Positive Results of an Early Intervention Strategy to Suppress a Spruce Budworm Outbreak after Five Years of Trials
Abstract
1. Introduction
2. Methods
2.1. Monitoring and Detection of SBW ‘Hot Spots’
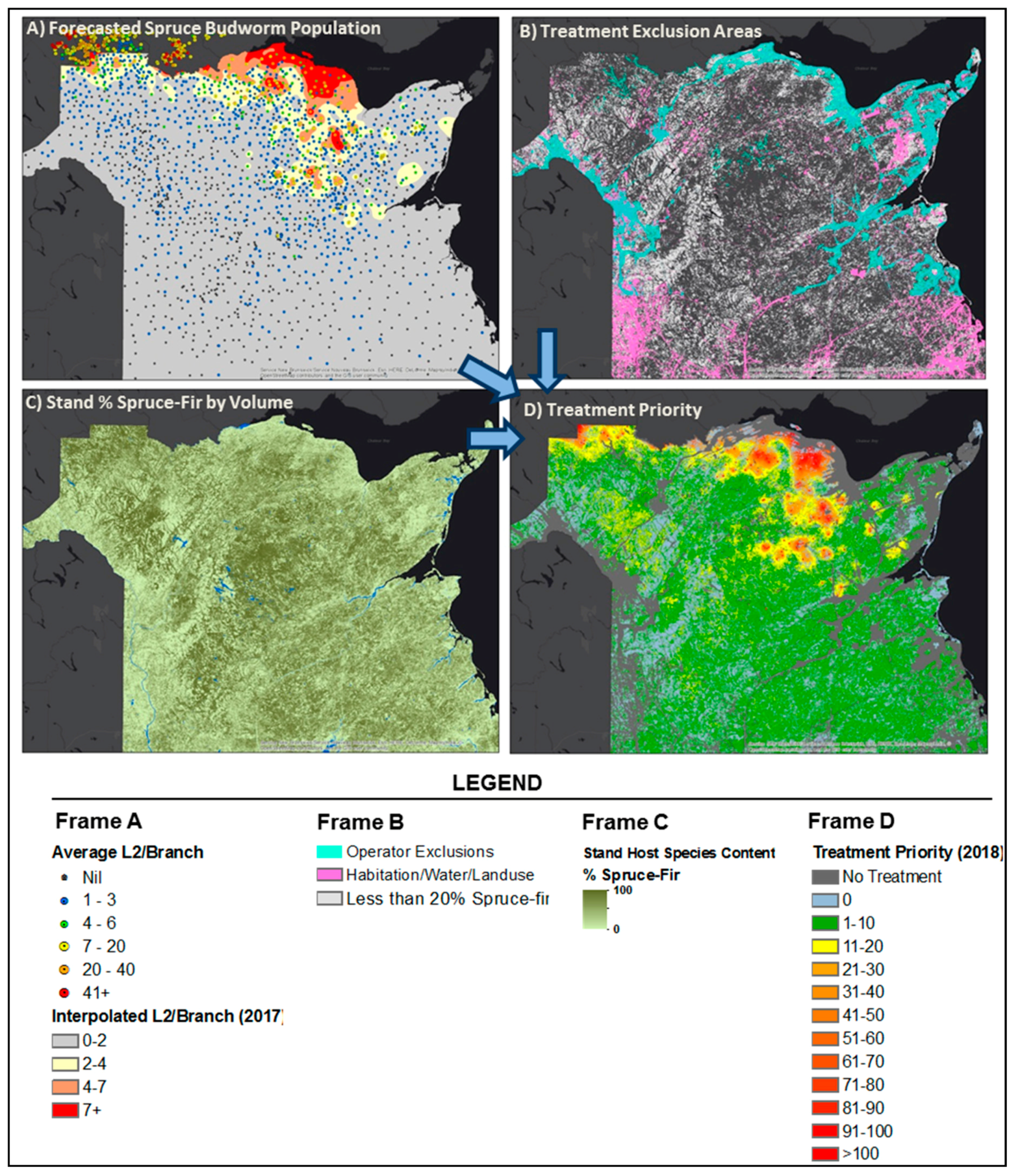
2.2. Incorporation of Effects of Forest Species Composition on SBW Dynamics
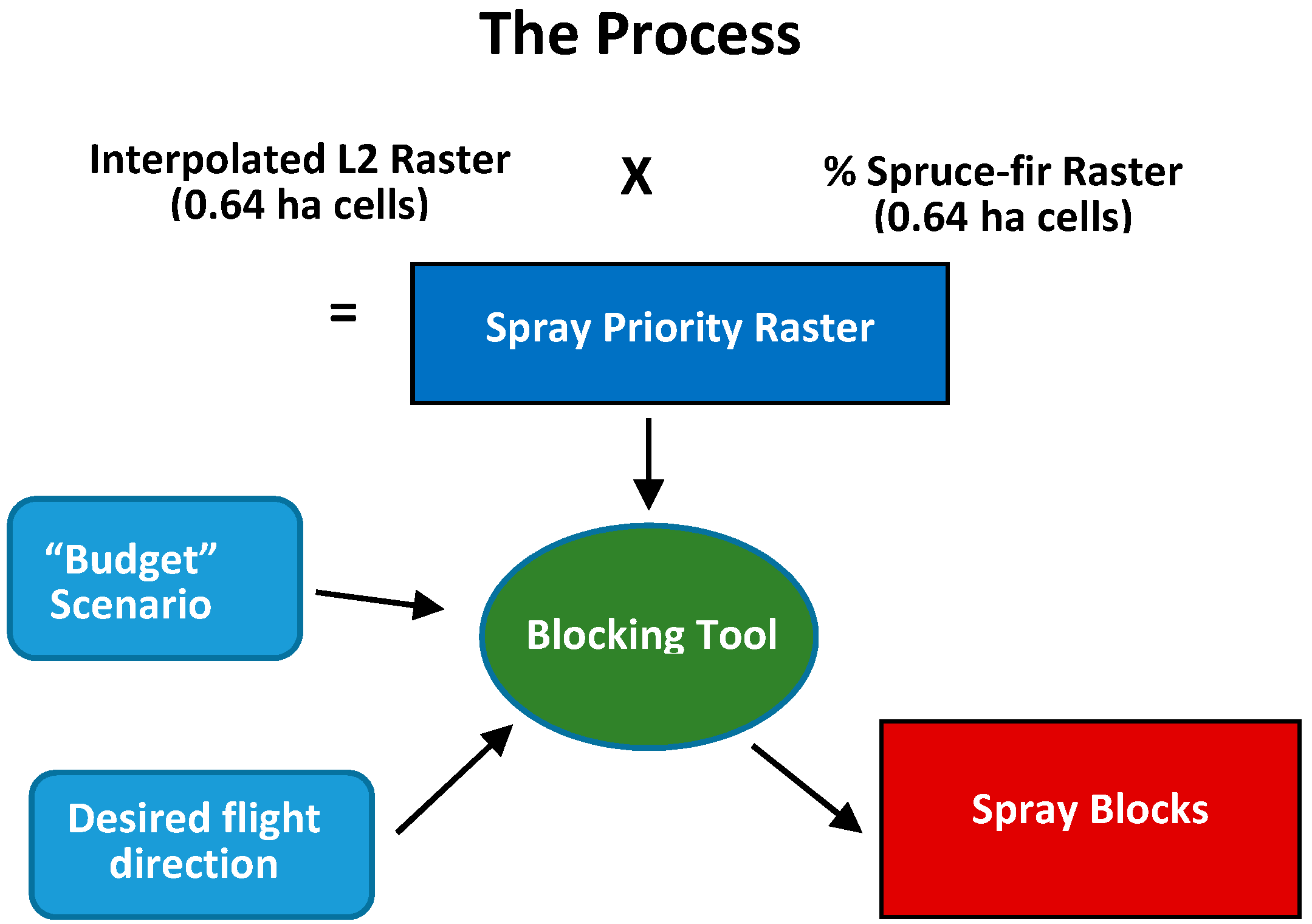
2.3. Development of the Optimum Pest Control Treatment Priority Model
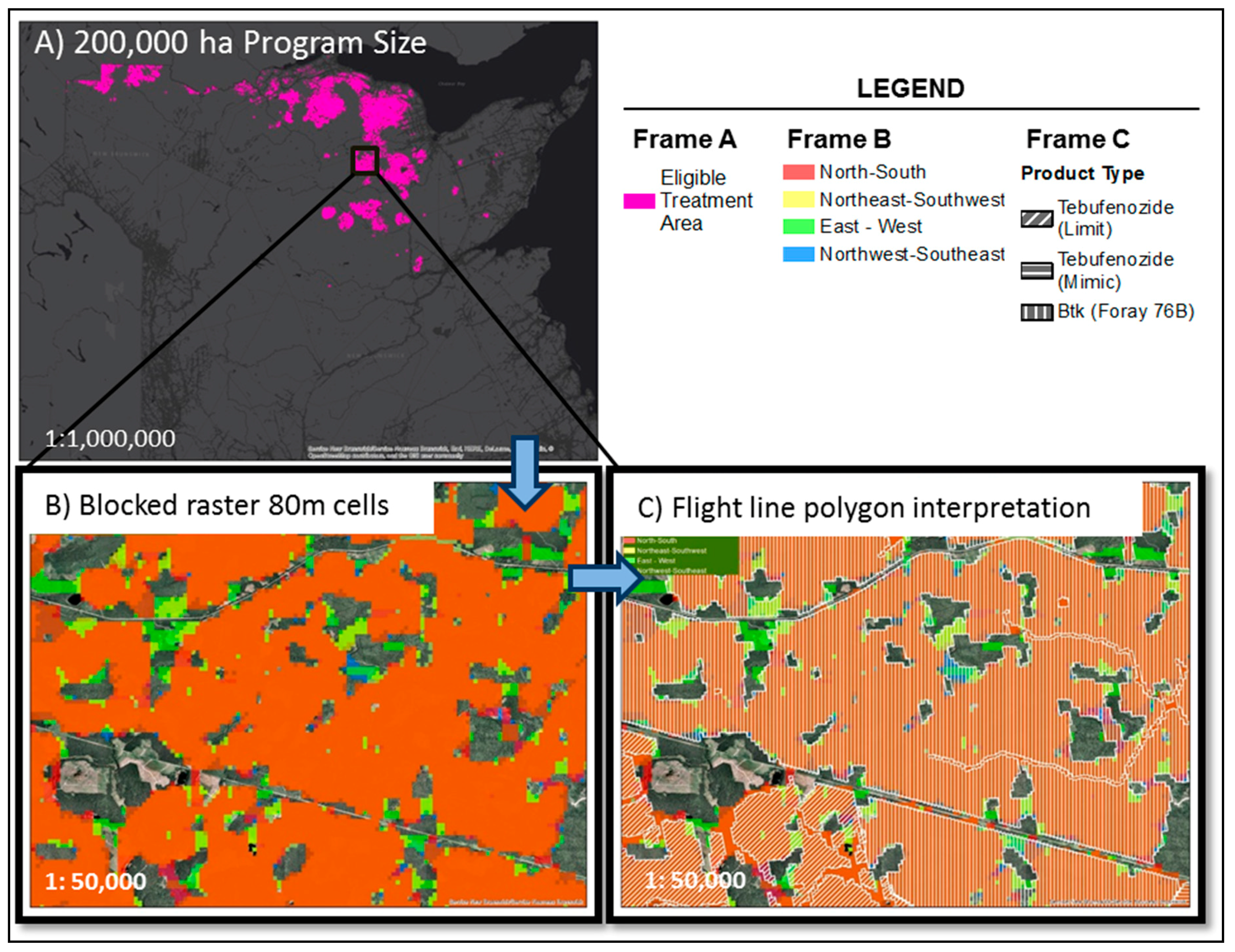
2.4. Insecticide Treatments for EIS against SBW
3. Results
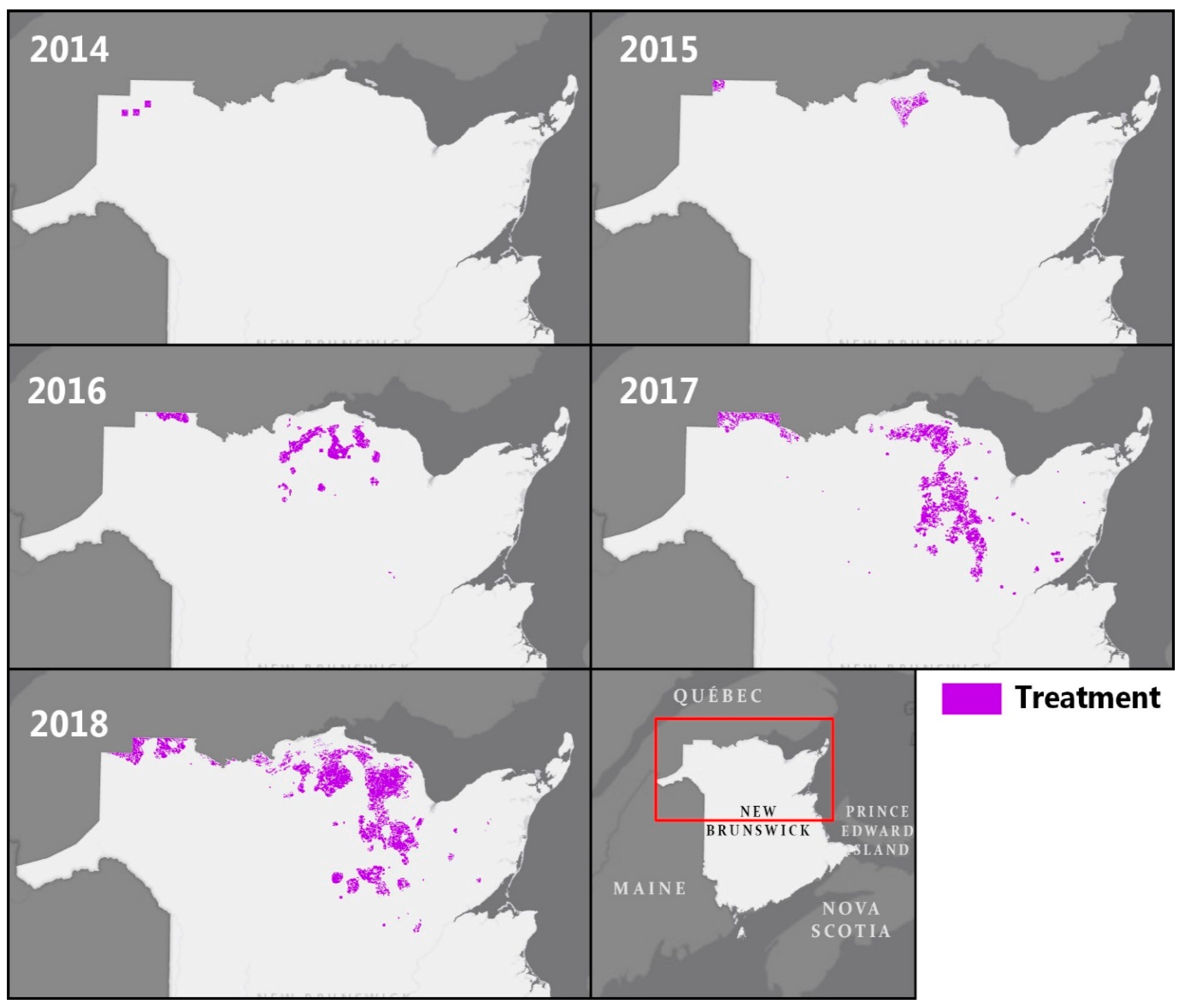
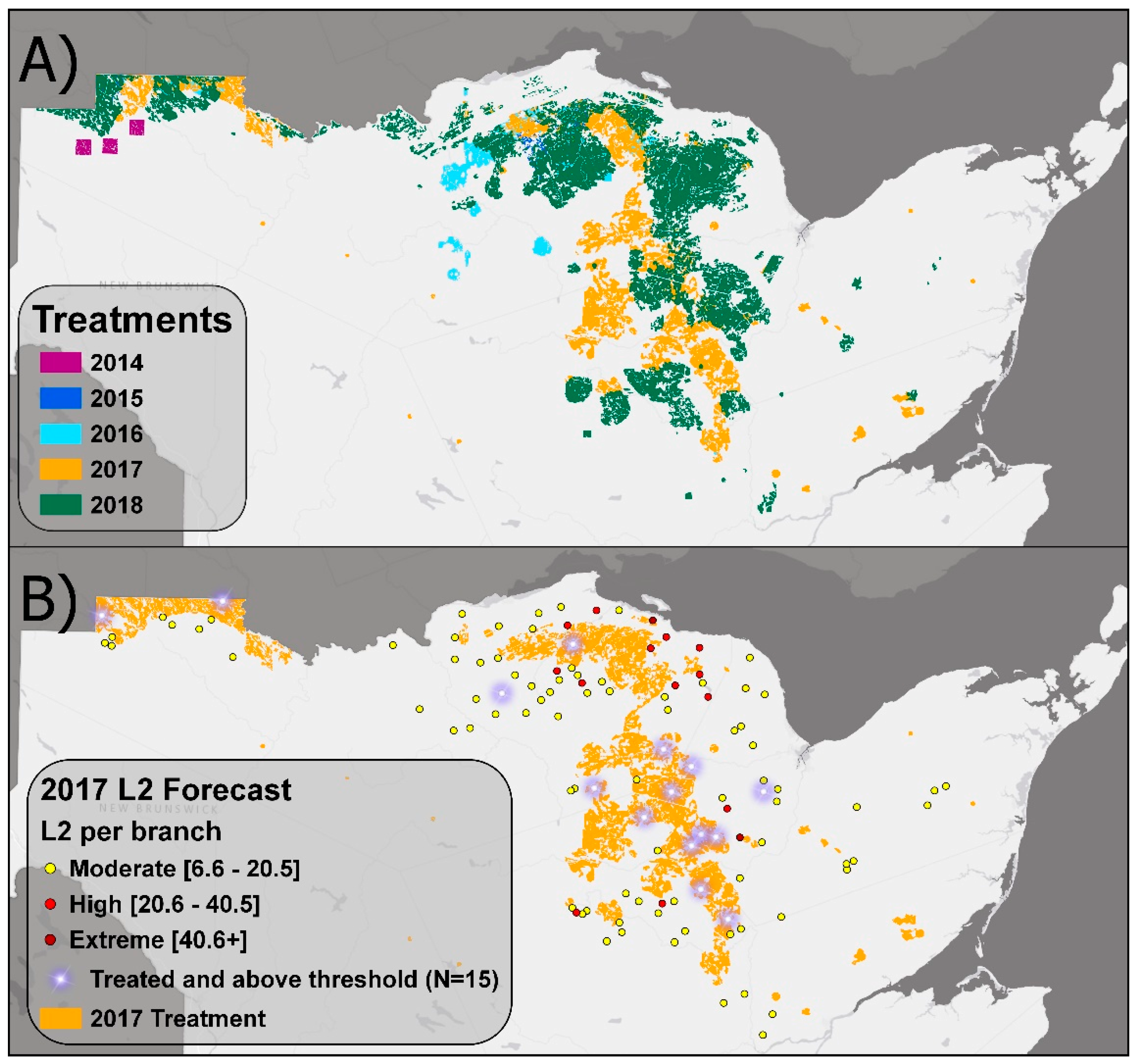
3.1. EIS Insecticide Treatments from 2014 to 2018
3.2. Efficacy of L2 Monitoring and Blocking Approach
3.3. Efficacy of Treatments for Suppressing Population Growth
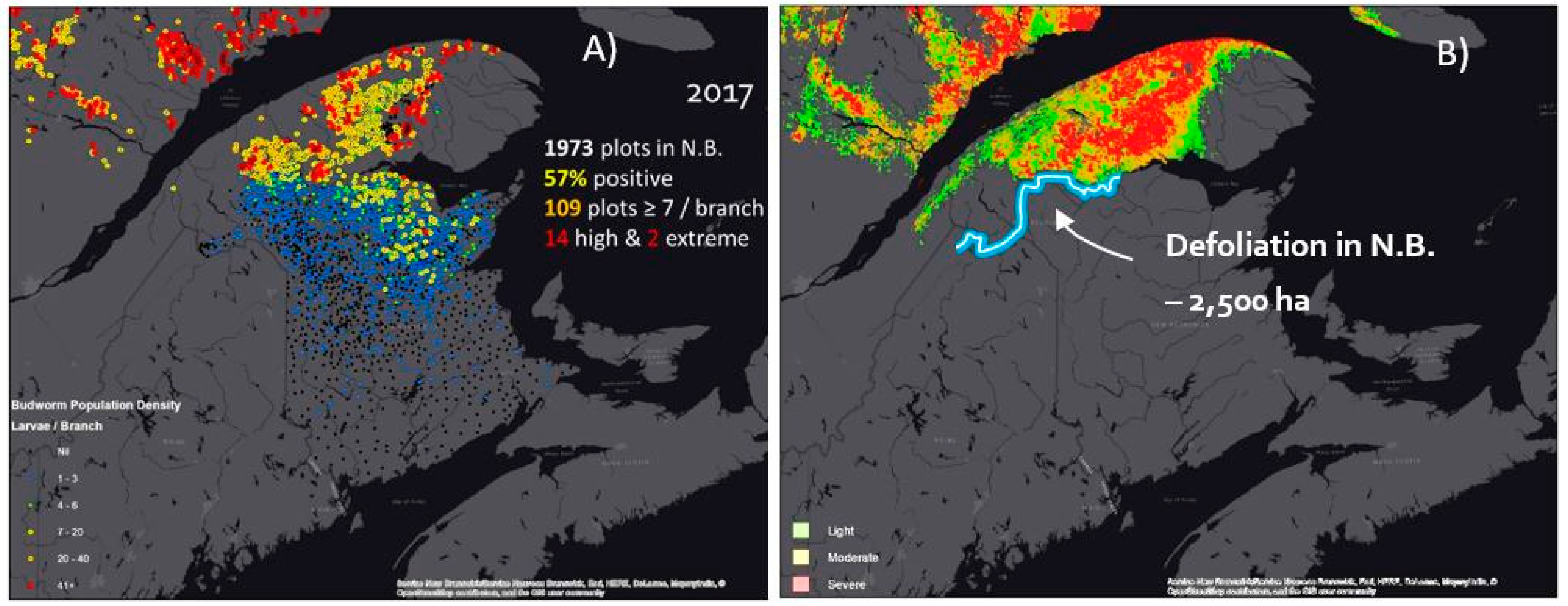
3.4. Comparison with SBW Populations and Defoliation in Adjacent Québec
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kettela, E.G. A Cartographic History of Spruce Budworm Defoliation 1967 to 1981 in Eastern North America; Canadian Forest Service: Ottawa, ON, Canada, 1983; p. 9.
- Ostaff, D.P.; MacLean, D.A. Patterns of balsam fir foliar production and growth in relation to defoliation by spruce budworm. Can. J. For. Res. 1995, 25, 1128–1136. [Google Scholar] [CrossRef]
- MacLean, D.A. Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks: A review and discussion. For. Chron. 1980, 56, 213–221. [Google Scholar] [CrossRef]
- Ostaff, D.P.; MacLean, D.A. Patterns of balsam fir mortality caused by an uncontrolled spruce budworm outbreak. Can. J. For Res. 1989, 19, 1087–1095. [Google Scholar]
- Virgin, G.V.; MacLean, D.A. Five decades of balsam fir stand development after spruce budworm-related mortality. For. Ecol. Manag. 2017, 400, 129–138. [Google Scholar] [CrossRef]
- Baskerville, G.L. Spruce budworm: Super silviculturist. For. Chron. 1975, 51, 138–140. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Lantz, V.A.; Hennigar, C.R.; MacLean, D.A. Economic impacts of spruce budworm (Choristoneura fumiferana Clem.) outbreaks and control in New Brunswick, Canada. Can. J. For. Res. 2012, 42, 490–505. [Google Scholar]
- James, P.M.A.; Robert, L.-E.; Wotton, B.M.; Martell, D.L.; Fleming, R.A.; Robert, L. Lagged cumulative spruce budworm defoliation affects the risk of fire ignition in Ontario, Canada. Ecol. Appl. 2017, 27, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Kneeshaw, D.; Sturtevant, B.R.; Cooke, B.; Work, T.; Pureswaran, D.; DeGrandpre, L.; MacLean, D.A. Insect disturbances in forest ecosystems. Chapter 7. In Routledge Handbook of Forest Ecology; Peh, K.S.-H., Corlett, R.T., Bergeron, Y., Eds.; Routledge: Oxon, UK, 2015; pp. 93–113. [Google Scholar]
- MacLean, D.A. Impacts of insect outbreaks on tree mortality, productivity, and stand development. Can. Entomol. 2016, 148, S138–S159. [Google Scholar] [CrossRef]
- Régnière, J.; Nealis, V.G. Ecological mechanisms of population change during outbreaks of the spruce budworm. Ecol. Entomol. 2007, 32, 461–477. [Google Scholar] [CrossRef]
- Johns, R.C.; Flaherty, L.; Carleton, D.; Edwards, S.; Morrison, A.; Owens, E. Population studies of tree-defoliating insects in Canada: A century in review. Can. Entomol. 2016, 148, S58–S81. [Google Scholar] [CrossRef]
- Royama, T.; Eveleigh, E.S.; Morin, J.R.B.; Pollock, S.J.; McCarthy, P.C.; McDougall, G.A.; Lucarotti, C.J. Mechanisms underlying spruce budworm outbreak processes as elucidated by a 14-year study in New Brunswick, Canada. Ecol. Monogr. 2017, 87, 600–631. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Cook, C.A. Aerial Spray Applications on Canadian Forests: 1945 to 1990; Forestry Canada: Ottawa, ON, Canada, 1993; p. 274. [Google Scholar]
- Coulombe Commission (Commission d’étude sur la gestion de la forêt publique québécoise). Chapitre 4: Protection, conservation et gestion multiressource: Des axes de changement. In Commission d’étude sur la gestion de la forêt publique québécoise, Rapport final; Ministère des Forêts, de la Faune et des Parcs: Quebec, QC, Canada, 2004; pp. 47–92. [Google Scholar]
- Lévesque, R.C.; Cusson, M.; Lucarotti, C. BEGAB: Budworm Eco-Genomics: Applications & Biotechnology (BEGAB); Institut de biologie intégrative et des systèmes, Université Laval: Québec City, QC, Canada, 2010. [Google Scholar]
- Hennigar, C.R.; MacLean, D.A.; Quiring, D.T.; Kershaw, J.A. Differences in spruce budworm defoliation among balsam fir and white, red, and black spruce. For. Sci. 2008, 54, 158–166. [Google Scholar]
- LeDuc, A.; Joyal, C.; Bergeron, Y.; Morin, H. Balsam fir mortality following the last spruce budworm outbreak in northwestern Quebec. Can. J. For. Res. 1995, 25, 1375–1384. [Google Scholar]
- Su, Q.; Needham, T.D.; MacLean, D.A. The influence of hardwood content on balsam fir defoliation by spruce budworm. Can. J. For. Res. 1996, 26, 1620–1628. [Google Scholar] [CrossRef]
- Campbell, E.M.; MacLean, D.A.; Bergeron, Y. The severity of budworm-caused growth reductions in balsam fir/spruce stands varies with the hardwood content of surrounding forest landscapes. For. Sci. 2008, 54, 195–205. [Google Scholar]
- Hennigar, C.; Erdle, T.; Gullison, J.; MacLean, D. Re-examining wood supply in light of future spruce budworm outbreaks: A case study in New Brunswick. For. Chron. 2013, 89, 42–53. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Lantz, V.A.; MacLean, D.A. Public attitudes about forest pest outbreaks and control: Case studies in two Canadian provinces. For. Ecol. Manag. 2009, 257, 1333–1343. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Lantz, V.A.; Hennigar, C.R.; MacLean, D.A. Benefit-cost analysis of spruce budworm (Choristoneura fumiferana Clem.) control: Incorporating market and non-market values. J. Environ. Manag. 2012, 93, 104–112. [Google Scholar] [CrossRef]
- QMFFP: Québec Ministère des Forêts, de la Faune et des Parcs. Aires infestées par la tordeuse des bourgeons de l’épinette au Québec en 2018; Gouvernement du Québec, Direction de la protection des forêts: Québec City, QC, Canada, 2018; 20p.
- Miller, C.A.; Kettela, E.G. Aerial control operations against the spruce budworm in New Brunswick, 1952–1973. In Aerial Control of Forest Insects in Canada; Prebble, M.L., Ed.; Department of the Environment: Ottawa, ON, Canada, 1975; pp. 94–112. [Google Scholar]
- Irving, H.J.; Webb, F.E. Forest protection against spruce budworm in New Brunswick—An aerial applicator’s perspective. Pulp Pap. Can. 1981, 82, 23–31. [Google Scholar]
- Kline, A.W.; Lavigne, D.R.; MacLean, D.A. Effectiveness of spruce budworm spraying in New Brunswick in protecting the spruce component of spruce–fir stands. Can. J. For. Res. 1984, 14, 163–176. [Google Scholar]
- Fuentealba, A.; Dupont, A.; Hébert, C.; Berthiaume, R.; Quezada-García, R.; Bauce, É. Comparing the efficacy of various aerial spraying scenarios using Bacillus thuringiensis to protect trees from spruce budworm defoliation. For. Ecol. Manag. 2019, 432, 1013–1021. [Google Scholar] [CrossRef]
- Régnière, J.; Delisle, J.; Pureswaran, D.S.; Trudel, R. Mate-finding allee effect in spruce budworm population dynamics. Entomol. Exp. Appl. 2013, 146, 112–122. [Google Scholar]
- Sharov, A.A.; Liebhold, A.M.; Roberts, A.E. Optimizing the use of barrier zones to slow the spread of gypsy moth (Lepidoptera: Lymantriidae) in North America. J. Econ. Entomol. 1998, 91, 165–174. [Google Scholar]
- Sharov, A.A.; Leonard, D.; Liebhold, A.M.; Roberts, E.A.; Dickerson, W. “Slow the spread”: A national program to contain the gypsy moth. J. For. 2002, 100, 30–36. [Google Scholar]
- Eveleigh, E.S.; McCann, K.S.; McCarthy, P.C.; Pollock, S.J.; Lucarotti, C.J.; Morin, B.; McDougall, G.A.; Strongman, D.B.; Huber, J.T.; Umbanhowar, J.; et al. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 2007, 104, 16976–16981. [Google Scholar] [CrossRef]
- Huber, J.; Eveleigh, E.; Pollock, S.; McCarthy, P. The Chalcidoid parasitoids and hyperparasitoids (Hymenoptera: Chalcidoidea) of Choristoneura species (Lepidoptera: Tortricidae) in America north of Mexico. Can. Entomol. 1996, 128, 1167–1220. [Google Scholar]
- Miller, J.D.; MacKenzie, S.; Foto, M.; Adams, G.W.; Findlay, J.A. Needles of white spruce inoculated with rugulosin-producing endophytes contain rugulosin reducing spruce budworm growth rate. Mycol. Res. 2002, 106, 471–479. [Google Scholar] [CrossRef]
- Frasz, S.L.; Walker, A.K.; Nsiama, T.K.; Adams, G.W.; Miller, J.D. Distribution of the foliar fungal endophyte Phialocephala scopiformis and its toxin in the crown of a mature white spruce tree as revealed by chemical and qPCR analyses. Can. J. For. Res. 2014, 44, 1138–1143. [Google Scholar] [CrossRef]
- MacLean, D.A.; Erdle, T.A.; MacKinnon, W.E.; Porter, K.B.; Beaton, K.P.; Cormier, G.; Morehouse, S.; Budd, M. The Spruce Budworm Decision Support System: Forest protection planning to sustain long-term wood supplies. Can. J. For. Res. 2001, 31, 1742–1757. [Google Scholar] [CrossRef]
- Hennigar, C.R.; MacLean, D.A.; Porter, K.B.; Quiring, D.T. Optimized harvest planning under alternative foliage-protection scenarios to reduce volume losses to spruce budworm. Can. J. For. Res. 2007, 37, 1755–1769. [Google Scholar] [CrossRef]
- Hennigar, C.R.; Wilson, J.S.; MacLean, D.A.; Wagner, R.G. Applying a spruce budworm decision support system to Maine: Projecting spruce-fir volume impacts under alternative management and outbreak scenarios. J. For. 2011, 109, 332–342. [Google Scholar]
- McLeod, I.M.; Lucarotti, C.J.; Hennigar, C.R.; MacLean, D.A.; Holloway, A.G.L.; Cormier, G.A.; Davies, D.C. Advances in aerial application technologies and decision support for integrated pest management. In Integrated Pest Management and Pest Control; Soloneski, S., Larramendy, M.L., Eds.; InTech Open Access Publisher: Rijeka, Croatia, 2012; pp. 651–668. ISBN 978-953-307-926-4. [Google Scholar]
- Miller, C.A.; Kettela, E.G.; McDougall, G.A. A Sampling Technique for Overwintering Spruce Budworm and Its Applicability to Population Surveys; Canadian Forestry Service: Fredericton, NB, Canada, 1971; p. 11.
- Zhang, B.; MacLean, D.A.; Johns, R.C.; Eveleigh, E.S. Effects of hardwood content on balsam fir defoliation during the building phase of a spruce budworm outbreak. Forests 2018, 9, 530. [Google Scholar] [CrossRef]
- Bognounou, F.; De Grandpré, L.; Pureswaran, D.S.; Kneeshaw, D. Temporal variation in plant neighborhood effects on the defoliation of primary and secondary hosts by an insect pest. Ecosphere 2017, 8, e01759. [Google Scholar] [CrossRef]
- Martel, V.; Johns, R.C.; Eveleigh, E.; McCann, K.; Pureswaran, D.; Sylvain, Z.; Morrison, A.; Morin, B.; Owens, E.; Hébert, C. Landscape level impacts of EIS on SBW, other herbivores and associated natural enemies (ACOA RD100 2.2.2). In Proceedings of the SERG-I Workshop, Fredericton, NB, Canada, 2–5 February 2017; pp. 112–118. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org (accessed on 23 April 2019).
- Mangiafico, S. Rcompanion: Functions to Support. Extension Education Program. Evaluation, Package Version 2.1.7; 2019. Available online: https://CRAN.R-project.org/package=rcompanion (accessed on 23 April 2019).
- De Rosario-Martinez, H. Phia: Post-Hoc Interaction Analysis, R Package Version 0.2-1; 2015. Available online: https://CRAN.R-project.org/package=phia (accessed on 23 April 2019).
- Van Frankenhuyzen, K. Development and current status of Bacillus thuringiensis for control of defoliating forest insects. In Forest Insect Pests in Canada; Armstrong, J.A., Ives, W.G.H., Eds.; Canadian Forest Service: Ottawa, ON, Canada, 1995; pp. 315–325. [Google Scholar]
- Kreutzweiser, D.; Nicholson, C. A simple empirical model to predict forest insecticide ground-level deposition from a compendium of field data. J. Environ. Sci. Health Part B 2007, 42, 107–113. [Google Scholar] [CrossRef]
- Sundaram, K.M.S. Persistence and mobility of tebufenozide in forest litter and soil ecosystems under field and laboratory conditions. Pestic. Sci. 1997, 51, 115–130. [Google Scholar] [CrossRef]
- Thompson, D.; Kreutzweiser, D. A review of the environmental fate and effects of natural ‘reduced risk’ pesticides in Canada. In Crop Protection Products for Organic Agriculture: Environmental, Health, and Efficacy Assessment; Racke, K.D., Felsot, A., Eds.; ACS Books, American Chemical Society: Washington, DC, USA, 2007; pp. 245–274. [Google Scholar]
- Sundaram, K.M.S. Photostability and rainfastness of tebufenozide deposits of fir foliage. In Biorational Pest Control Agents; American Chemical Society: Washington, DC, USA, 1995; pp. 134–152. [Google Scholar]
- Addison, J.A. Safety testing of tebufenozide, a new molt-inducing insecticide for effects on non-target forest soil invertebrates. Ecotoxicol. Environ. Saf. 1996, 33, 55–61. [Google Scholar] [CrossRef]
- Kreutzweiser, D.; Capell, S.; Wainio-Keizer, K.; Eichenberg, D. Toxicity of new molt inducing insecticide (RH-5992) to aquatic macroinvertebrates. Ecotoxicol. Environ. Saf. 1994, 28, 14–24. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Gunn, J.M.; Thompson, D.G.; Pollard, H.G.; Faber, M.J. Zooplankton community responses to a novel forest insecticide, tebufenozide (RH-5992), in littoral lake enclosures. Can. J. Fish. Aquat. Sci. 1998, 55, 639–648. [Google Scholar] [CrossRef]
- US Department of Agriculture, Forest Service. Gypsy Moth Management in the United States: A Cooperative Approach: Final Supplementary Environmental Impact Statement; US Department of Agriculture Forest Service: Newtown Square, PA, USA, 2012; Volume 1, p. 11.
- Royama, T.; MacKinnon, W.E.; Kettela, E.G.; Carter, N.E.; Hartling, L.K. Analysis of spruce budworm outbreak cycles in New Brunswick, Canada, since 1952. Ecology 2005, 86, 1212–1224. [Google Scholar] [CrossRef]
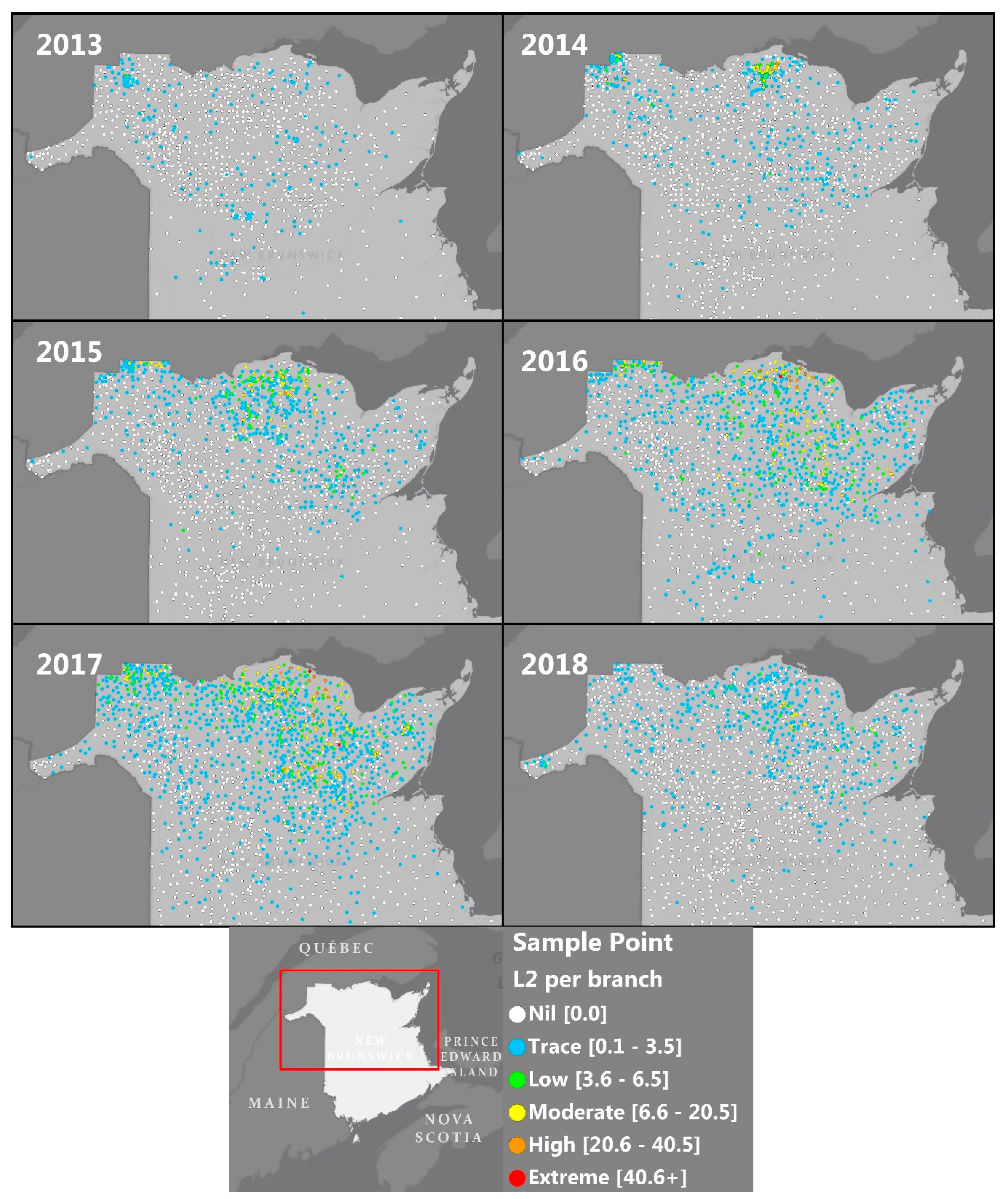
| Year | % of L2 Samples by L2/Branch Class (in Parentheses) | No. Sample Points | |||||
|---|---|---|---|---|---|---|---|
| Nil (0) | Trace (0.1–3.5) | Low (3.6–6.5) | Moderate (6.6–20.5) | High (20.6–40.5) | Extreme (>40.5) | ||
| 2013 | 83 | 17 | 0.0 | 0.0 | 0.0 | 0.0 | 1136 |
| 2014 | 82 | 17 | 0.5 | 0.5 | 0.0 | 0.0 | 1503 |
| 2015 | 68 | 26 | 3.4 | 2.2 | 0.1 | 0.0 | 1561 |
| 2016 | 48 | 40 | 6.4 | 4.5 | 0.6 | 0.1 | 1649 |
| 2017 | 43 | 44 | 7.1 | 4.7 | 0.7 | 0.1 | 1964 |
| 2018 | 74 | 25 | 0.9 | 0.5 | 0.0 | 0.0 | 1851 |
| Year | Area Treated by Active Ingredient (ha) | |||
|---|---|---|---|---|
| Bacillus thuringiensis K. | Tebufenozide | Pheromone | Total | |
| 2014 | 169 | 4472 | 490 | 5131 |
| 2015 1 | 12,093 | 3263 | 271 | 15,627 |
| 2016 2 | 36,889 | 19,719 | 1000 | 57,608 |
| 2017 | 79,088 | 68,142 | 0 | 147,230 |
| 2018 3 | 104,660 | 94,403 | 633 | 199,696 |
| Total | 232,899 | 189,999 | 2394 4 | 425,292 |
| Year | Treatment 1 | Pre-Treatment 2 L2/Branch | Post-Treatment 2 L2/Branch | Difference (Pre-Post Treatment) 4 | F 5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| N 3 | Mean | SEM | N 3 | Mean | SEM | ||||
| 2015 | Treated (0 km) | 201 | 2.9 | 0.31 | 65 | 1.8 | 0.32 | −1.1 | 3.4 * |
| Untreated < 3 km | 116 | 2.0 | 0.30 | 46 | 3.5 | 0.74 | +1.4 | 8.9 *** | |
| Untreated 3–6 km | 33 | 0.47 | 0.14 | 30 | 2.0 | 0.44 | +1.6 | 6.6 * | |
| 2016 | Treated (0 km) | 77 | 6.2 | 0.63 | 84 | 3.8 | 0.70 | −2.4 | 15 *** |
| Untreated < 3 km | 156 | 1.8 | 0.23 | 121 | 3.7 | 0.63 | +1.9 | 10 *** | |
| Untreated 3–6 km | 95 | 1.1 | 0.15 | 73 | 3.8 | 0.91 | +2.7 | 12 *** | |
| 2017 | Treated (0 km) | 149 | 7.5 | 0.68 | 158 | 3.0 | 0.48 | −4.5 | 65 *** |
| Untreated < 3 km | 171 | 2.4 | 0.35 | 195 | 4.6 | 0.48 | +2.2 | 25 *** | |
| Untreated 3–6 km | 149 | 1.3 | 0.17 | 192 | 3.2 | 0.34 | +1.8 | 20 *** | |
| 2018 | Treated (0 km) | 209 | 7.3 | 0.53 | 209 | 0.34 | 0.05 | −7.0 | 619 *** |
| Untreated < 3 km | 262 | 2.4 | 0.24 | 191 | 0.88 | 0.14 | −1.5 | 75 *** | |
| Untreated 3–6 km | 185 | 1.4 | 0.12 | 142 | 0.80 | 0.15 | −0.62 | 48 *** | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLean, D.A.; Amirault, P.; Amos-Binks, L.; Carleton, D.; Hennigar, C.; Johns, R.; Régnière, J. Positive Results of an Early Intervention Strategy to Suppress a Spruce Budworm Outbreak after Five Years of Trials. Forests 2019, 10, 448. https://doi.org/10.3390/f10050448
MacLean DA, Amirault P, Amos-Binks L, Carleton D, Hennigar C, Johns R, Régnière J. Positive Results of an Early Intervention Strategy to Suppress a Spruce Budworm Outbreak after Five Years of Trials. Forests. 2019; 10(5):448. https://doi.org/10.3390/f10050448
Chicago/Turabian StyleMacLean, David A., Peter Amirault, Luke Amos-Binks, Drew Carleton, Chris Hennigar, Rob Johns, and Jacques Régnière. 2019. "Positive Results of an Early Intervention Strategy to Suppress a Spruce Budworm Outbreak after Five Years of Trials" Forests 10, no. 5: 448. https://doi.org/10.3390/f10050448
APA StyleMacLean, D. A., Amirault, P., Amos-Binks, L., Carleton, D., Hennigar, C., Johns, R., & Régnière, J. (2019). Positive Results of an Early Intervention Strategy to Suppress a Spruce Budworm Outbreak after Five Years of Trials. Forests, 10(5), 448. https://doi.org/10.3390/f10050448





