No Ontogenetic Shifts in C-, N- and P-Allocation for Two Distinct Tree Species along Elevational Gradients in the Swiss Alps
Abstract
1. Introduction
2. Materials and Methods
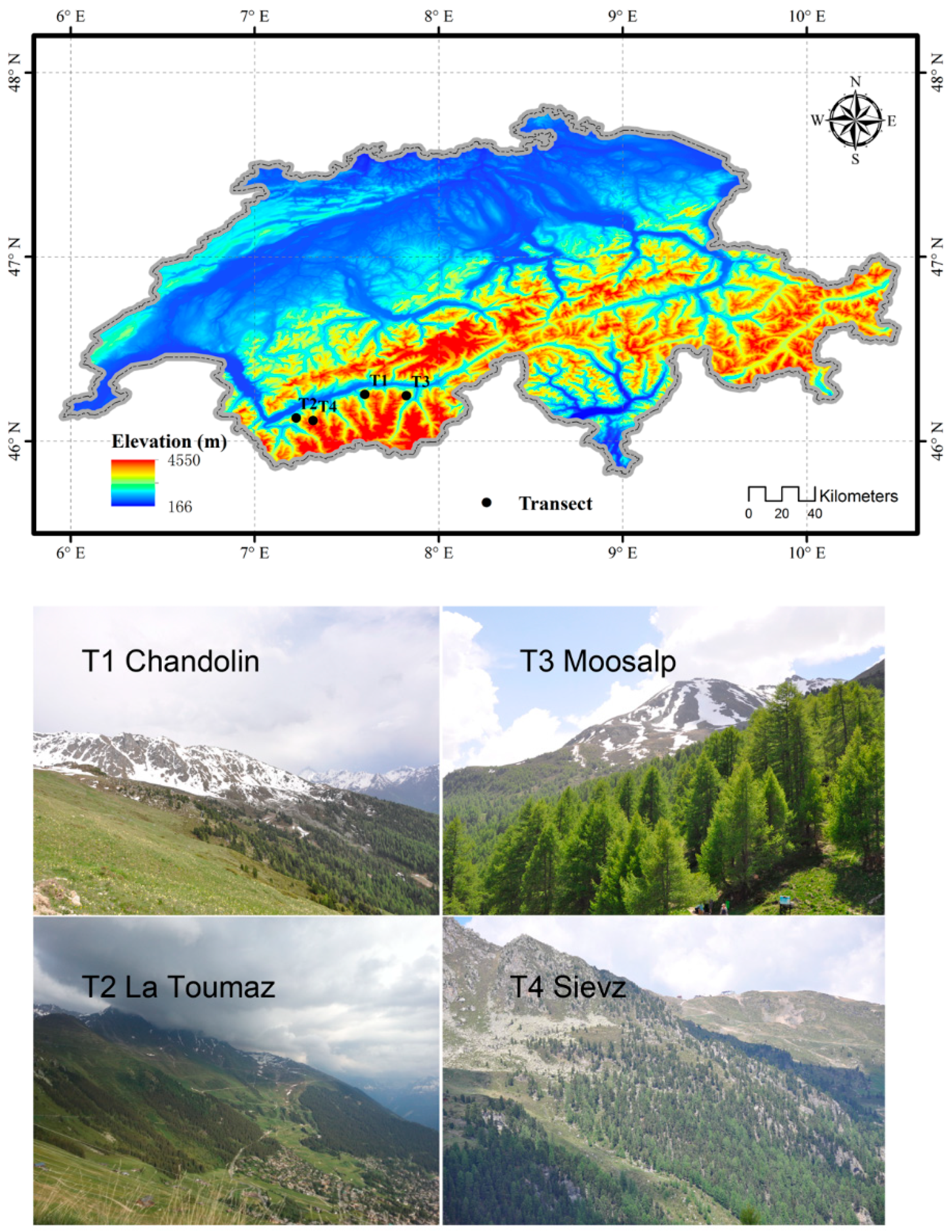
2.1. Description of Sites and Species
2.2. Sampling Protocol
2.3. Plant Analysis
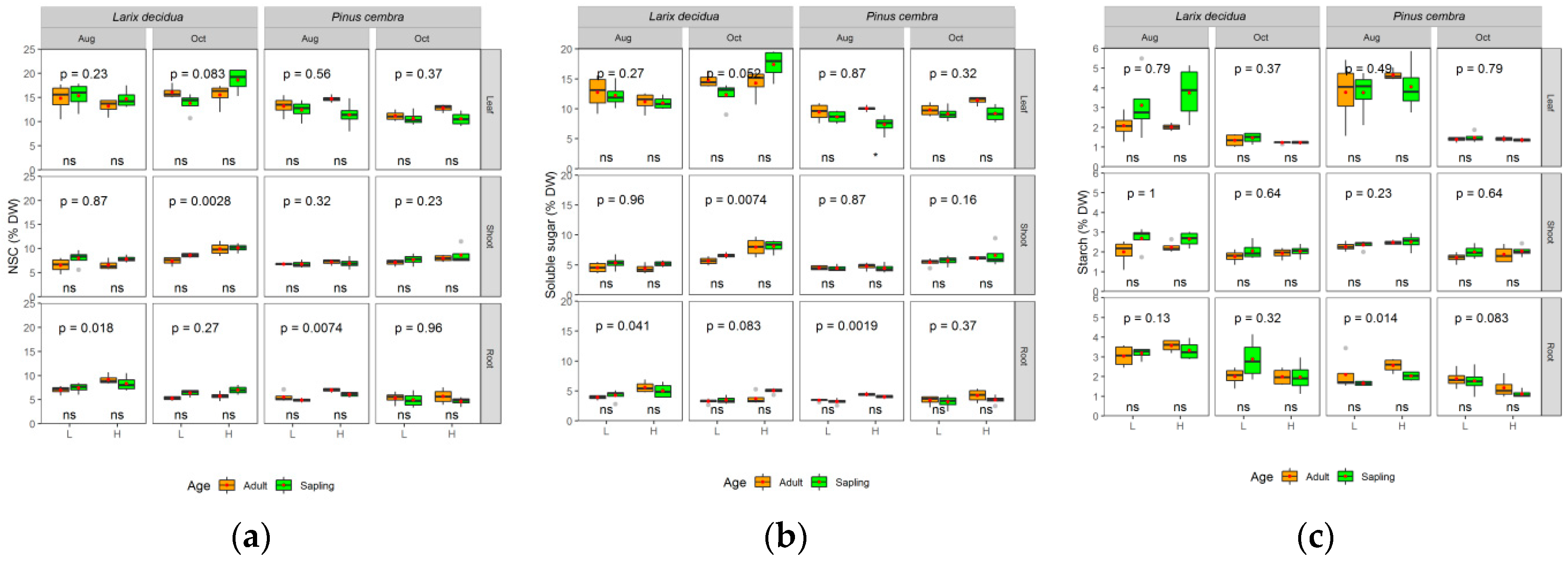
2.3.1. Non-Structural Carbohydrates
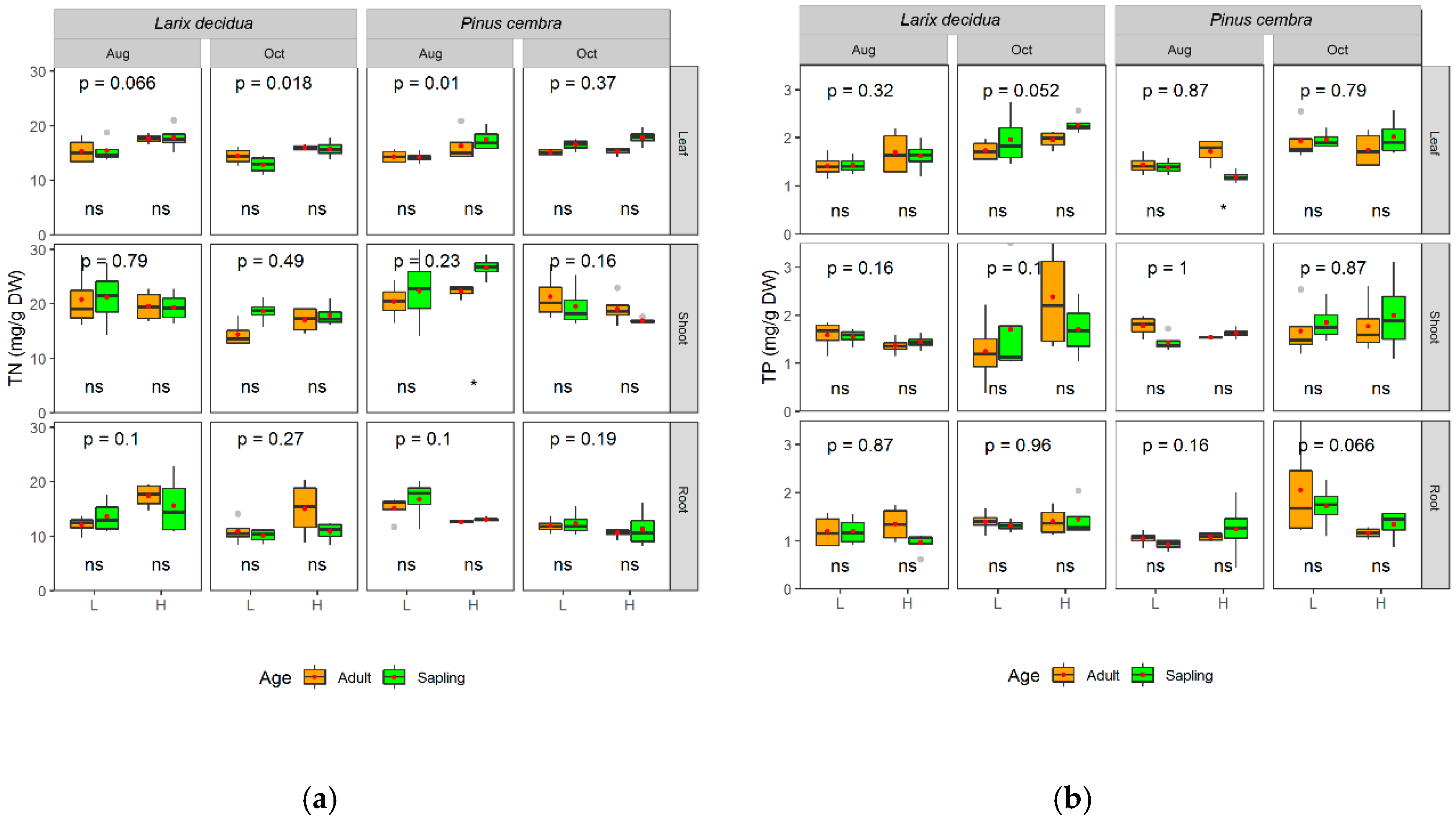
2.3.2. Nitrogen and Phosphorus Concentrations
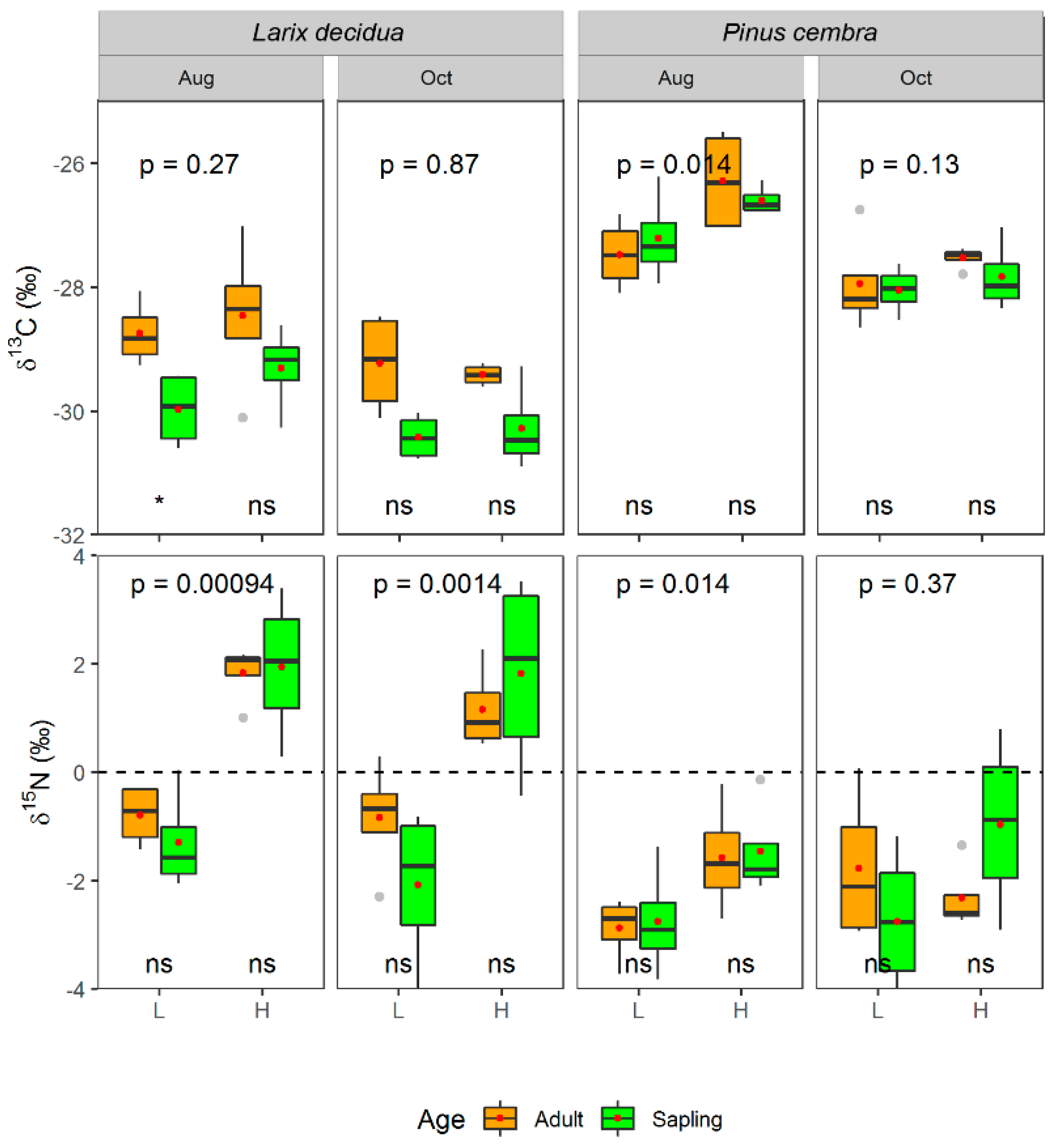
2.3.3. Carbon and Nitrogen Isotopic Abundance
2.4. Statistical Analyses
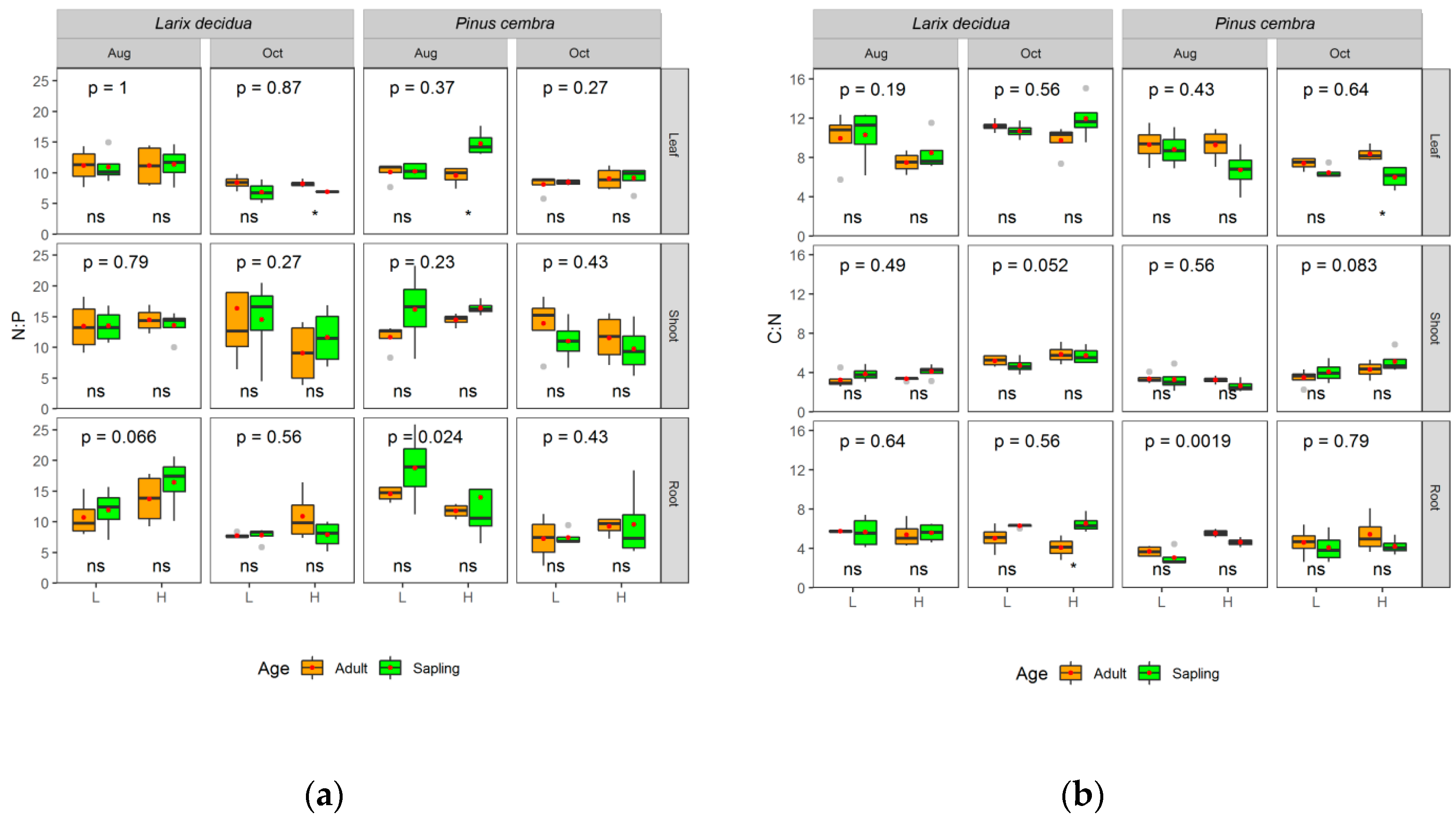
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steffen, W.; Sanderson, R.A.; Tyson, P.D.; Jäger, J.; Matson, P.A.; Moore, B., III; Oldfield, F.; Richardson, K.; Schellnhuber, H.J.; Turner, B.L.; et al. Global Change and the Earth System: A Planet under Pressure; Springer Science & Business Media: Heidelberg, Germany, 2006. [Google Scholar]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Fröberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cook, B.I.; Allen, J.M.; Crimmins, T.M.; Betancourt, J.L.; Travers, S.E.; Pau, S.; Regetz, J.; Davies, T.J.; Kraft, N.J.B.; et al. Warming experiments underpredict plant phenological responses to climate change. Nature 2012, 485, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Steppe, K.; Niinemets, Ü.; Teskey, R.O. Tree size-and age-related changes in leaf physiology and their influence on carbon gain. In Size-and Age-Related Changes in Tree Structure and Function; Springer: New York, NY, USA, 2011; pp. 235–253. [Google Scholar]
- Portsmuth, A.; Niinemets, Ü.; Truus, L.; Pensa, M. Biomass allocation and growth rates in Pinus sylvestris are interactively modified by nitrogen and phosphorus availabilities and by tree size and age. Can. J. For. Res. 2005, 35, 2346–2359. [Google Scholar] [CrossRef]
- Gedroc, J.J.; McConnaughay, K.D.M.; Coleman, J.S. Plasticity in Root/Shoot Partitioning: Optimal, Ontogenetic, or Both? Funct. Ecol. 1996, 10, 44–50. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Sardans, J.; Pérez-Trujillo, M.; Estiarte, M.; Peñuelas, J. Strong relationship between elemental stoichiometry and metabolome in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 4181–4186. [Google Scholar] [CrossRef]
- Álvarez-Yépiz, J.C.; Cueva, A.; Dovčiak, M.; Teece, M.; Yepez, E.A. Ontogenetic resource-use strategies in a rare long-lived cycad along environmental gradients. Conserv. Physiol. 2014, 2, cou034. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Bazzaz, F.A. Changes in drought response strategies with ontogeny in Quercus rubra: Implications for scaling from seedlings to mature trees. Oecologia 2000, 124, 8–18. [Google Scholar] [CrossRef]
- Bansal, S.; Germino, M.J. Variation in ecophysiological properties among conifers at an ecotonal boundary: Comparison of establishing seedlings and established adults at timberline. J. Veg. Sci. 2010, 21, 133–142. [Google Scholar] [CrossRef]
- Körner, C. The nutritional status of plants from high altitudes. Oecologia 1989, 81, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Li, M.-H.; Xiao, W.-F.; Wang, S.-G.; Cheng, G.-W.; Cherubini, P.; Cai, X.-H.; Liu, X.-L.; Wang, X.-D.; Zhu, W.-Z. Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Marqués, L.; Camarero, J.J.; Gazol, A.; Zavala, M.A. Drought impacts on tree growth of two pine species along an altitudinal gradient and their use as early-warning signals of potential shifts in tree species distributions. Forest Ecol. Manag. 2016, 381, 157–167. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef]
- Peng, G.; Wu, C.; Xu, X.; Yang, D. The age-related changes of leaf structure and biochemistry in juvenile and mature subalpine fir trees (Abies faxoniana Rehder & E.H. Wilson.) along an altitudinal gradient. Pol. J. Ecol. 2012, 60, 311–321. [Google Scholar]
- Zhao, C.; Chen, L.; Ma, F.; Yao, B.; Liu, J. Altitudinal differences in the leaf fitness of juvenile and mature alpine spruce trees (Picea crassifolia). Tree Physiol. 2008, 28, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Körner, C.; Hoch, G. A test of the growth-limitation theory for alpine tree line formation in evergreen and deciduous taxa of the eastern Himalayas. Funct. Ecol. 2008, 22, 213–220. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F.I.; Pfund, L.; Körner, C.; Hoch, G. Variation of mobile carbon reserves in trees at the alpine treeline ecotone is under environmental control. New Phytol. 2012, 195, 794–802. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F.I.; Hoch, G. Similar variation in carbon storage between deciduous and evergreen treeline species across elevational gradients. Ann. Bot. 2013, 112, 623–631. [Google Scholar] [CrossRef]
- Rabasa, S.G.; Granda, E.; Benavides, R.; Kunstler, G.; Espelta, J.M.; Ogaya, R.; Peñuelas, J.; Scherer-Lorenzen, M.; Gil, W.; Grodzki, W.; et al. Disparity in elevational shifts of European trees in response to recent climate warming. Glob. Change Biol. 2013, 19, 2490–2499. [Google Scholar] [CrossRef]
- Máliš, F.; Kopecký, M.; Petřík, P.; Vladovič, J.; Merganič, J.; Vida, T. Life stage, not climate change, explains observed tree range shifts. Glob. Chang. Biol. 2016, 22, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.M.; Bradford, J.B.; Lauenroth, W.K. Early indicators of change: Divergent climate envelopes between tree life stages imply range shifts in the western United States. Glob. Ecol. Biogeogr. 2014, 23, 168–180. [Google Scholar] [CrossRef]
- Bansal, S.; Germino, M.J. Temporal variation of nonstructural carbohydrates in montane conifers: Similarities and differences among developmental stages, species and environmental conditions. Tree Physiol. 2009, 29, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Baber, O.; Slot, M.; Celis, G.; Kitajima, K. Diel patterns of leaf carbohydrate concentrations differ between seedlings and mature trees of two sympatric oak species. Botany 2014, 92, 535–540. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. Growth and carbon relations of tree line forming conifers at constant vs. variable low temperatures. J. Ecol. 2009, 97, 57–66. [Google Scholar] [CrossRef]
- Gruber, A.; Pirkebner, D.; Oberhuber, W. Seasonal dynamics of mobile carbohydrate pools in phloem and xylem of two alpine timberline conifers. Tree Physiol. 2013, 33, 1076–1083. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Cao, M.; Wang, S.G.; Xiao, W.F.; Li, M.H. Seasonal dynamics of mobile carbon supply in Quercus aquifolioides at the upper elevational limit. PLoS ONE 2012, 7, e34213. [Google Scholar] [CrossRef][Green Version]
- Li, M.H.; Jiang, Y.; Wang, A.; Li, X.; Zhu, W.; Yan, C.F.; Du, Z.; Shi, Z.; Lei, J.; Schönbeck, L. Active summer carbon storage for winter persistence in trees at the cold alpine treeline. Tree Physiol. 2018. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. The carbon charging of pines at the climatic treeline: A global comparison. Oecologia 2003, 135, 10–21. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Xiang, J.S.; Wang, S.G.; Li, M.H. Resprouting ability and mobile carbohydrate reserves in an oak shrubland decline with increasing elevation on the eastern edge of the Qinghai-Tibet Plateau. For. Ecol. Manag. 2012, 278, 118–126. [Google Scholar] [CrossRef]
- Li, M.H.; Hoch, G.; Körner, C. Spatial variability of mobile carbohydrates within Pinus cembra trees at the alpine treeline. Phyton-Ann. Rei. Bot. A 2001, 41, 203–213. [Google Scholar]
- Seifter, S.; Dayton, S.; Novic, B.; Muntwyler, E. The Estimation of Glycogen with the Anthrone Reagent. Arch. Biochem. 1950, 25, 191–200. [Google Scholar] [PubMed]
- Osaki, M.; Shinano, T.; Tadano, T. Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil Sci. Plant Nutr. 1991, 37, 117–128. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis. Part 2. Chemical and microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Gebauer, G.; Schulze, E.-D. Carbon and nitrogen isotope ratios in different compartments of a healthy and a declining Picea abies forest in the Fichtelgebirge, NE Bavaria. Oecologia 1991, 87, 198–207. [Google Scholar] [CrossRef]
- Remy, E.; Wuyts, K.; Boeckx, P.; Ginzburg, S.; Gundersen, P.; Demey, A.; Van Den Bulcke, J.; Van Acker, J.; Verheyen, K. Strong gradients in nitrogen and carbon stocks at temperate forest edges. For. Ecol. Manag. 2016, 376, 45–58. [Google Scholar] [CrossRef]
- Mayor, J.R.; Sanders, N.J.; Classen, A.T.; Bardgett, R.D.; Clément, J.-C.; Fajardo, A.; Lavorel, S.; Sundqvist, M.K.; Bahn, M.; Chisholm, C.; et al. Elevation alters ecosystem properties across temperate treelines globally. Nature 2017, 542, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Millard, P.; Grelet, G.-a. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef]
- Piper, F.I.; Fajardo, A. Foliar habit, tolerance to defoliation and their link to carbon and nitrogen storage. J. Ecol. 2014, 102, 1101–1111. [Google Scholar] [CrossRef]
- Pardo, L.H.; Semaoune, P.; Schaberg, P.G.; Eagar, C.; Sebilo, M. Patterns in δ15N in roots, stems, and leaves of sugar maple and American beech seedlings, saplings, and mature trees. Biogeochemistry 2013, 112, 275–291. [Google Scholar] [CrossRef][Green Version]
- Reich, A.; Ewel, J.; Nadkarni, N.; Dawson, T.; Evans, R.D. Nitrogen isotope ratios shift with plant size in tropical bromeliads. Oecologia 2003, 137, 587–590. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Yan, E.-R.; Choi, W.-J.; Salifu, F.; Tan, X.; Chen, Z.C.; Zeng, D.-H.; Chang, S. Soil nitrification and foliar δ15N declined with stand age in trembling aspen and jack pine forests in northern Alberta, Canada. Plant Soil 2014, 376, 399–409. [Google Scholar] [CrossRef]
- Hiltbrunner, E.; Schwikowski, M.; Körner, C. Inorganic nitrogen storage in alpine snow pack in the Central Alps (Switzerland). Atmos. Environ. 2005, 39, 2249–2259. [Google Scholar] [CrossRef]
- Chen, L.; Flynn, D.F.B.; Zhang, X.; Gao, X.; Lin, L.; Luo, J.; Zhao, C. Divergent patterns of foliar δ13C and δ15N in Quercus aquifolioides with an altitudinal transect on the Tibetan Plateau: An integrated study based on multiple key leaf functional traits. J. Plant Ecol. 2015, 8, 303–312. [Google Scholar] [CrossRef]
- Bai, E.; Boutton, T.; Liu, F.; Wu, X.B.; Archer, S.; Hallmark, C.T. Spatial variation of the stable nitrogen isotope ratio of woody plants along a topoedaphic gradient in a subtropical savanna. Oecologia 2009, 159, 493–503. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Li, J.; Wang, Q. Nitrogen isotope composition characteristics of modern plants and their variations along an altitudinal gradient in Dongling Mountain in Beijing. Sci. China Ser. D-Earth Sci. 2010, 53, 128–140. [Google Scholar] [CrossRef]
- Garten, C.T., Jr.; Miegroet, H.V. Relationships between soil nitrogen dynamics and natural 15N abundance in plant foliage from Great Smoky Mountains National Park. Can. J. Forest Res. 1994, 24, 1636–1645. [Google Scholar] [CrossRef]
- Chen, Y.; Han, W.; Tang, L.; Tang, Z.; Fang, J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 2013, 36, 178–184. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.-P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Sah, S.; Brumme, R. Altitudinal gradients of natural abundance of stable isotopes of nitrogen and carbon in the needles and soil of a pine forest in Nepal. J. Forest Sci. 2003, 49, 19–26. [Google Scholar] [CrossRef]
- Huber, E.; Wanek, W.; Gottfried, M.; Pauli, H.; Schweiger, P.; Arndt, S.; Reiter, K.; Richter, A. Shift in soil–plant nitrogen dynamics of an alpine–nival ecotone. Plant Soil 2007, 301, 65–76. [Google Scholar] [CrossRef]
- Averill, C.; Finzi, A. Increasing plant use of organic nitrogen with elevation is reflected in nitrogen uptake rates and ecosystem δ15N. Ecology 2010, 92, 883–891. [Google Scholar] [CrossRef]
- Keller, G. Utilization of inorganic and organic nitrogen sources by high-subalpine ectomycorrhizal fungi of Pinus cembra in pure culture. Mycol. Res. 1996, 100, 989–998. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Metcalfe, D.B.; Inselsbacher, E.; Stangl, Z.; Oren, R.; Näsholm, T.; Högberg, P. Greater carbon allocation to mycorrhizal fungi reduces tree nitrogen uptake in a boreal forest. Ecology 2016, 97, 1012–1022. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-F.; Jiang, Z.-P.; Schaub, M.; Gessler, A.; Ni, Y.-Y.; Xiao, W.-F.; Li, M.-H. No Ontogenetic Shifts in C-, N- and P-Allocation for Two Distinct Tree Species along Elevational Gradients in the Swiss Alps. Forests 2019, 10, 394. https://doi.org/10.3390/f10050394
Liu J-F, Jiang Z-P, Schaub M, Gessler A, Ni Y-Y, Xiao W-F, Li M-H. No Ontogenetic Shifts in C-, N- and P-Allocation for Two Distinct Tree Species along Elevational Gradients in the Swiss Alps. Forests. 2019; 10(5):394. https://doi.org/10.3390/f10050394
Chicago/Turabian StyleLiu, Jian-Feng, Ze-Ping Jiang, Marcus Schaub, Arthur Gessler, Yan-Yan Ni, Wen-Fa Xiao, and Mai-He Li. 2019. "No Ontogenetic Shifts in C-, N- and P-Allocation for Two Distinct Tree Species along Elevational Gradients in the Swiss Alps" Forests 10, no. 5: 394. https://doi.org/10.3390/f10050394
APA StyleLiu, J.-F., Jiang, Z.-P., Schaub, M., Gessler, A., Ni, Y.-Y., Xiao, W.-F., & Li, M.-H. (2019). No Ontogenetic Shifts in C-, N- and P-Allocation for Two Distinct Tree Species along Elevational Gradients in the Swiss Alps. Forests, 10(5), 394. https://doi.org/10.3390/f10050394




