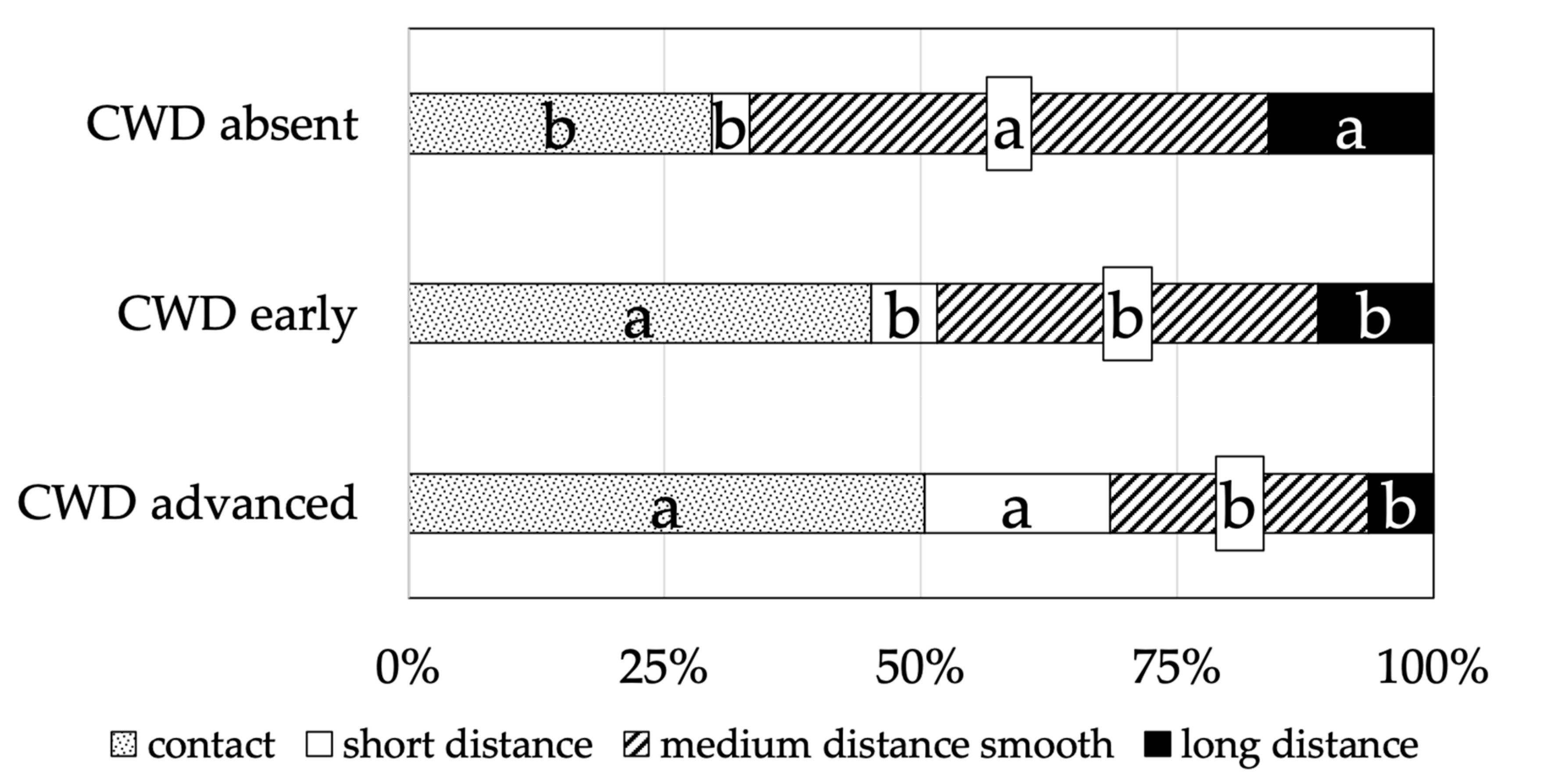
3.2. Mycorrhizal Colonisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Anderson, I.C.; Cairney, J.W.G. Ectomycorrhizal fungi: Exploring the mycelial frontier. FEMS Microbiol. Rev. 2007, 31, 388–406. [Google Scholar] [CrossRef]
- Karst, J.; Marczak, L.; Jones, M.D.; Turkington, R. The mutualism-parasitism continuum in ectomycorrhizas: A quantitative assessment using meta-analysis. Ecology 2008, 89, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Sillo, F.; Zampieri, E.; Giordano, L.; Lione, G.; Colpaert, J.V.; Balestrini, R.; Gonthier, P. Identification of genes differentially expressed during the interaction between the plant symbiont Suillus luteus and two plant pathogenic allopatric Heterobasidion species. Mycol. Prog. 2015, 14, 106. [Google Scholar] [CrossRef]
- Horton, T.R.; Bruns, T.D. The molecular revolution in ectomycorrhizal ecology: Peeking into the black-box. Mol. Ecol. 2001, 10, 1855–1871. [Google Scholar] [CrossRef]
- Buée, M.; Vairelles, D.; Garbaye, J. Year round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech (Fagus sylvatica) forest subjected to two thinning regimes. Mycorrhiza 2005, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Bruns, T.D. Thoughts on the processes that maintain local species diversity of ectomycorrhizal fungi. Plant Soil 1995, 170, 63–73. [Google Scholar] [CrossRef]
- Dickie, I.A.; Xu, B.; Koide, R.T. Vertical niche differentiation of ectomycorrhizal hyphae in soil as shown by T.-RFLP analysis. New Phytol. 2002, 156, 527–535. [Google Scholar] [CrossRef]
- Tedersoo, L.; Köljalg, U.; Hallenberg, N.; Larsson, K.H. Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol. 2003, 159, 153–165. [Google Scholar] [CrossRef]
- Buée, M.; Courty, P.E.; Mignot, D.; Garbaye, J. Soil niche effect on species diversity and catabolic activities in an ectomycorrhizal fungal community. Soil Biol. Biochem. 2007, 39, 1947–1955. [Google Scholar] [CrossRef]
- Bzdyk, R.; Olchowik, J.; Studnicki, M.; Nowakowska, J.A.; Oszako, T.; Urban, A.; Hilszczańska, D. Ectomycorrhizal Colonisation in Declining Oak Stands on the Krotoszyn Plateau, Poland. Forests 2019, 10, 30. [Google Scholar] [CrossRef]
- Montecchio, L.; Causin, R.; Rossi, S.; Mutto Accordi, S. Changes in ectomycorrhizal diversity in a declining Quercus ilex coastal forest. Phytopathol. Mediterr. 2004, 43, 26–34. [Google Scholar]
- Jung, T.; Blaschke, H.; Osswald, W. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 2000, 49, 706–718. [Google Scholar] [CrossRef]
- Kovacs, G.; Pausch, M.; Urban, A. Diversity of ectomycorrhizal morphotypes and oak decline. Phyton Ann. Rei. Bot. A. 2000, 40, 109–116. [Google Scholar]
- Mosca, E.; Montecchio, L.; Sella, L.; Garbaye, J. Short-term effect of removing tree competition on the ectomycorrhizal status of a declining pedunculate oak forest (Quercus robur L.). For. Ecol. Manag. 2007, 244, 129–140. [Google Scholar] [CrossRef]
- Kuikka, K.; Härmä, E.; Markkola, A.; Rautio, P.; Roitto, M.; Saikkonen, K.; Ahonen-Jonnarth, U.; Finlay, R.; Tuomi, J. Severe defoliation of Scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology 2003, 84, 2051–2061. [Google Scholar] [CrossRef]
- Finlay, R.D.; Söderström, B. Mycorrhiza and carbon flow to the soil. In Mycorrhizal Functioning an Integrative Plant-Fungal Process; Allen, M.J., Ed.; Chapman and Hall: New York, NY, USA, 1992; pp. 134–160. [Google Scholar]
- Tedersoo, L.; Gates, G.; Dunk, C.; Lebel, T.; May, T.W.; Dunk, C.; Lebel, T.; Kõljalg, U.; Jairus, T. Establishment of ectomycorrhizal fungal community on isolated Nothofagus cunninghamii seedlings regenerating on dead wood in Australian wet temperate forests: Does fruit-body type matter? Mycorrhiza 2009, 19, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.L.; Brewer, J.S.; Jackson, C.R.; Hoeksema, J.D. Tree thinning and fire affect ectomycorrhizal fungal communities and enzyme activities. Ecosphere 2018, 9, e02471. [Google Scholar] [CrossRef]
- Väre, H. Influence of decaying birch logs to Scots pine mycorrhizae at clear-cutted ploughed sites in northern Finland. Agr. Ecosyst. Environ. 1989, 28, 539–545. [Google Scholar] [CrossRef]
- Maser, C.; Anderson, R.G.; Cromack, K.; Williams, J.T.; Martin, R.E. Dead and Down Woody Material. In Wildlife Habitats in Managed Forests: The Blue Mountains of Oregon and Washington; Thomas, J.W., Ed.; US Department of Agriculture Forest Service: Washington, DC, USA, 1979; pp. 78–95. [Google Scholar]
- Harvey, A.E.; Larsen, M.J.; Jurgensen, M.F. Comparative distribution of ectomycorrhizae in soils of three western Montana forest habitat types. For. Sci. 1979, 25, 350–358. [Google Scholar]
- Harvey, A.E.; Jurgensen, M.F.; Larsen, M.J. Organic reserves: Importance to ectomycorrhizae in forest soils in western Montana. For. Sci. 1981, 27, 442–445. [Google Scholar]
- Tedersoo, L.; Naadel, T.; Bahram, M.; Pritsch, K.; Buegger, F.; Leal, M.; Koljalg, U.; Poldmaa, K. Enzymatic activities and stable isotope patterns of ectomycorrhizal fungi in relation to phylogeny and exploration types in an afrotropical rain forest. New Phytol. 2012, 195, 832–843. [Google Scholar] [CrossRef]
- Hupperts, S.F.; Karst, J.; Pritsch, K.; Landhausser, S.M. Host phenology and potential saprotrophism of ectomycorrhizal fungi in the boreal forest. Funct. Ecol. 2016, 31, 116–126. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae. A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Faliński, J.B. Vegetation dynamics in temperate lowland primeval forests. Ecological studies in Białowieża Forest. In Geobotany; Dr W. Junk Publishers: Dordrecht, The Netherlands, 1986; pp. 1–537. [Google Scholar]
- Brzeziecki, B.; Bielak, K.; Bolibok, L.; Drozdowski, S.; Zajączkowski, J.; Żybura, H. Structural and compositional dynamics of strictly protected woodland communities with silvicultural implications, using Białowieża Forest as an example. Ann. For. Sci. 2018, 75, 89. [Google Scholar] [CrossRef]
- Mojski, J.E. Nizina Podlaska. In Geomorfologia Polski; Galon, R., Ed.; PWN: Warszawa, Poland, 1972; pp. 318–373. [Google Scholar]
- Renvall, P. Community stucture and dynamics of wood-rotting Basidomycetes on decomposing conifer trunks in northern Finland. Karstenia 1995, 35, 1–51. [Google Scholar] [CrossRef]
- Waddell, D.R. Estimating Load Weights with Huber’s cubic volume formula: A. Field Trial. USDA For. Serv. 1989, 484, 12. [Google Scholar]
- Agerer, R. Colour Atlas of Ectomycorrhizae, 1st ed.; Einhorn-Verlag: Munich, Germany, 1987. [Google Scholar]
- Olchowik, J.; Bzdyk, R.; Studnicki, M.; Bednarska-Błaszczyk, M.; Urban, A.; Aleksandrowicz-Trzcińska, M. The effects of silver and copper nanoparticles on the condition of English oak (Quercus robur L.) seedlings in a container nursery experiment. Forests 2017, 8, 310. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 7.5., 2005. Available online: http:// www.purl.oclc.org/estimates (accessed on 15 February 2019).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- ISO 10390. Soil Quality. Determination of pH; International Organization for Standardization: Geneva, Switzerland, 1997. [Google Scholar]
- ISO 13878. Soil Quality. Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”); International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- ISO 10694. Soil Quality. Determination of Organic and Total Carbon after Dry Combustion (“Elementary Analysis”); International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Schlichting, E.; Blume, H.P.; Stahr, K. Bodenkundliches Praktikum; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1995. [Google Scholar]
- ISO 11260. Soil Quality. Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://www.R.-project.org/ (accessed on 15 February 2019).
- Pregitzer, K.S.; Hendrick, R.L.; Fogel, R. The demography of fine roots in response to patches of water and nitrogen. New Phytol. 1993, 125, 575–580. [Google Scholar] [CrossRef]
- Drew, M.C. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Christensen, M. Fungal diversity on decaying beech logs—Implications for sustainable forestry. Biodiv. Conserv. 2003, 12, 953–997. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Aude, E.; Christensen, M. Cryptogam communities on decaying deciduous wood–does tree species diversity matter? Biodiv. Conserv. 2005, 14, 2061–2078. [Google Scholar] [CrossRef]
- Hilszczanska, D.; Ciesielska, A.; Sierota, Z. Enzymatic activity of Thelephora terrestris and Hebeloma crustuliniforme in cultures and mycorrhizal association with Scots pine seedlings. Pol. J. Environ. Stud. 2008, 17, 881–886. [Google Scholar]
- Courty, P.E.; Buée, M.; Diedhiou, A.G.; Pascale, F.K.; Le Tacon, F.; Rineau, F.; Turpault, M.P.; Uroz, S.; Garbaye, J. The role of ectomycorrhizal communities in forest ecosystem processes: New perspectives and emerging concepts. Soil Biol. Biochem. 2010, 42, 679–698. [Google Scholar] [CrossRef]
- Edman, M.; Jonsson, B.G. Spatial pattern of downed logs and wood-decaying fungi in an old-growth Picea abies forest. J. Veget. Sci. 2001, 12, 609–620. [Google Scholar] [CrossRef]
- Abrego, N.; Salcedo, I. Variety of woody debris as the factor influencing wood-inhabiting fungal richness and assemblages: Is it a question of quantity or quality? For. Ecol. Manag. 2013, 291, 377–385. [Google Scholar] [CrossRef]
- Goodman, D.M.; Trofymow, J.A. Distribution of ectomycorrhizas in microhabitats in mature and old-growth stands of Douglas-fir on southeastern Vancouver Island. Soil. Biol. Biochem. 1998, 30, 2127–2138. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Bruns, T.D. Nitrogen and ectomycorrhizal fungal communities: What we know, what we need to know. New Phytol. 2001, 149, 156–158. [Google Scholar] [CrossRef]
- Visser, S. Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol. 1995, 129, 389–401. [Google Scholar] [CrossRef]
- Taylor, D.L.; Bruns, T.D. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: Minimal overlap between the mature forest and resistant propagules communities. Mol. Ecol. 1999, 8, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Healy, R.A.; Horner, H.T.; Bonito, G.; McLaughlin, D.J.; Smith, M.E. An ultrastructural study of spore wall development and septal pores in species of the Pachyphlodes (Pezizaceae, Pezizales) lineage, with a description of the new species Pachyphlodes annagardnerae. Mycol. Prog. 2018, 17, 45. [Google Scholar] [CrossRef]
- Healy, R.; Hobart, C.; Tocci, G.E.; Bóna, L.; Merényi, Z.; Paz Conde, A.; Smith, M.E. Fun with the discomycetes: Revisiting collections of Korf’s anamorphic Pezizales and Thaxter’s New England truffles leads to a connection between forms and the description of two new truffle species: Pachyphlodes pfisteri and P. nemoralis. Ascomycete 2015, 7, 357–366. [Google Scholar]
- Hobbie, E.A.; van Diepen, L.T.A.; Lilleskov, E.A.; Ouimette, A.P.; Finzi, A.C.; Hofmocke, K.S. Fungal functioning in a pine forest: evidence from a 15N-labeled global change experiment. New Phytol. 2014, 201, 1431–1439. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Hart, M.M. Animal nitrogen swap for plant carbon. Nature 2001, 410, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Velmala, S.M.; Rajala, T.; Heinonsalo, J.; Taylor, A.F.S.; Pennanen, T. Profiling functions of ectomycorrhizal diversity and root structuring in seedlings of Norway spruce (Picea abies) with fast- and slow growing phenotypes. New Phytol. 2014, 201, 610–622. [Google Scholar] [CrossRef]
- Wallander, H.; Johansson, U.; Sterkenburg, E.; Brandström, D.M.; Lindahl, B.D. Production of ectomycorrhizal mycelium peaks during canopy closure in Norway spruce forests. New Phytol. 2010, 187, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Agerer, R.; Raidl, S. Distance-related semi-quantitative estimation of the extramatrical ectomycorrhizal mycelia of Cortinarius obtusus and Tylospora asterophora. Mycol. Prog. 2004, 3, 57–64. [Google Scholar] [CrossRef]
- Baier, R.; Ettl, R.; Hahn, C.; Göttlein, A. Early development and nutrition of Norway spruce (Picea abies (L.) Karst.) seedlings on different seedbeds in the Bavarian limestone Alps—A bioassay. Ann. For. Sci. 2006, 63, 339–348. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.V.D.; Sylvia, D.M.; Fox, A.J. Contribution of ectomycorrhizal to the potential nutrient-absorbing surface of pine. New Phytol. 1994, 128, 639–644. [Google Scholar] [CrossRef]
- Kjøller, R.; Nilsson, L.-O.; Hansen, K.; Schmidt, I.K.; Vesterdal, L.; Gundersen, P. Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol. 2012, 194, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Suz, L.M.; Barsoum, N.; Benham, S.; Dietrich, H.P.; Fetzer, K.D.; Fischer, R.; García, P.; Gehrman, J.; Kristöfel, F.; Manninger, M.; et al. Environmental drivers of ectomycorrhizal communities in Europe’s temperate oak forests. Mol. Ecol. 2014, 23, 5628–5644. [Google Scholar] [CrossRef] [PubMed]
- Nygren, C.M.; Eberhardt, U.; Karlsson, M.; Parrent, J.L.; Lindahl, B.D.; Taylor, A.F. Growth on nitrate and occurrence of nitrate reductase-encoding genes in a phylogenetically diverse range of ectomycorrhizal fungi. New Phytol. 2008, 180, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Mäkipää, R.; Rajala, T.; Schigel, D.; Rinne, K.T.; Pennanen, T.; Abrego, N.; Ovaskainen, O. Interactions between soil and dead wood-inhabiting fungal communities during the decay of Norway spruce logs. ISME J. 2017, 11, 1964–1974. [Google Scholar] [CrossRef]

| CWDabsent (m3/500 m2) | CWDearly (m3/500 m2) | CWDadvanced (m3/500 m2) | ||
|---|---|---|---|---|
| Decay class | I | - | 16.45 | - |
| II | - | 0.05 | - | |
| III | - | 0.90 | - | |
| IV | - | - | 11.99 | |
| V | - | - | 13.61 |
| pHKCl | Ntotal (%) | Corg (%) | C/N | P2O5 (mg/100g) | Ca (cmol/kg) | Mg (cmol/kg) | K (cmol/kg) | Relative Soil Humidity (%) | OM (%) (Organic Matter) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CWDabsent | 3.1 | b | 0.1 | c | 2.8 | c | 25.3 | a | 7.72 | c | 0.7 | c | 0.1 | c | 0.1 | b | 13.1 | c | 4.8 | c |
| CWDearly | 4.1 | a | 0.5 | a | 6.8 | a | 13.5 | c | 8.2 | b | 13.1 | a | 0.9 | a | 0.3 | a | 25.8 | a | 12 | a |
| CWDadvanced | 3.7 | b | 0.2 | b | 4.5 | b | 20.4 | b | 16.6 | a | 5.32 | b | 0.6 | b | 0.2 | a | 20.6 | b | 7.7 | b |
| BLAST Top-Hit | CWDabsent | CWDearly | CWDadvanced | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Identification | Closest Match | NCBI | Identity (%) | Putative Ecology | Freq. | Abun. | Freq. | Abun. | Freq. | Abun. |
| Basidiomycota | ||||||||||
| Tomentella sp. | Tomentella sp. | MK583527 | 95 | ECM | 30 | 7.8 | 45 | 8.1 | 55 | 5.4 |
| Xerocomellus sp. | Xerocomellus sp. | MK583530 | 95 | ECM | 5 | 2.6 | 5 | 2.6 | 5 | 0.4 |
| Laccaria sp. | Laccaria sp. | MK583519 | 95 | ECM | 15 | 2.3 | 20 | 1.1 | 15 | 1.1 |
| Clavulina sp. | Clavulina sp. | - | 96 | ECM | 10 | 1.7 | 5 | 1.5 | 10 | 0.9 |
| Scleroderma citrinum Pers. | Scleroderma citrinum | MK583525 | 97 | ECM | 5 | 1.1 | 5 | 0.2 | 5 | 0.2 |
| Thelephora terrestris Ehrh. | Thelephora terrestris | MK583526 | 99 | ECM | 5 | 0.6 | 10 | 0.4 | 5 | 0.4 |
| Tylospora asterophora (Bonord.) Donk. | Tylospora asterophora | MK583528 | 98 | ECM | 5 | 0.3 | 5 | 1.1 | 10 | 2.8 |
| Xerocomellus porosporus (Imler ex G. Moreno & Bon) Šutara | Xerocomellus porosporus | MK583529 | 99 | ECM | - | - | 5 | 0.2 | 5 | 1.1 |
| Russula ochroleuca (Pers.) Fr. | Russula ochroleuca | MK583524 | 99 | ECM | 5 | 0.6 | - | - | 15 | 1.5 |
| Russula nigricans (Bull.) Fr. | Russula nigricans | MK583523 | 97 | ECM | 5 | 1.1 | - | - | - | - |
| Russula amoenolens Romagn. | Russula amoenolens | MK583522 | 98 | ECM | 20 | 4.3 | ||||
| Inocybe lanuginose (Bull.) P. Kumm. | Inocybe lanuginosa | MK583518 | 97 | ECM | - | - | - | - | 10 | 1.1 |
| Amanita citrina Pers. | Amanita citrina | MK583515 | 97 | ECM | - | - | 5 | 0.4 | - | - |
| Ascomycota | ||||||||||
| Pachyphlodes nemoralis Hobart, Bóna & Conde | Pachyphlodes nemoralis | MK583521 | 100 | ECM | 25 | 4.0 | 10 | 0.9 | 40 | 6.6 |
| Cenococcum geophilum Fr. | Cenococcum geophilum | MK583516 | 98 | ECM | 10 | 0.6 | 10 | 0.7 | 15 | 1.1 |
| Humaria hemisphaerica (F.H. Wigg.) | Humaria hemisphaerica | MK583517 | 97 | ECM | 5 | 0.3 | 15 | 3.7 | 5 | 0.4 |
| Hyaloscypha bicolor (Hambl. & Sigler) Vohník, Fehrer & Réblová | Hyaloscypha bicolor | MK583520 | 97 | ECM | - | - | 20 | 2.2 | - | - |
| Phialocephala fortinii Wang & Wilcox | Phialocephala fortinii | - | 98 | Endoph. | - | - | - | - | 15 | 4.7 |
| Mycorrhizal fungal species richness (n) | 12 | 13 | 14 | |||||||
| Degree of mycorrhization (%) | 23.27 | 27.07 | 27.19 | |||||||
| Estimated species richness: | ||||||||||
| Chao1 | 1.3 | 1.7 | 2.2 | |||||||
| Diversity | ||||||||||
| Shannon–Wiener (H’) | 0.23 b | 0.41 ab | 0.66 a | |||||||
| Simpson 1/D | 0.26 b | 0.40 ab | 0.55 a | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olchowik, J.; Hilszczańska, D.; Bzdyk, R.M.; Studnicki, M.; Malewski, T.; Borowski, Z. Effect of Deadwood on Ectomycorrhizal Colonisation of Old-Growth Oak Forests. Forests 2019, 10, 480. https://doi.org/10.3390/f10060480
Olchowik J, Hilszczańska D, Bzdyk RM, Studnicki M, Malewski T, Borowski Z. Effect of Deadwood on Ectomycorrhizal Colonisation of Old-Growth Oak Forests. Forests. 2019; 10(6):480. https://doi.org/10.3390/f10060480
Chicago/Turabian StyleOlchowik, Jacek, Dorota Hilszczańska, Roman Mariusz Bzdyk, Marcin Studnicki, Tadeusz Malewski, and Zbigniew Borowski. 2019. "Effect of Deadwood on Ectomycorrhizal Colonisation of Old-Growth Oak Forests" Forests 10, no. 6: 480. https://doi.org/10.3390/f10060480
APA StyleOlchowik, J., Hilszczańska, D., Bzdyk, R. M., Studnicki, M., Malewski, T., & Borowski, Z. (2019). Effect of Deadwood on Ectomycorrhizal Colonisation of Old-Growth Oak Forests. Forests, 10(6), 480. https://doi.org/10.3390/f10060480






