Abstract
To understand whether the process of seasonal nitrogen resorption and biomass allocation are different in CO2-enriched plants, seedlings of red maple (Acer rubrum L.) were exposed to three CO2 concentrations (800 µL L−1 CO2 treatments—A800, 600 µL L−1 CO2 treatments—A600, and 400 µL L−1 CO2 treatments—A400) in nine continuous stirred tank reactor (CSTR) chambers. Leaf mass per area, leaf area, chlorophyll index, carbon (C), nitrogen (N) contents, nitrogen resorption efficiency (NRE), and biomass allocation response were investigated. The results indicated that: (1) Significant leaf N decline was found in senescent leaves of two CO2 treatments, which led to an increase of 43.4% and 39.7% of the C/N ratio in A800 and A600, respectively. (2) Elevated CO2 induced higher NRE, with A800 and A600 showing significant increments of 50.3% and 46.2%, respectively. (3) Root biomass increased 33.1% in A800 and thus the ratio of root to shoot ratio was increased by 25.8%. In conclusion, these results showed that to support greater nutrient and water uptake and the continued response of biomass under elevated CO2, Acer rubrum partitioned more biomass to root and increased leaf N resorption efficiency.
1. Introduction
The initial effect of elevated CO2 increases net primary production (NPP) by enhancing leaf photosynthesis in most plant communities. To sustain the high rates of forest NPP observed under elevated CO2, the requirement for nitrogen would also increase the close relationship of photosynthesis [1] and the synthesis of proteins required for the construction and maintenance of living tissue. However, studies confirm that growth at elevated CO2 results in significant reductions in N content in leaves (mass basis) and a relatively higher C/N [2] ratio. Elevated CO2 which may cause nitrogen (N) concentrations to decline in green leaves has been well investigated [3]. The possible reasons for N decline in leaf include dilution effects [4], increase of less N demand (increased use of photosynthetic nitrogen efficiency and downregulation of photosynthetic enzymes) [5,6,7], less N available (carbon enrichment of the rhizosphere leads to progressively greater limitations of the nitrogen available to plants) [7,8,9], and CO2 inhibition of nitrate assimilation [10]. Apart from this direct effect on photosynthesis, sugar sensing and signaling pathways are reported as important pathways to indirectly regulate the photosynthesis process [11].
It is well known that elevated CO2 increases plant growth and leads to greater biomass production. However, which plant organ is allocated the greater production of carbohydrates varies within and between species. There is a wide range of root responses from large increases [12], to no response [13,14], and decreased responses [15]. The functional balance of shoot root biomass partitioning models predict that as plant tissue C/N ratios rise, root biomass allocation will increase to ensure greater uptake of N to balance the extra C input due to elevated CO2 [16]. It is also reported that greater root growth is related to the sugar signaling pathway which acts on regulating nutrient uptake and transport [11]. In addition, there has been considerable debate concerning whether elevated CO2 results in shifts in the root to shoot ratio (R/S). Thus, identifying the ecological attributes that predict root responses remains challenging.
Nutrient resorption is defined as the process by which nutrients are mobilized from senescent leaves and transported to other plant parts [17]. The differences between nitrogen (N) in green leaves and N of abscised leaves are dependent on the process of N resorption [18,19]. Nitrogen resorption efficiency is calculated as N resorption divided by the initial N content (N in green leaves). However, the reports of the effects of elevated CO2 on litter N are less than on green leaves [3,20]. Litter N inherently is more difficult to detect because factors that affect senescence and resorption are increasingly variable. Although significant declines in green leaf N content in elevated CO2 have been reported [18], few significant effects have been reported on N resorption independently of warming, lacking light, soil nitrogen, and water conditions. Nitrogen resorption may become an increasingly important source of N as a higher net primary production induced by elevated CO2. Biomass allocation will be a critical feature to address the environmental challenges, and its relationship to foliar N resorption is poorly understood.
Red maple (Acer rubrum L.) was selected for this experiment because it is one of the most common and widespread deciduous trees of eastern and central North America, growing on a wide range of soil types in both moist and dry biomass. The main objective of the experiment is to study the leaf N content, N resorption efficiency, and biomass allocation responses to elevated CO2, independently of warming, lacking light, soil nitrogen, and water conditions. We want to know whether the process of seasonal leaf N resorption and biomass allocation are different in CO2-enriched plants. The hypothesis tested is that elevated CO2 would increase biomass allocation to the roots and increase leaf N resorption for more N demand in a condition of adequate nutrients and water.
2. Materials and Methods
2.1. Experimental System and Design
The experiment was investigated in nine continuously stirred tank reactor (CSTR) chambers (Figure 1), which were located inside a glass greenhouse at the University of Massachusetts, Amherst. More details of the operation of our CSTR chambers and CO2 control system have been described previously [21,22,23] and the CO2 concentration fluctuation range was ±10 µL L−1 around the target CO2 concentrations. The time course of irradiance inside the greenhouse was adjusted the day before depending on the weather forecast, so that the irradiance inside the greenhouse was similar to the natural environment. The photoperiod varied with the season outside the greenhouse, mostly from 6 a.m. to 7:30 p.m. All seedlings were exposed to natural light since the chambers were located in a separate single glass greenhouse, but a supplementary irradiance was also supplied with nine 400 W metal Halide lights (Metal Arc, Sylvania, Worcester, MA, USA) in order to better simulate outdoors irradiance.

Figure 1.
The nine continuously stirred tank reactor (CSTR) chambers in the experiment.
The irradiance inside was 75%–85% of that outside. The maximum radio flux was 630 lx. The temperature inside the greenhouse (only analyzed the day we collected data) varied from 18.16 °C to 35.8 °C and the average value was 35.8 °C. The relative humidity varied from 33.99% to 81.96%, and the average value was 58%. The temperature and relative humidity were on average 4.8 °C higher and 15% lower than outside, respectively. The average temperature and relative humidity in the 800 and 600 μL L−1 chambers were 0.34 °C higher and 2% lower, respectively, than in the control chamber [24]. CO2 concentration enrichment was started from June 26, 2014 to November 28, 2014 (156 days) with pure CO2 supplied continuously for 24 hours. One LI–7000 CO2/H2O analyzer monitor was used to measure and record the CO2 concentration (Li–Cor Inc., Lincoln, NE, USA). The CO2 concentration inside every chamber was checked and adjusted every two days to make sure the target CO2 concentration settings could be reached. Three CO2 concentration treatments (800 μL L−1—A800, 600 μL L−1—A600, and 400 μL L−1—A400) were set up randomly among nine chambers with three replications each. Twenty-seven seedlings, uniform in basal diameter and height, were carefully picked up and divided into nine groups, so there were three seedlings grown inside each chamber. All seedlings in chambers were watered every other day with the same volume of water as needed.
2.2. Plant Material and Management
Two-year-old nursery-grown red maple (Acer rubrum L.) seedlings grew from seeds (natural settings) were obtained from the Forrest Keeling nursery (fknursery.com) in Elsberry. Red maple, one of the most common and widespread deciduous trees of eastern and central North America, is adaptable to a very wide range of site conditions. All seedlings were transplanted immediately in 1-gallon pots to the greenhouse on June 3. The growing medium used in pots was an inorganic potting mixture (MM 300 growing medium) (SunGro, Washington, WA, USA). All seedlings were acclimated to the greenhouse environment from June 3 to June 25 (23 days) until all seedlings grew well, then all seedlings with pots were moved into the CSTR chambers on June 26. All seedlings were well-watered every other day. All seedlings were fertilized with a soluble fertilizer (16–17–18; Peters Professional; Scotts, OH, USA) (3.9 g L−1) every week and a micronutrient soluble trace element mix (36.9 mg L−1) [22] was applied once on August 8.
2.3. Leaf Chlorophyll Index
The chlorophyll index was measured weekly on the same marked leaves used for leaf area, measuring N content weekly from September 5 until complete senescence using a nondestructive SPAD-502 chlorophyll meter (Konica Minolta, Tokyo, Japan). SPAD, a surrogate expressed for chlorophyll index, estimated the amount of chlorophyll present by measuring the amount of light transmitting through a leaf. The Minolta SPAD has two LEDs that emit red (650 nm, chlorophyll absorbs light and is unaffected by carotene) and infrared (940 nm, no absorption occurs) wavelengths through an intact leaf sample. Minolta SPAD data was found to be quite well correlated with leaf N [25] and leaf chlorophyll contents [26], and therefore it has been used to estimate the leaf chlorophyll index nondestructively in other atmospheric CO2 enrichment studies [27]. The SPAD value in this paper was represented as the average value of the first, second, and third bud-break leaves.
2.4. Elemental Carbon (C), Nitrogen (N) Contents of Senescent Leaves and Nitrogen Resorption Efficiency (NRE)
In the plants, natural leaf abscission was preceded by the autumnal leaf senescence, a process involving changes in the color of the leaves and a decline in their photosynthetic capacity [28]. In our experiment, the onset of senescence was established on October 7. Senescent leaves were easily identified in the red maple by displaying yellow colors and by becoming detached from the plants with very gentle shaking. The greenhouse did not have wind or extremely low temperatures. Some leaves remained attached to the stem without photosynthetic activity, these were tested by very gentle shaking to determine whether they could be considered to be falling leaves. The senescent leaves were collected, and the elemental contents of carbon and nitrogen were measured using an NC2500 elemental analyzer (CE Instruments, Milan, Italy). The N content (mg g−1) in green leaves was estimated by the regression equation between SPAD and N in senescent leaves (YN = 0.0639XSPAD + 0.0113, R2 = 0.51, p < 0.05). Nitrogen resorption efficiency (NRE) was calculated as the percentage reduction of nitrogen between green and senescent leaves using the following formula [19,27]:
NRE = 100% × [(green leaf N − senescent leaf N)/green leaf N]
2.5. Leaf Mass per Area (LMA) and Whole Leaf Area
Leaf mass per area determinations (LMA, g m−2) were collected on September 15t. LMA was calculated by weighting the dry mass of leaf disks of a known area. The leaf area of red maple was calculated using the formula [29]:
where L and W are the longest length and the width of red maple leaves, respectively. Whole plant leaf area was calculated by the sum of the products of leaf number and leaf area in upper, middle, and latter leaves, respectively.
Leaf area = 3.676 + 0.49 × L × W
2.6. Biomass Measurements
Root, stem, and leaves were separated and collected at the end of the experiment. The dry mass was determined after drying at 85 °C for 48 hours to constant mass.
2.7. Data Analysis
There are three chambers at each CO2 concentration and three CO2 levels.
The effects of elevated CO2 on all parameters except SPAD and whole leaf area were analyzed by one-way ANOVA with CO2 concentration treatments as the treatment factor. For SPAD and whole leaf area, split-plot RM-ANOVA was applied as CO2 levels, time and the interaction of CO2 and time as the treatment factor. When the CO2 effect was significant, post-hoc comparisons were taken using the LSD test. Normality (Kolmogorov–Smirnov test) and homogeneity of variance (Levene’s test) of the data were checked prior to analysis. Results were considered significant when p < 0.05. All the analyses were performed by the SPSS statistics software (Version 18.0, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Leaf Mass per Area (LMA), Leaf SPAD, and Whole Plant Area
The LMA showed no responses to elevated CO2 (Figure 2). For SPAD measurements, a decreasing trend was observed during the experiment in all treatments (Figure 3). SPAD values in A800 and A600 were 22.3% and 17.3% lower, respectively, on August 20, when the largest difference between treatments was observed (Figure 3). The whole plant leaf area was not significantly different among treatments (Figure 3).
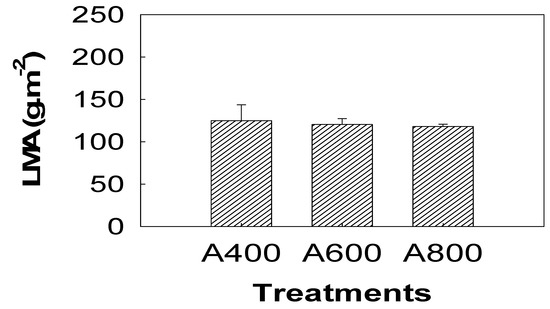
Figure 2.
Effects of elevated CO2 (A400—400, A600—600, and A800—800 µL L−1) on leaf mass per area (LMA) of red maple (Acer rubrum L.). The values shown are mean ± SD. There were no significant differences among (CO2) treatments when p < 0.05, n = 3.
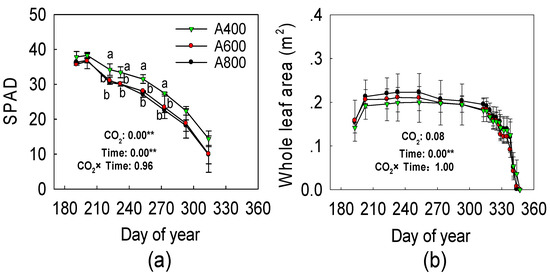
Figure 3.
Effects of elevated CO2 (A400—400, A600—600, and A800—800 µL L−1) on mean SPAD value (a) and whole plant leaf area (b) of red maple (Acer rubrum L.). The values shown are mean ± SD. Different lowercase letters above bars mean significant multiple comparison results among [CO2] treatments when p < 0.05, n = 3. The ANOVA results of (CO2) treatments (CO2), time, and the interaction (CO2 × time) are shown in the figure, ** means p < 0.01.
3.2. Leaf Carbon (C), Nitrogen (C) Contents, and Nitrogen Resorption Efficiency (NRE)
The results indicated that N content of senescent leaves in A800 and A600 significantly decreased by 28.1% and 27.5%, respectively (Figure 4). The C content experienced no response to elevated CO2 (Figure 4). The C/N ratio in A800 and A600 was 43.4% and 39.7% higher, respectively, than in A400. The N resorption efficiency in A800 and A600 increased 50.3% and 46.2%, respectively (Figure 5).
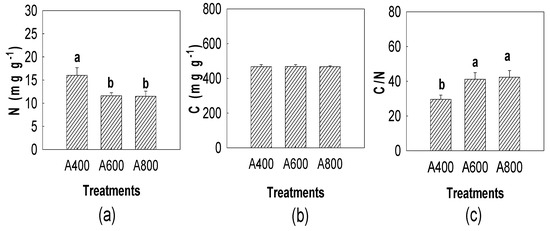
Figure 4.
Effects of elevated CO2 (A400—400, A600—600, and A800—800 µL L−1) on leaf mass-based N contents (a) leaf mass-based C contents (b) and C/N ratio (c) in senescent leaves of red maple (Acer rubrum L.). The values shown are mean ± SD. Different lowercase letters above bars mean significant multiple comparison results among CO2 treatments when p < 0.05, n = 3.
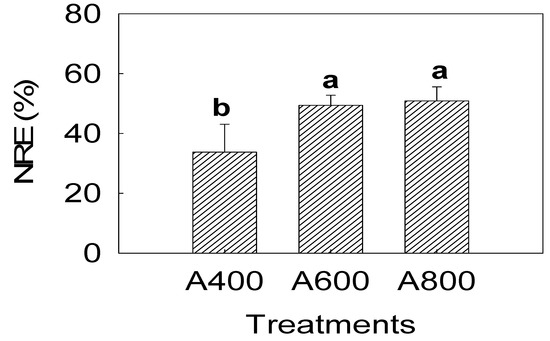
Figure 5.
Effects of elevated CO2 (A400—400, A600—600, and A800—800 µL L−1) on nitrogen resorption efficiency (NRE) of red maple (Acer rubrum L.) under three CO2 concentration treatments (A400—400, A600—600, and A800—800 µL L−1). The values shown are mean ± SD. Different lowercase letters above bars mean significant multiple comparison results among CO2 treatments when p < 0.05, n = 3.
3.3. Biomass
With respect to A400, the total dry biomass significantly increased in A800 (by 19.2%) but effects were not significant for A600. The root biomass of A800 also increased by 33.1% and thus the mass ratio of roots to shoots was increased by 25.8% (Figure 6). However, leaf, stem, and leaves did not allocate more biomass under elevated CO2.
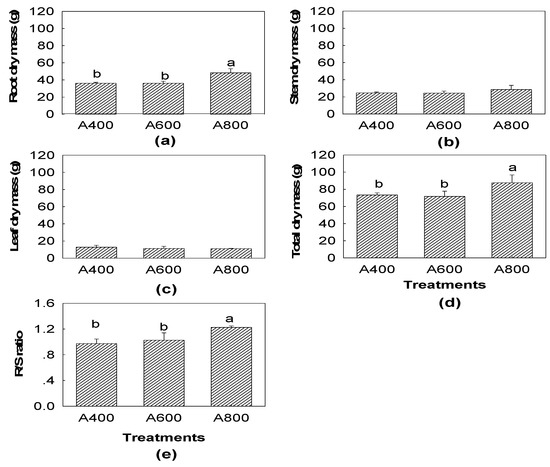
Figure 6.
Dry mass of roots (a) stem, (b) leaves, (c) total, (d) mass ratio of roots to shoots (R/S ratio, and (e) of red maple (Acer rubrum L.) under three CO2 concentration treatments (A400—400, A600—600, and A800—800 µL L−1). The values shown are mean ± SD. Different lowercase letters above bars mean significant multiple comparison results among CO2 treatments when p < 0.05, n = 3.
4. Discussion
A long-lived leaf usually acts as a storage organ for a while, especially for Rubisco (Ribulose-1,5-bisphosphate) enzyme which may play a significant role as an instantaneous storage protein [30]. Decreased leaf photosynthesis and nitrogen in the autumn is the first step of leaf senescence (decreased N and increased C/N ratio) for deciduous trees. In our study, N in A800 and A600 all significantly decreased (28.1% and 27.5%, respectively, Figure 4). These results are comparable with Norby et al (2000) [18], who found that CO2 enrichment reduced leaf N content by 25% in red maple (Acer rubrum L.) under elevated (+300 µL L−1) atmospheric CO2 content. Unlike Curtis (1998) [2], who found reduced N content only when using a leaf mass basis N, but not when using a leaf area basis (due to increased leaf area and leaf weight rather than N reallocation), we found the N mass-based contents decreased without changes in the leaf mass per area (LMA) and the leaf area (Figure 2 and Figure 3). This result suggested that in Acer rubrum the significant decrease in leaf N contents under CO2 enrichment is not caused by changes in leaf density. Meanwhile, the increased C/N ratio of senescent leaf litter is a determinant of long-term allocational carbon and nitrogen and growth responses to CO2 enrichment. It was reported that although less nitrogen is invested in Rubisco total nitrogen demand of green tissues would reduce, which comprised up to 60% of soluble leaf protein with nitrogen [31,32]. However, although the Rubisco content decreased because more nitrogen was allocated to RuBP (Ribulose-1,5-bisphosphate) regeneration and to Pi (phosphate) regeneration [11,33], the specific activity of plants increased and thus maintained sufficient photosynthetic capacity during the growing season [31].
Nitrogen (N) resorption is defined as the process where nitrogen and other nutrients are mobilized from senescent leaves and then transported to other plant organs [17]. The N resorption efficiency (mass-based) was 50.8% at A800 and 49.4% at A600 in leaves of red maple (Figure 5), which are comparable to a mean resorption efficiency of 49%–59% of some plants obtained by other researchers [34,35,36] but lower than previous measurements of resorption (around 70%) in Acer rubrum [37]. The N resorption efficiency in our experiment should be underestimated because N reduction in green leaves was more pronounced than senescent leaves [33] while we estimated N in green leaves through the relationship between SPAD and N in senescent leaves.
More N resorbed and stored in permanent tissues or organs, more nitrogen can be used for early growth in the next year, especially in the case when N availability is insufficient to meet the demands of developing foliage [38]. However, senescence is an aging process of losing functions and structures, whose ultimate goal is to remove nutrients from senescent leaves and transport them to other plant organs [17,39].
Elevated CO2 increases carbohydrate production by stimulating photosynthesis. The proportion (amounts) of the extra carbohydrates accumulated under elevated CO2 in leaves partitioning among plant organs is not well known. It is also affected by species, plant growth method [40], availability of carbon sinks [41], duration of the experiment [42], or nonstructural carbohydrate accumulation by its role as a signaling molecule (sugar sensing and signaling pathways) [11], etc. Our results indicated that the root to shoot ratio increased and more biomass was allocated to root than to shoot and leaf (Figure 6). There are many similar research results on a general increase in root biomass and root to shoot ratio [43]. Growth in roots would be stimulated when carbohydrates are transported more to roots, and therefore the balance of nutrient/water uptake and transport would also be improved [11]. Root biomass in A800 treatments increased 33.1%, which is comparable with a meta-analysis by Nie et al. (2013), who found the biomass of total root was increased by 28.8% [43]. Under elevated CO2, biomass, distribution among root, stems, and foliage should be due to the proportion of new C allocation to different tissue types, different turnover rates of the tissue types, or by the two processes in combination [20]. Therefore, increased root biomass is the more typical mechanism supporting greater N uptake and the continued response of NPP under elevated CO2 [44].
It is noted that the impact of our results also has limitations. First, seedlings and saplings in a natural environment must deal with the far greater seasonal amplitude of environmental variability than adult trees. Two-year seedlings grown from seeds in ambient air are possibly more characteristic [45,46] to elevated CO2 than mature, well-established trees for accelerating growth instead of acclimation. Second, the seedlings may experience a sort of priming effect and not suffer from acclimation of photosynthesis in a relatively short CO2 fumigation period. Thus, our results only occurred when soil nutrition and soil water were not limited, and complementary long-term experiments would be needed.
5. Conclusions
In summary, we found that red maple (Acer rubrum L.) allocated more biomass to roots, leaf C/N ratio decreased, and N resorption from senescent leaves increased under 800 µL L−1 CO2 concentration treatment after one growing season. More growth in root may be helpful to improving nutrient and water uptake, and therefore would help to maintain the balance of nutrients within the plant as a sink. The nitrogen resorption is an increasingly important N source especially when facing high CO2 concentration future. Higher N resorption efficiency of senescent leaves makes possible more nitrogen transfer to other plant organs for early growth next year. Biomass allocation and proportion will be a critical feature to address the environmental challenges. Nitrogen partitioning in plants and leaf senescence dynamics in forests grown under elevated CO2 is paramount and complementary long-term experiments will also be needed.
Author Contributions
Conceptualization, W.M.; methodology, W.M. and L.L.; Formal Analysis, L.L.; investigation, L.L.; data curation, L.L.; writing—original draft preparation, L.L.; writing—Review and editing, X.W.; supervision, W.M. and X.W.; project administration, W.M.; funding acquisition, W.M. and L.L.
Funding
This work was funded by the China Scholarship Council (CSC), the National Natural Science for Youth Foundation of China (31700439), and China Postdoctoral Foundation (2018M631595).
Acknowledgments
I appreciate David Ratkowsky at the Tasmanian Institute of Agriculture and Vicent Calatayud at the CEAM Foundation for their great help in English polishing and revising suggestions. We thank anonymous reviewers for their time and efforts in improving this paper. I also thank Mary Gibler from the Forrest Keeling nursery (fknursery.com) for her kind help with the uniform tree seedlings.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reich, P.B.; Walters, M.B.; Kloeppel, B.D.; Ellsworth, D.S. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia 1995, 104, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.S.; Wang, X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 1998, 113, 299–313. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob. Chang. Biol. 2010, 4, 43–54. [Google Scholar] [CrossRef]
- Curtis, P.S. A meta-analysis of leaf gas exchange and nitrogen in trees grown under elevated carbon dioxide. Plant Cell Environ. 1996, 19, 127–137. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants face the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Fangmeier, A.; Chrost, B.H.; Gy, P.; Krupinska, K. CO2 enrichment enhances flag leaf senescence in barley due to greater grain nitrogen sink capacity. Environ. Exp. Bot. 2000, 44, 151–164. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.G.; Rogers, H.H. Spatial and temporal deployment of crop roots in CO2-enriched environments. New Phytol. 2000, 147, 55–71. [Google Scholar] [CrossRef]
- Bassirirad, H.; Gutschick, V.P.; Lussenhop, J. Root system adjustments: regulation of plant nutrient uptake and growth responses to elevated CO2. Oecologia 2001, 126, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, J.P., Jr. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front Physiol. 2017, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Iversen, C.M.; Joanne, L.; Norby, R.J. CO2 enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytol. 2010, 179, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.P.; Day, F.P.; Hungate, B.A.; Drake, B.G.; Hinkle, C.R. Root biomass and nutrient dynamics in a scrub-oak ecosystem under the influence of elevated atmospheric CO2. Plant Soil 2007, 292, 219–232. [Google Scholar] [CrossRef]
- Handa, I.T.; Hagedorn, F.; Hättenschwiler, S. No stimulation in root production in response to 4 years of in situ CO2 enrichment at the Swiss treeline. Funct. Ecol. 2008, 22, 348–358. [Google Scholar] [CrossRef]
- Aguirrezabal, L.A.N.; Pellerin, S.; Tardieu, F. Carbon nutrition, root branching and elongation: Can the present state of knowledge allow a predictive approach at a whole-plant level? Environ. Exp. Bot. 1993, 33, 121–130. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Thornley, J.H.M. A shoot: Root partitioning model. Ann. Bot. 1982, 49, 585–597. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Litterfall dynamics and element use efficiency in a Kansas gallery forest. Am. Midl. Nat. 1986, 116, 180–189. [Google Scholar] [CrossRef]
- Norby, R.J.; Long, T.M.; Hartzrubin, J.S.; O’Neill, E.G. Nitrogen resorption in senescing tree leaves in a warmer, CO2-enriched atmosephere. Plant Soil. 2000, 224, 15–29. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Reich, P.B.; Yunjian, L.; Bradford, J.B.; Hendrik, P.; Perry, C.H.; Jacek, O. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. PNAS 2014, 111, 13721–13726. [Google Scholar] [CrossRef]
- Heck, W.W.; Philbeck, R.B.; Dunning, F.A. A Continuous Stirred Tank Reactor (CSTR) System for Exposing Plants to Gaseous or Vapor Contaminants: Theory, Specifications, Construction, and Operation; U.S. Dept. of Agriculture, Agricultural Research Service, Southern Region: New Orleans, LA, USA, 1978; pp. 1–32.
- Elagöz, V.; Han, S.S.; Manning, W.J. Acquired changes in stomatal characteristics in response to ozone during plant growth and leaf development of bush beans (Phaseolus vulgaris L.) indicate phenotypic plasticity. Environ. Pollut. 2006, 140, 395–405. [Google Scholar]
- Manning, W.J.; Krupa, S.V. Experimental methodology for studying the effects of ozone on crops and trees. In Surface Level Ozone Exposures and Their Effects on Vegetation; Lefohn, A.S., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1992; pp. 93–156. [Google Scholar]
- Li, L.; Manning, W.J.; Wang, X.K. Autumnal leaf abscission of sugar maple is not delayed by atmospheric CO2 enrichment. Photosynthetica 2018, 56, 1134–1139. [Google Scholar] [CrossRef]
- Lopez-Bellido, R.J.; Shepherd, C.E.; Barraclough, P.B. Predicting post-anthesis N requirements of bread wheat with a Minolta SPAD meter. Eur. J. Agron. 2004, 20, 313–320. [Google Scholar] [CrossRef]
- Hoel, B.O.; Solhaug, K.A. Effect of irradiance on chlorophyll estimation with the minolta SPAD-502 leaf chlorophyll meter. Ann. Bot-London. 1998, 82, 389–392. [Google Scholar] [CrossRef]
- Herrick, J.D.; Thomas, R.B. Leaf senescence and late-season net photosynthesis of sun and shade leaves of overstory sweetgum (Liquidambar styraciflua) grown in elevated and ambient carbon dioxide concentrations. Tree Physiol. 2003, 23, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kikuzawa, K.; Lechowicz, M.J. Ecology of Leaf Longevity; Springer: Berlin/Heidelberg, Germany, 2011; pp. 23–39. [Google Scholar]
- Wargo, P.M. Correlations of Leaf Area with Length and Width Measurements of Leaves of Black Oak, White Oak, and Sugar Maple; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Broomall, PA, USA, 1978.
- Warren, C.R.; Adams, M.A. What determines rates of photosynthesis per unit nitrogen in Eucalyptus seedlings? Funct. Plant Biol. 2004, 31, 10350–10354. [Google Scholar] [CrossRef]
- Urban, O.; Hrstka, M.; Zitová, M.; Holisová, P.; Sprtová, M.; Klem, K.; Calfapietra, C.; Angelis, P.D.; Marek, M.V. Effect of season, needle age and elevated CO2 concentration on photosynthesis and Rubisco acclimation in Picea abies. Plant Physiol. Biochem. 2012, 58, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Greitner, C.; Drake, B.G. Acclimation of photosynthesis in relation to Rubisco and nonstructural carbohydrate contents and in situ carboxylase activity in Scirpus olneyi grown at elevated CO2 in the field. Plant Cell Environ. 1995, 18, 875–884. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Chapin, F.S.; Kedrowski, R.A. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 1983, 64, 376–391. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Q.; Luo, Y.; Vogel, J.; Shi, Z.; Ruan, H.; Xu, X. Nitrogen and phosphorus resorption in planted forests worldwide. Forests 2019, 10, 201. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Briones, M.A.J.I.; Ineson, P. Elevated CO2 affects field decomposition rate and palatability of tree leaf litter: Importance of changes in substrate quality. Soil Biol. Biochem. 1998, 30, 1565–1571. [Google Scholar] [CrossRef]
- Kang, S.M.; Ko, K.C.; Titus, J.S. Mobilization and metabolism of protein and soluble nitrogen during spring growth of apple trees. J. Am. Soc. Hortic. Sci. 1982, 107, 209–213. [Google Scholar]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Suter, D.; Frehner, M.; Fischer, B.U.; Nösberger, J.; Lüsche, A. Elevated CO2 increases carbon to the roots of Lolium perenne under free-air CO2 enrichment but not in a controlled environment. New Phytol. 2002, 154, 65–75. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrerizo, P.M.; Arrese-Igor, C.; Aparicio-Tejo, P.M. Pea plant responsiveness under elevated [CO2] is conditioned by the N source (N2 fixation versus NO3−fertilization). Environ. Exp. Bot. 2013, 95, 34–40. [Google Scholar] [CrossRef]
- De Graaff, M.; van Groenigen, K.; Six, J.; van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Glob. Chang. Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Nie, M.; Lu, M.; Bell, J.; Raut, S.; Pendall, E. Altered root traits due to elevated CO2: A meta-analysis. Glob. Ecol. Biogeogr. 2013, 22, 1095–1105. [Google Scholar] [CrossRef]
- Finzi, A.C.; Norby, R.J.; Carlo, C.; Anne, G.B.; Birgit, G.; Holmes, W.E.; Hoosbeek, M.R.; Iversen, C.M.; Jackson, R.B.; Kubiske, M.E. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. PNAS 2007, 104, 14014–14019. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E.; Lee, T.; Ellsworth, D.S.; West, J.B.; Tilman, D.; Knops, J.M.H.; Naeem, S.; Trost, J. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 2006, 440, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Huxman, T.E.; Hamerlynck, E.P.; Jordan, D.N.; Salsman, K.J.; Smith, S.D. The effects of parental CO2 environment on seed quality and subsequent seedling performance in Bromusrubens. Oecologia 1998, 114, 202–208. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).