Pretreatment with High-Dose Gamma Irradiation on Seeds Enhances the Tolerance of Sweet Osmanthus Seedlings to Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Collection, Gamma Irradiation and Storage
2.2. Salt Stress and Tolerance Assays
2.3. Sampling for Physiological Index
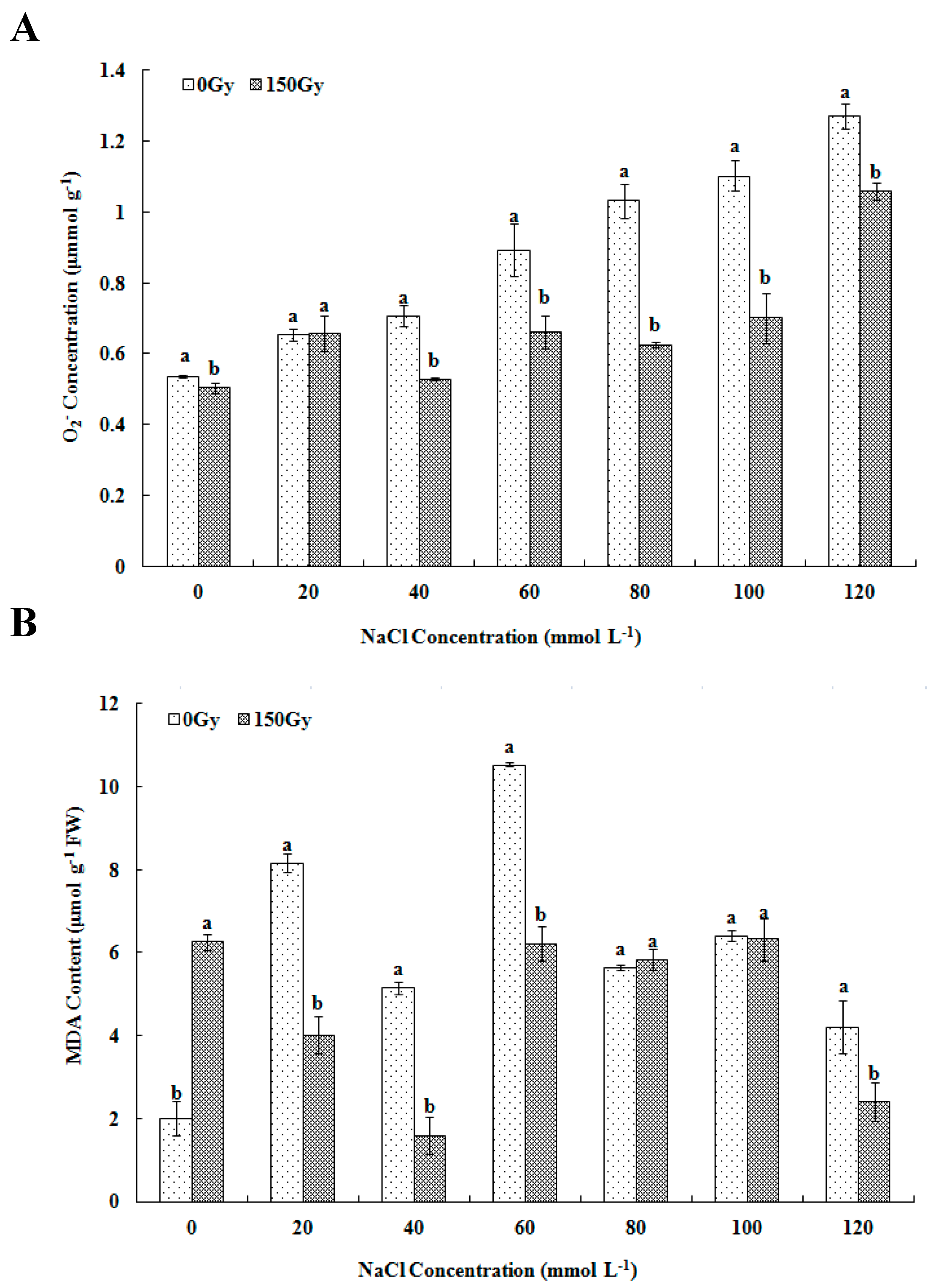
2.3.1. Measurement of O2− Content and MDA Level
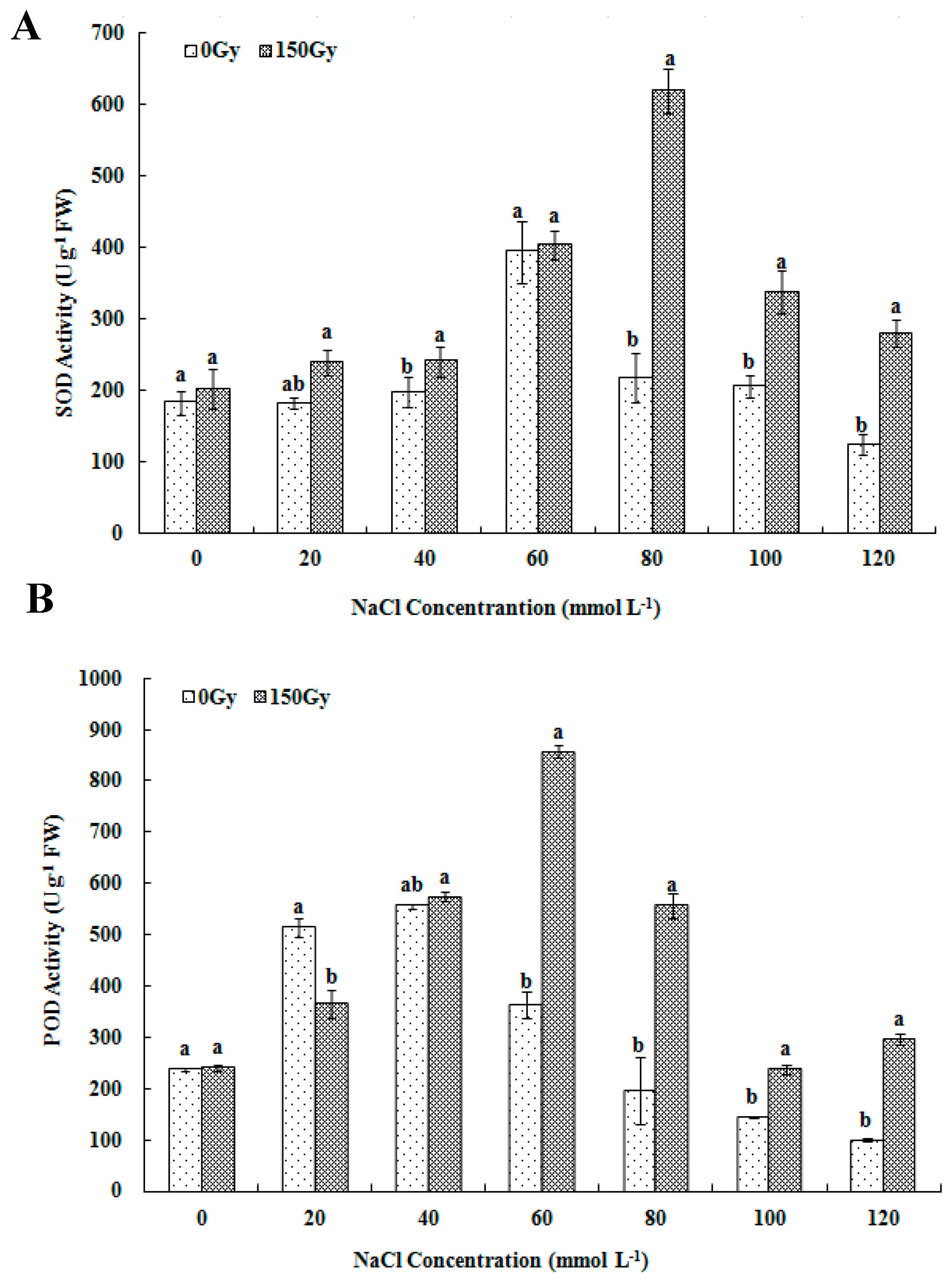
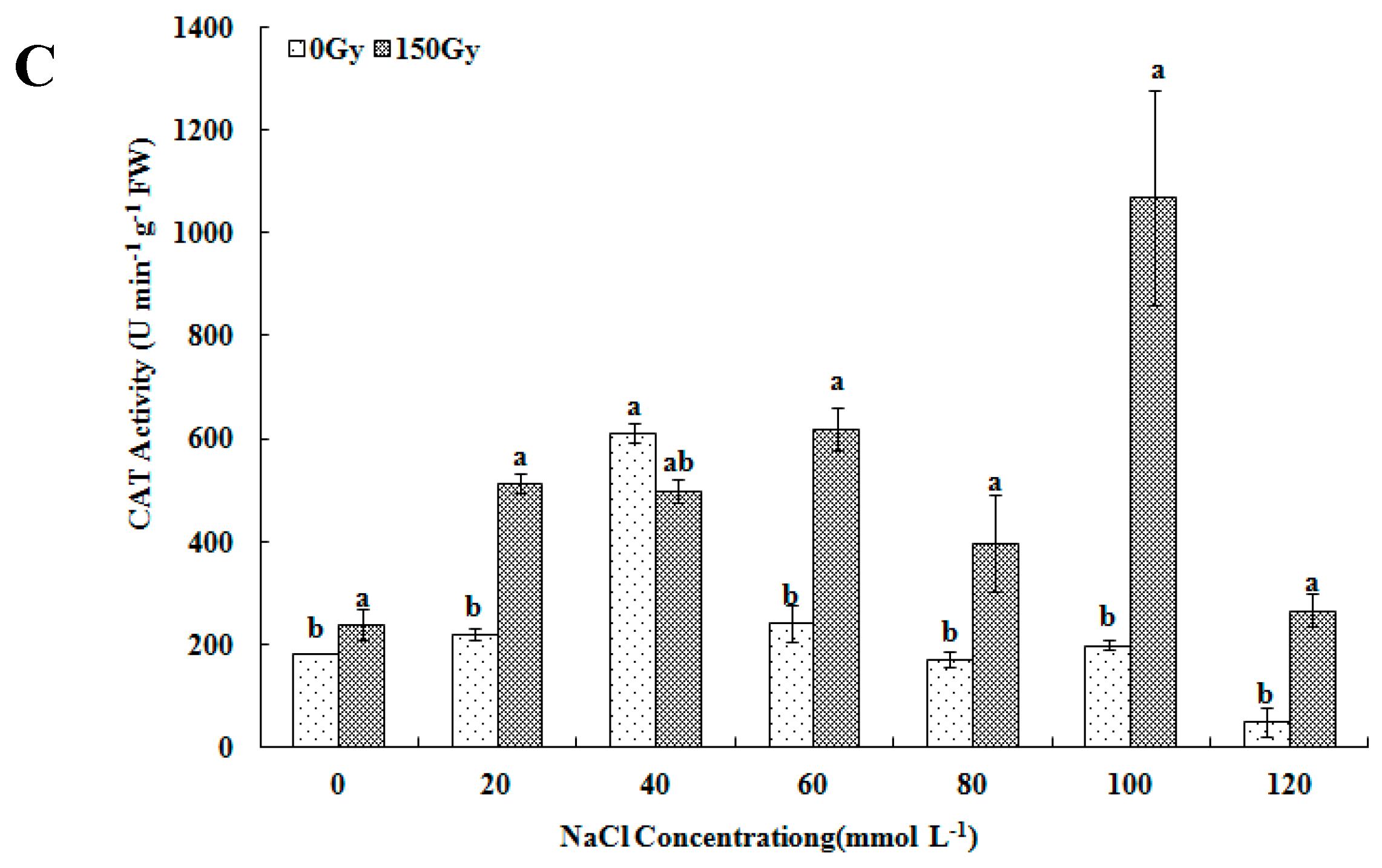
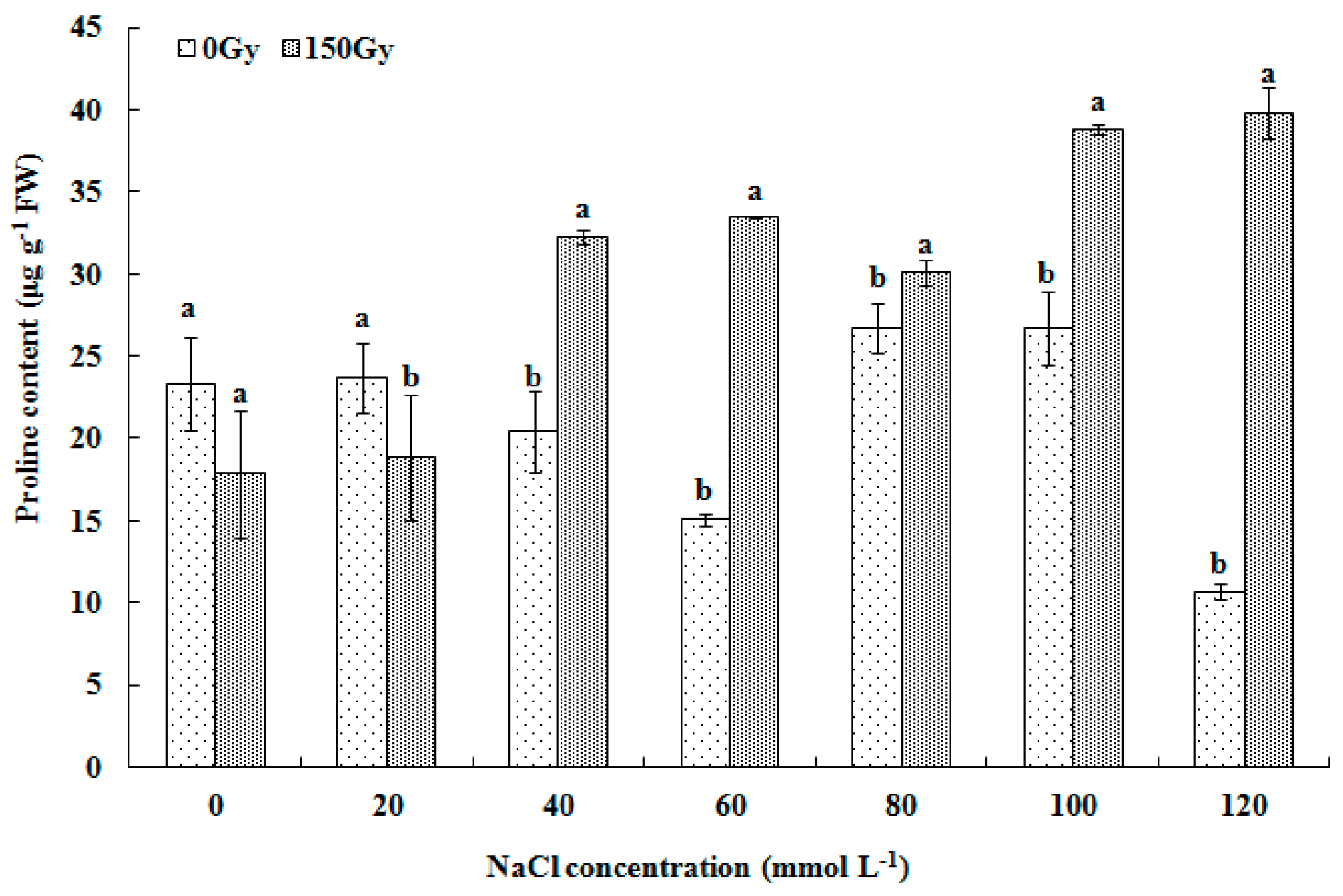
2.3.2. Determination of Antioxidant Enzyme Activity and Proline Level
2.4. Data Analyses
3. Results
3.1. Tolerance in Irradiated Seedlings to Salinity Stress
3.2. Effects of High-Doses Gamma Irradiation on ROS Level and Lipid Peroxidation in Response to Salt Stress
3.3. Effects of High-Doses Gamma Irradiation on ROS Scavenging Systems in Response to Salt Stress
3.4. Effects of High-Doses Gamma Irradiation on Proline Level in Response to Salt Stress
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murtaza, G.; Ghafoor, A.; Owens, G.; Qadir, M.; Kahlon, U.Z. Environmental and economic benefits of saline-sodic soil reclamation using low-quality water and soil amendments in conjunction with a rice-wheat cropping system. J. Agron. Crop Sci. 2009, 195, 124–136. [Google Scholar] [CrossRef]
- Rozema, J.; Flowers, T. Ecology crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good! Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.P.J.C.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Carvalho, M.D. Drought stress and reactive oxygen species, production, scavenging and signaling. Plant Signal Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species, metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Kai, H.; Hirashima, K.; Matsuda, O.; Ikegami, H.; Winkelmann, T.; Nakahara, T.; Iba, K. Thermotolerant cyclamen with reduced acrolein and methyl vinyl ketone. J. Exp. Bot. 2012, 63, 4143–4150. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Wi, S.G.; Chung, B.Y.; Kim, J.S.; Kim, J.H.; Baek, M.H.; Lee, J.W.; Kim, Y.S. Ultrastructural changes of cell organelles in Arabidopsis stem after gamma irradiation. J. Plant Biol. 2005, 48, 195–200. [Google Scholar] [CrossRef]
- Wi, S.G.; Chung, B.Y.; Kim, J.S.; Kim, J.H.; Baek, M.H.; Lee, J.W.; Kim, Y.S. Effects of gamma irradiation on morphological changes and biological responses in plants. Micron 2007, 38, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, R.; Shu, C.; Zhang, X.C. Screening for cold-resistant tomato under radiation mutagenesis and observation of the submicroscopic structure. Acta Physiol. Plant 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, H.S.; Pai, H.S. Expression patterns of diverse genes in response to gamma irradiation in Nicotiana tabacum. J. Plant Biol. 2000, 43, 82–87. [Google Scholar] [CrossRef]
- Moussa, H.R. Gamma irradiation effects on antioxidant enzymes and G6PDH activities in Vicia Faba plants. J. New Seeds 2008, 9, 89–99. [Google Scholar] [CrossRef]
- Zaka, R.; Vandecasteele, C.M.; Misset, M.T. Effects of low chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipa capillata (Poaceae). J. Exp. Bot. 2002, 53, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chung, B.Y.; Kim, J.S.; Wi, S.G. Effects of in planta gamma-irradiation on growth, photosynthesis, and antioxidative capacity of red pepper (Capsicum annuum L.). J. Plant Biol. 2005, 48, 47–56. [Google Scholar] [CrossRef]
- Chen, S.; Chai, M.L.; Jia, Y.F.; Gao, Z.S.; Zhang, L.; Gu, M.X. In Vitro Selection of salt tolerant variants following 60Co gamma irradiation of long-term callus cultures of zoysia matrella [L.] merr. Plant Cell Tiss. Organ Cult. 2011, 107, 493–500. [Google Scholar] [CrossRef]
- Qi, W.C.; Zhang, L.; Xu, H.B.; Wang, L.; Jiao, Z. Physiological and molecular characterization of the enhanced salt tolerance induced by low-dose gamma irradiation in Arabidopsis seedlings. Biochem. Biophys. Res. Commun. 2014, 450, 1010–1015. [Google Scholar] [CrossRef]
- Xiang, Q.B.; Liu, Y.L. An Illustrated Monograph of the Sweet Osmanthus Cultivars in China; Zhejiang Science and Technology Press: Hangzhou, China, 2008. [Google Scholar]
- Ke, D.S.; Wang, A.G.; Sun, G.C.; Dong, L.F. The effect of active oxygen on the activity of ACC synthase induced by exogenous IAA. Acta Bot. Sin. 2002, 44, 551–556. [Google Scholar]
- Geng, X.M.; Liu, J.; Lu, J.G.; Hu, F.R.; Okubo, H. Effects of cold storage and different pulsing treatments on postharvest quality of cut OT Lily ‘Mantissa’ flowers. J. Facul. Agric. Kyushu Univ. 2009, 54, 41–45. [Google Scholar]
- Dhindsa, R.A.; Plumb, D.P.; Thorpe, T.A. Leaf senescence, correlated with increased permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Exp. Bot. 1981, 126, 93–101. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination offree proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Geng, X.M.; Liu, X.; Ji, M.; Hoffmann, W.A.; Grunden, A.; Xiang, Q.Y.J. Enhancing heat tolerance of the little dogwood cornus canadensis L. f. with introduction of a superoxide reductase gene from the hyperthermophilic archaeon pyrococcus furiosus. Front. Plant Sci. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Marcu, D.; Cristea, V.; Daraban, L. Dose-dependent effects of gamma radiation on lettuce (Lactuca sativa var. capitata) seedlings. Int. J. Radiat. Biol. 2013, 89, 219–223. [Google Scholar] [CrossRef]
- Geng, X.M.; Wang, L.G.; Li, N.; Yang, X.L. Study on the seed germination and seedling growth of Osmanthus fragrans under 6°Co-γ irradiation. J. Nuclear Agric. Sci. 2016, 30, 0216–0223, (In Chinese with English abstract). [Google Scholar]
- Shereen, A.; Ansari, R.; Mumtaz, S.; Bughio, H.R.; Mujtaba, S.M.; Shirazi, M.U.; Khan, M.A. Impact of gamma irradiation induced changes on growth and physiological responses of rice under saline conditions. Pakist. J. Bot. 2009, 41, 2487–2495. [Google Scholar]
- He, S.Z.; Han, Y.F.; Wang, Y.P.; Zhai, H.; Liu, Q.C. In vitro selection and identification of sweet potato (Ipomoea batatas (L.) Lam.) plants tolerant to NaCl. Plant Cell Tiss. Organ Cult. 2009, 96, 69–74. [Google Scholar] [CrossRef]
- Haruhiko, W.; Koshiba, T.; Matsui, T.; Satô, M. Involvement of peroxidase in differential sensitivity to γ-radiation in seedlings of two Nicotiana species. Plant Sci. 1998, 132, 109–119. [Google Scholar]
- Zaka, R.; Chenal, C.; Misset, M.T. Effects of low doses of short-term gamma irradiation on growth and development through two generations of Pisum sativum. Sci. Total Environ. 2004, 320, 121–129. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Helaly, M.N.M.; El-Hosieny, A.M.R. Effectiveness of gamma irradiated protoplasts on improving salt tolerance of lemon (Citrus limon L. Burm. f.). Am. J. Plant Physiol. 2011, 6, 190–208. [Google Scholar] [CrossRef]
- Qi, W.C.; Zhang, L.; Feng, W.S.; Xu, H.B.; Wang, L.; Jiao, Z. ROS and ABA Signaling Are Involved in the Growth Stimulation Induced by Low-Dose Gamma Irradiation in Arabidopsis Seedling. Appl. Biochem. Biotechnol. 2015, 175, 1490–1506. [Google Scholar] [CrossRef]
- Goh, E.J.; Kim, J.B.; Kim, W.J.; Ha, B.K.; Kim, S.H.; Kang, S.Y.; Seo, Y.W.; Kim, D.S. Physiological changes and anti-oxidative responses of Arabidopsis plants after acute and chronic γ-irradiation. Radiat. Environ. Biophys. 2014, 53, 677–693. [Google Scholar] [CrossRef]
- Aghaei, K.; Ehsanpour, A.A.; Komatsu, S. Potato responds to salt stress by increased activity of antioxidant enzymes. J. Integr. Plant Biol. 2009, 51, 1095–1103. [Google Scholar] [CrossRef]
- Nikam, A.A.; Devarumath, R.M.; Ahuja, A.; Babu, H.; Shitole, M.G.; Suprasanna, P. Radiation-induced in vitro mutagenesis system for salt tolerance and other agronomic characters in sugarcane (Saccharum officinarum L.). Crop J. 2015, 3, 46–56. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 1998, 36, 767–772. [Google Scholar] [CrossRef]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Hossain, M.A.; Fujita, M. Evidence for a role of exogenous glycinebetaine and proline inantioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol. Mol. Biol. Plants 2010, 16, 19–29. [Google Scholar] [CrossRef] [PubMed]
| Grade | Salinity Damage Level | Description |
|---|---|---|
| 0 | No damage | No morphological damage symptoms of the whole plant |
| 1 | Mild | Less than 20% of the leaves have scorched margin and dehydration symptoms |
| 2 | Moderate | Nearly 50% of the leaves scorch, yellow with rust or wither |
| 3 | Severe | More than 50% of the leaves scorch, yellow with rust or wither |
| 4 | Very severe | More than 90% of the leaves scorch and wither or even the whole plant dies |
| NaCl Concentration (mmol/L) | Salt Injury Index | Salt Injury Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 Gy | 50 Gy | 100 Gy | 150 Gy | 0 Gy | 50 Gy | 100 Gy | 150 Gy | |
| 0 | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a |
| 20 | 0.08 ± 0.01 a | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.00 ± 0.00 b | 63.33 a | 30.00 b | 26.67 c | 0.00 d |
| 40 | 0.55 ± 0.05 a | 0.37 ± 0.03 b | 0.36 ± 0.01 b | 0.28 ± 0.04 c | 100.0 a | 90.00 b | 70.00 c | 33.33 d |
| 60 | 0.70 ± 0.05 a | 0.56 ± 0.06 b | 0.52 ± 0.06 b | 0.40 ± 0.04 c | 100.0 a | 100.0 a | 96.67 b | 80.00 c |
| 80 | 0.90 ± 0.02 a | 0.74 ± 0.05 b | 0.67 ± 0.05 c | 0.54 ± 0.04 c | 100.0 a | 100.0 a | 100.0 a | 100.0 a |
| 100 | 0.90 ± 0.01 a | 0.89 ± 0.01 a | 0.78 ± 0.06 c | 0.85 ± 0.03 b | 100.0 a | 100.0 a | 100.0 a | 100.0 a |
| 120 | 0.98 ± 0.01 a | 0.98 ± 0.01 a | 0.95 ± 0.01 b | 0.97 ± 0.01 a | 100.0 a | 100.0 a | 100.0 a | 100.0 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Zhang, Y.; Wang, L.; Yang, X. Pretreatment with High-Dose Gamma Irradiation on Seeds Enhances the Tolerance of Sweet Osmanthus Seedlings to Salinity Stress. Forests 2019, 10, 406. https://doi.org/10.3390/f10050406
Geng X, Zhang Y, Wang L, Yang X. Pretreatment with High-Dose Gamma Irradiation on Seeds Enhances the Tolerance of Sweet Osmanthus Seedlings to Salinity Stress. Forests. 2019; 10(5):406. https://doi.org/10.3390/f10050406
Chicago/Turabian StyleGeng, Xingmin, Yuemiao Zhang, Lianggui Wang, and Xiulian Yang. 2019. "Pretreatment with High-Dose Gamma Irradiation on Seeds Enhances the Tolerance of Sweet Osmanthus Seedlings to Salinity Stress" Forests 10, no. 5: 406. https://doi.org/10.3390/f10050406
APA StyleGeng, X., Zhang, Y., Wang, L., & Yang, X. (2019). Pretreatment with High-Dose Gamma Irradiation on Seeds Enhances the Tolerance of Sweet Osmanthus Seedlings to Salinity Stress. Forests, 10(5), 406. https://doi.org/10.3390/f10050406




