Heterodichogamy, Pollen Viability, and Seed Set in a Population of Polyploidy Cyclocarya Paliurus (Batal) Iljinskaja (Juglandaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Plantation of C. paliurus
2.3. Investigation of Flowering Phenology
2.4. Pollen Dispersal
2.5. Seed Collection
3. Results
3.1. Sex Expression of the Juvenile Population
3.1.1. Sexual Diversity
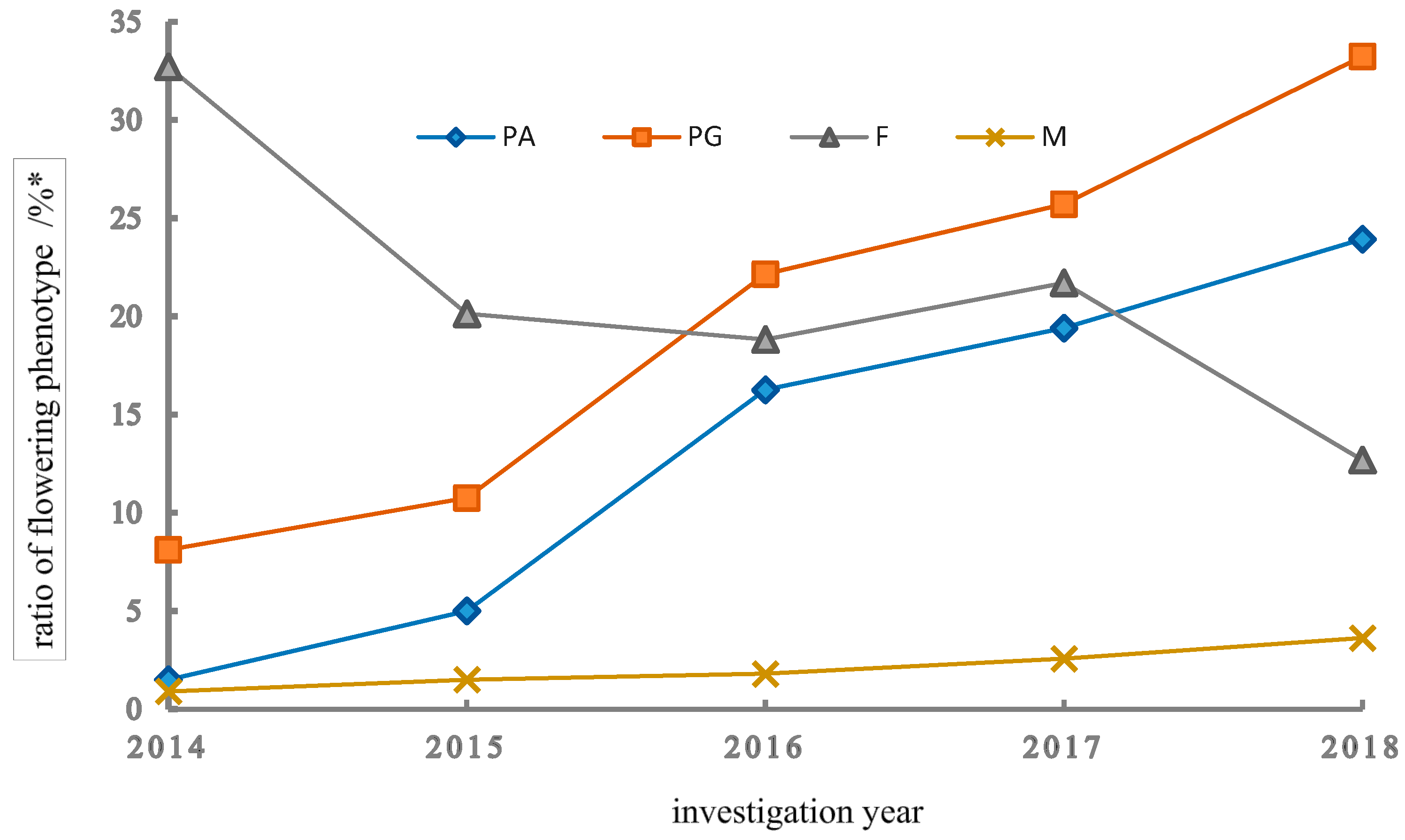
3.1.2. Expression Features of Various Flowering Phenotypes
3.1.3. Changing Patterns of Mating Types across Years
3.1.4. Reciprocal Transitions between Mating Types
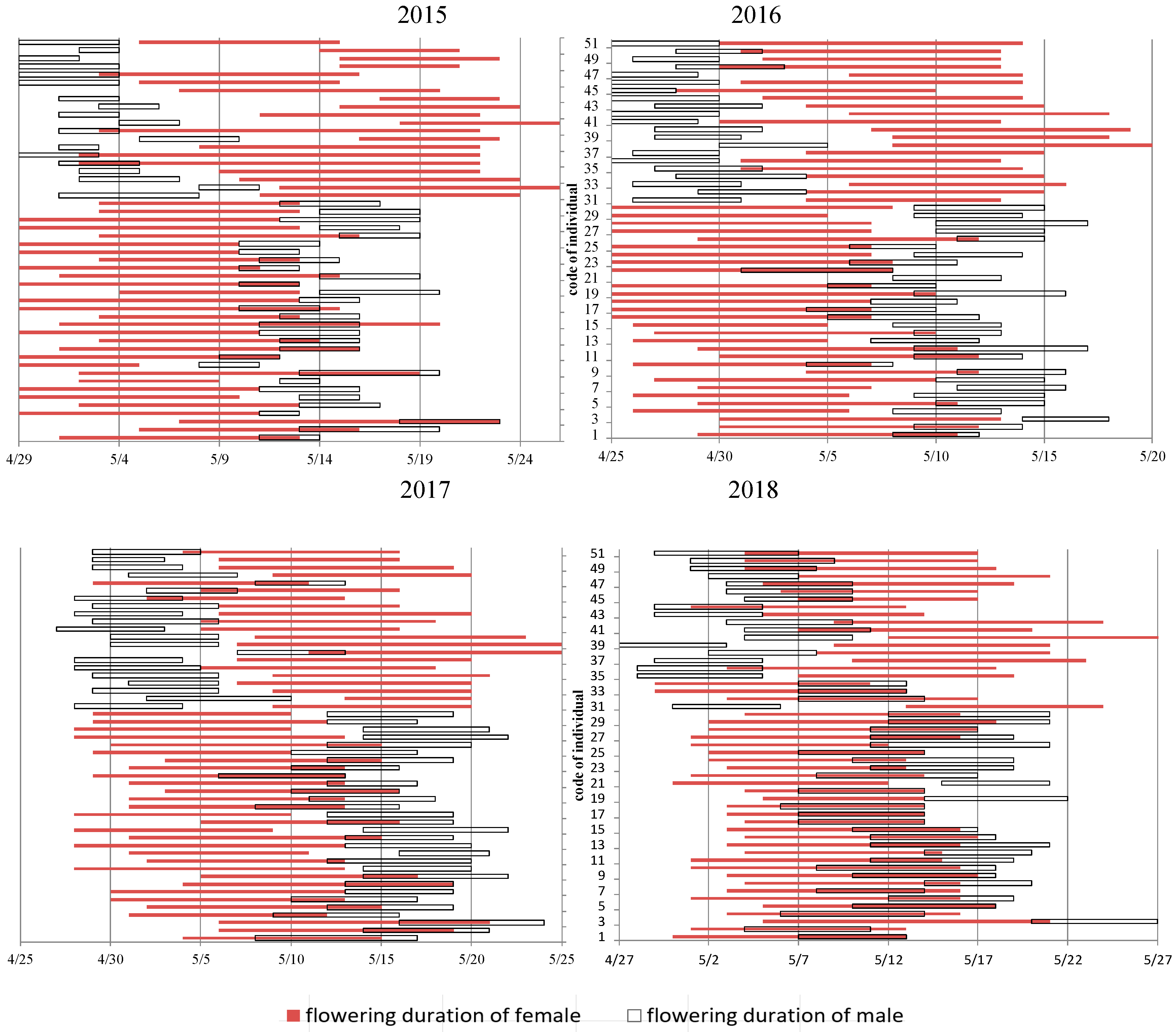
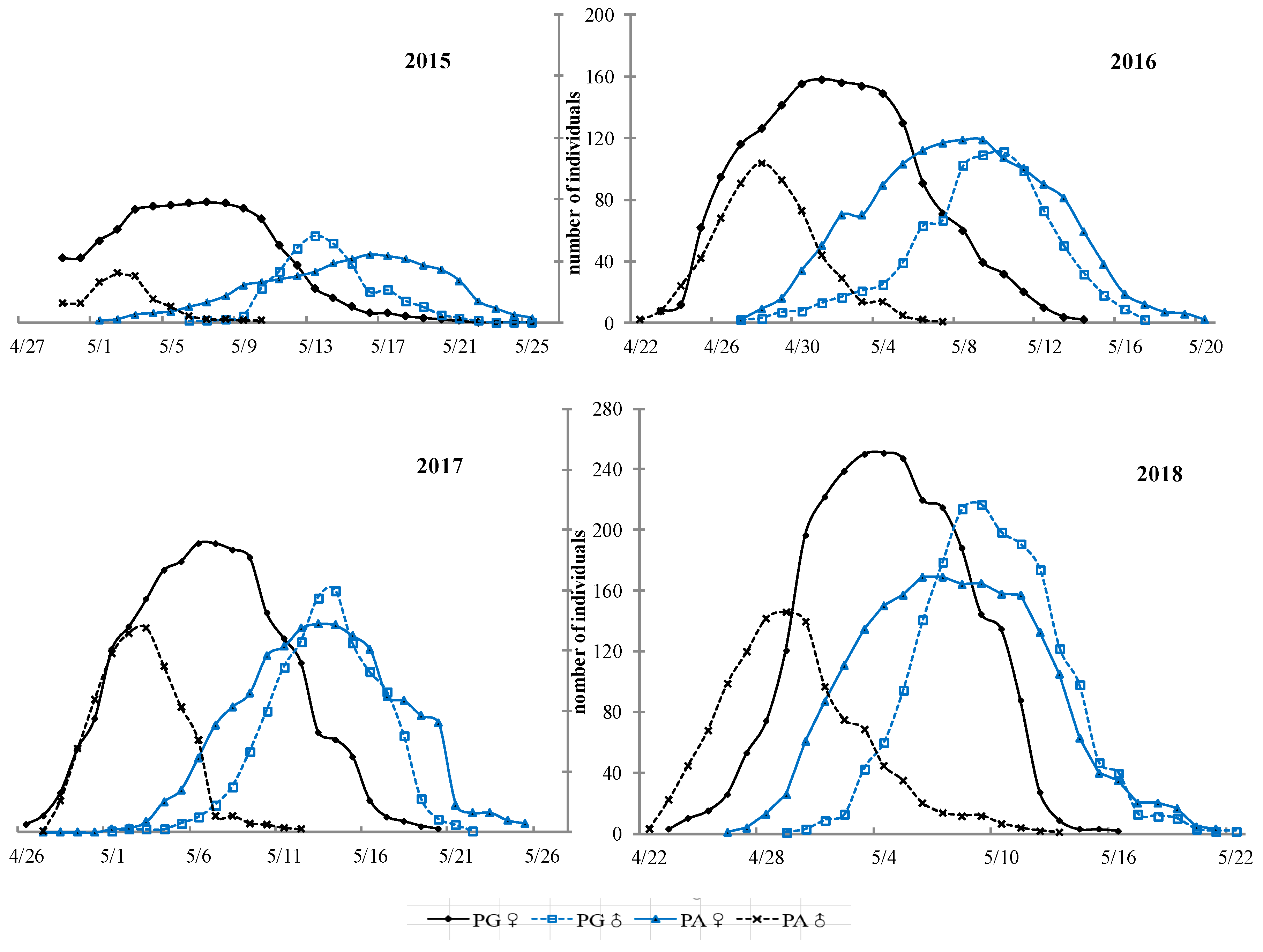
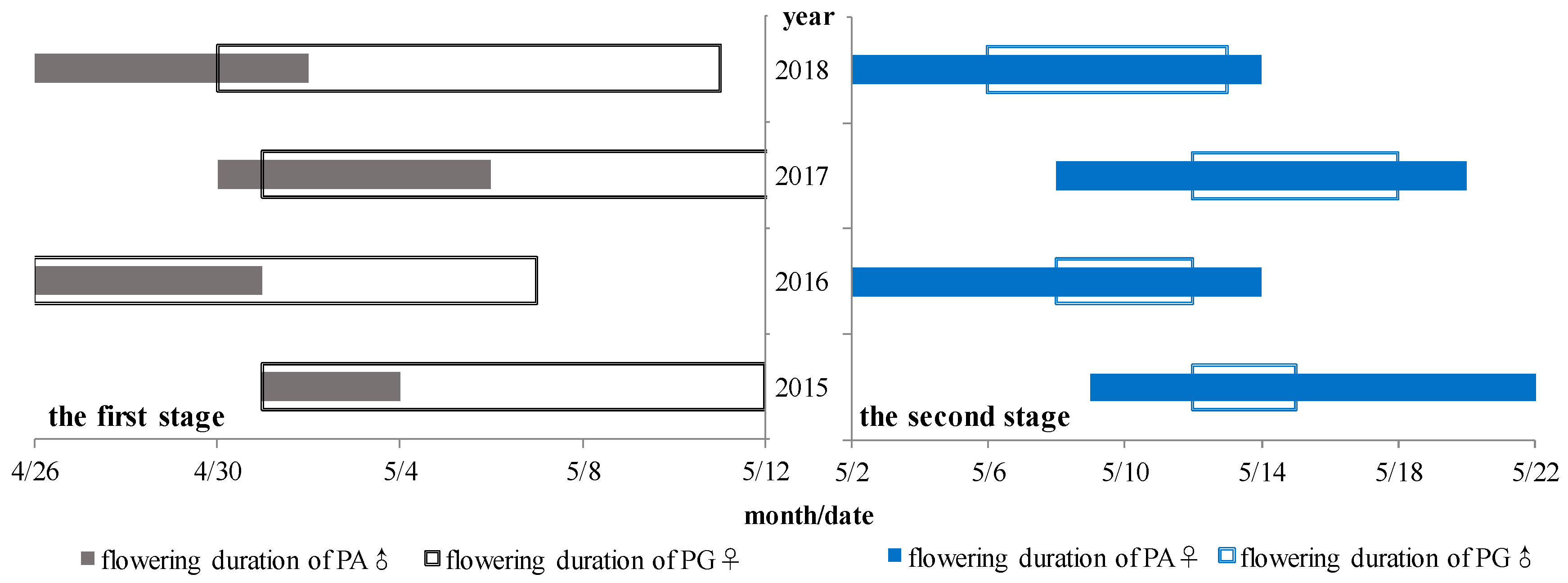
3.2. Flowering Phenology of PG and PA Types across Years
3.3. Phenological Characteristics of the Population
3.4. Pollen Dispersal Characteristics of the Population
4. Discussion
4.1. Sexual Polymorphism and Bias Associated with Nutrient Conditions and Climatic Factors
4.2. Morph Ratio and Transition
4.3. Flowering Phenology of the Individual and Population
4.4. Reasons for Low Seed Success in C. paliurus
4.5. Polyploid C. Paliurus Relating to Characters of Flowering and Seed Success
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Renner, S.S. How common is heterodichogamy? Trends Ecol. Evol 2002, 16, 595–597. [Google Scholar] [CrossRef]
- Barrett, S.C. The evolution of plant sexual diversity. Nat. Rev. Genet. 2002, 3, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Lloyd, D.G. The avoidance of interference between the presentation of pollen and stigmas in angiosperms II. Herkogamy. N. Z. J. Bot. 1986, 24, 16. [Google Scholar] [CrossRef]
- Bai, W.N.; Zeng, Y.F.; Liao, W.J.; Zhang, D.Y. Flowering phenology and wind-pollination efficacy of heterodichogamous Juglans mandshurica (Juglandaceae). Ann. Bot. 2006, 98, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Partridge, L.; Robertson, A. Frequency-Dependent Selection; Cambridge University Press: New York, NY, USA, 1988. [Google Scholar]
- Gleeson, S.K. Heterodichogamy in walnuts: Inheritance and s ratios. Evolution 1982, 36, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.E.; Romberg, L.D. Inheritance of heterodichogamy in pecan. Heredity 1985, 76, 456–458. [Google Scholar] [CrossRef]
- Liu, J.J.; Mao, X.; Li, X.C. A review on flowering mechanism in heterodichogamous plants. J. Nanjing For. Univ. 2016, 40, 147–154. (In Chinese) [Google Scholar] [CrossRef]
- Fukuhara, T.; Tokumaru, S. Inflorescence dimorphism, heterodichogamy and thrips pollination in Platycarya strobilacea (Juglandaceae). Ann. Bot. 2014, 113, 467–476. [Google Scholar] [CrossRef]
- Mao, X.; Liu, J.J.; Li, X.C.; Qin, J.; Fu, X.X. Flowering biological characteristics and mating system in immature plantations of heterodichogamous Cyclocarya paliurus. J. Nanjing For. Univ. 2016, 40, 47–55. (In Chinese) [Google Scholar] [CrossRef]
- Bai, W.N.; Zeng, Y.F.; Zhang, D.Y. Mating patterns and pollen dispersal in a heterodichogamous tree, Juglans mandshurica (Juglandaceae). New Phytol. 2007, 176, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.G.; Xu, C.R.; Li, L.N.; Zhi, L.Y. Cyclocariosides II and III: Two secodammarane triterpenoid saponins from Cyclocarya paliurus. Planta Med. 1995, 61, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.Y.; Wang, Y.X.; Wen, H.L.; Yi, X. Determination of flavonoid compounds and vitamins in the leaves of Cyclocarya paliurus (Batal.) Iljinsk. Food Sci. 2001, 22, 66–68. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Z.F.; Meng, F.C.; Jiang, C.H.; Zhao, M.G.; Shang, X.L.; Fang, S.Z.; Ye, W.C.; Zhang, Q.W.; Zhang, J.; Yin, Z.Q. Triterpenoids from Cyclocarya paliurus and their inhibitory effect on the secretion of apoliprotein B48 in Caco-2 cells. Phytochemistry 2017, 142, 76–84. [Google Scholar] [CrossRef]
- Yang, H.M.; Yin, Z.Q.; Zhao, M.G.; Jiang, C.H.; Zhang, J.; Pan, K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry 2018, 151, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.L.; Fang, S.Z.; Zhou, M.M.; Qin, J. Responses of morphology, gas Exchange, photochemical activity of photosystem II, and antioxidant balance in Cyclocarya paliurus to light spectra. Front. Plant Sci. 2018, 23, 1042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qian, C.Y.; Ding, S.H.; Shang, X.L.; Yang, W.X.; Fang, S.Z. Effect of light regime and provenance on leaf characteristics, growth and flavonoid accumulation in Cyclocarya paliurus (Batal) Iljinskaja coppices. Bot. Stud. 2016, 57, 28–41. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.L.; Wang, T.L.; Fang, S.Z. Leaf nitrogen and phosphorus stoichiometry of Cyclocarya paliurus across China. Forests 2018, 9, 771. [Google Scholar] [CrossRef]
- Fang, S.Z.; Wang, J.Y.; Wei, Z.Y.; Zhu, Z.X. Methods to break seed dormancy in Cyclocarya paliurus (Batal). Iljinskaja. Sci. Hortic. 2006, 110, 305–309. [Google Scholar] [CrossRef]
- Li, X.C.; Fu, X.X.; Shang, X.L.; Yang, W.X.; Fang, S.Z. Natural population structure and genetic differentiation for heterodicogamous plant: Cyclocarya paliurus (Batal.) Iljinskaja (Juglandaceae). Tree Genet. Genomes 2017, 13, 80. [Google Scholar] [CrossRef]
- De Jong, P.C. Flowering and Sex Expression in Acer L.: A Biosystematic Study; Mededelingen Land Bouwboge School: Wageningen, The Netherlands, 1976; Volume 76, pp. 1094–1098. [Google Scholar]
- Mao, X.; Li, X.C.; Liu, J.J.; Fu, X.X. Scanning electron microscope observation on pollen morphology of six tree species in Juglandaceae. J. Plant Resour. Environ. 2016, 25, 104–106. (In Chinese) [Google Scholar] [CrossRef]
- Dommée, B.; Bompar, J.L.; Denelle, N. Sexual tetra-morphism in Thymelaea hirsuta (Thymelaeaceae): Evidence of the pathway from heterodichogamy to dioecy at the infra-specific level. Am. J. Bot. 1990, 77, 1449–1462. [Google Scholar] [CrossRef]
- Asai, T. Dichogamy in Full Moon Maple (Acer japonicum Thunb.); Bulletin of the Hokkaido Forestry Research Institute: Tokyo, Japan, 2000; Volume 37, pp. 27–40. [Google Scholar]
- Sato, T. Phenology of sex expression and gender variation in a heterodichogamous maple, Acer japonicum. Ecology 2002, 83, 1226–1238. [Google Scholar] [CrossRef]
- Levy, Y.Y.; Dean, C. The transition to flowering. Plant Cell 1998, 10, 1973–1990. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, P.; Olimpieri, I.; De Simoni, G.; Gras, M.; Malvolti, M. Barriers to interspecific hybridization between Juglans nigra L. and J. regia L. species. Tree Genet. Genomes 2013, 9, 291–305. [Google Scholar] [CrossRef]
- Tal, O. Acer pseudoplatanus (Sapindaceae): Heterodichogamy and thrips pollination. Plant Syst. Evol. 2009, 278, 211–221. [Google Scholar] [CrossRef]
- Kimura, M.; Seiwa, K.; Suyama, Y.; Ueno, N. Flowering system of heterodichogamous Juglans ailanthifolia. Plant Species Biol. 2003, 18, 75–84. [Google Scholar] [CrossRef]
- McCarthy, B.C.; Quinn, J.A. Reproductive ecology of system of two sympatric tree species. Am. J. Bot. 1990, 77, 261–273. [Google Scholar] [CrossRef]
- Knuth, P. Handbook of Flower Pollination; Clarendon Press: Oxford, UK, 1906. [Google Scholar]
- Darwin, C. The Different Forms of Flowers on Plants of the Same Species; John Murray: London, UK, 1877. [Google Scholar]
- Polito, V.S.; Pinney, K. The relationship between phenology of pistillate flower organogenesis and mode of heterodichogamy in Juglans regia L. (Juglandaceae). Sex. Plant Reprod. 1997, 10, 36–39. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.R.; Li, Y. Pecan production in China. Sci. Hortic. 2015, 197, 719–727. [Google Scholar] [CrossRef]
- Solis Neffa, V.G.; Fernández, A. Chromosome studies in Turnera (Turneraceae). Genet. Mol. Biol. 2000, 23, 925–930. [Google Scholar] [CrossRef]

| Flowering Group (FP) | Mating Type | Observed Year | ||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | ||
| Monoecious group (MO) | number of PA | 12 | 40 | 127 | 151 | 185 |
| number of PG | 65 | 86 | 173 | 200 | 257 | |
| PA:PG | 5.42:1 | 2.15:1 | 1.36:1 | 1.32:1 | 1.39:1 | |
| PG/MO (%) | 84.4 | 68.3 | 57.7 | 57.0 | 58.1 | |
| PG/FP (%) | 18.8 | 28.8 | 37.5 | 37.0 | 45.2 | |
| MO/T (%) | 11.1 | 15.8 | 38.4 | 45.1 | 57.2 | |
| Unisexual group (UN) | number of F | 262 | 161 | 147 | 169 | 98 |
| number of M | 7 | 12 | 14 | 20 | 28 | |
| F:M | 37.4:1 | 13.4:1 | 10.5:1 | 8.5:1 | 3.5:1 | |
| F/UN (%) | 97.4 | 93.1 | 91.3 | 89.4 | 77.8 | |
| F/FP (%) | 75.7 | 53.8 | 39.0 | 31.3 | 17.3 | |
| UN/T (%) | 33.6 | 21.6 | 20.6 | 24.3 | 16.3 | |
| FP/T (%) | 43.2 | 37.4 | 59.0 | 69.4 | 73.5 | |
| Total plants in the population (T) * | 801 | 800 | 781 | 778 | 773 | |
| Transition Pattern | Number of Transitions between Phenotypes/Total Number of Transitions (%) | ||||
|---|---|---|---|---|---|
| 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | ||
| UF↔FP | UF→F, F→UF | 53/20.2, 101/38.4 | 91/30.3, 3/1.0 | 85/31.7, 30/11.2 | 38/13.3, 32/11.2 |
| UF→M, M→UF | 5/1.9, 4/1.5 | 9/3.0, -- | 3/1.1, 4/1.5 | 5/1.8, 2/0.7 | |
| UF→PG, PG→UF | 6/2.3, 8/3.0 | 42/14.0, -- | 15/5.6, 3/1.1 | 24/8.4, 10/3.5 | |
| UF→PA, PA→UF | 3/1.1, 1/0.4 | 38/12.7, -- | 11/4.1, 4/1.5 | 23/8.1, 12/4.2 | |
| Subtotal of UF | 181/68.8 | 183/61.0 | 155/57.8 | 146/51.2 | |
| UN↔MO | F→PG, PG→F | 36/13.7, 8/3.0 | 46/15.3, 5/1.7 | 36/13.4, 14/5.2 | 48/16.8, 2/0.7 |
| F→PA, PA→F | 28/10.6, 4/1.5 | 43/14.3, 5/1.7 | 18/6.7, 18/6.7 | 39/13.7, 10/3.5 | |
| M→PG, PG→M | 2/0.8, 2/0.8 | 4/1.3, 2/0.7 | 9/3.4, 3/1.1 | 3/1.1, 11/3.9 | |
| M→PA, PA→M | --, -- | 8/2.7, 1/0.3 | 7/2.6, 1/0.4 | 6/2.1, 2/0.7 | |
| subtotal | 80/30.1 | 114/38.0 | 106/39.6 | 121/42.5 | |
| MO↔MO | PG→PA, PA→PG | --, -- | 1/0.3 | --, -- | 5/1.8, 3/1.1 |
| UN↔UN | F→M, M→F | 1/0.4, 1/0.4 | 2/0.7, -- | 5/1.7, 2/0.7 | 9/3.2, 1/0.4 |
| total | 263 | 300 | 268 | 285 | |
| Flowering Stage | Observation Date (Month/Date) | Pollen Density (Grain/cm2) | Pollen Viability (%) | ||
|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | ||
| First stage | 4/30 | 136 ± 25 | 305 ± 56 | 20.5 ± 3.9 | 18.3 ± 3.9 |
| 5/01 | / | 262 ± 31 | 16.4 ± 4.2 | 27.6 ± 6.5 | |
| 5/03 | 178 ± 34 | / | 6.0 ± 3.2 | 11.0 ± 5.0 | |
| 5/04 | / | 47 ± 12 | / | 6.5 ± 2.6 | |
| 5/06 | 105 ± 12 | / | 13.9 ± 2.9 | / | |
| Second stage | 5/08 | 151 ± 16 | / | 20.2 ± 6.8 | 26.4 ± 7.1 |
| 5/10 | 247 ± 33 | 281 ± 29 | 28.6 ± 4.7 | 31.1 ± 5.9 | |
| 5/12 | 216 ± 26 | / | 20.0 ± 4.4 | 28.8 ± 3.4 | |
| 5/14 | 128 ± 23 | 33 ± 7 | 20.3 ± 5.7 | 13.9 ± 4.3 | |
| 5/16 | 23 ± 5 | 9 ± 4 | 5.6 ± 5.6 | 16.6 ± 12.6 | |
| 5/18 | 12 ± 5 | 0 | 0 | 0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Fu, X.-X.; Huang, P.; Chen, X.-L.; Qu, Y.-Q. Heterodichogamy, Pollen Viability, and Seed Set in a Population of Polyploidy Cyclocarya Paliurus (Batal) Iljinskaja (Juglandaceae). Forests 2019, 10, 347. https://doi.org/10.3390/f10040347
Mao X, Fu X-X, Huang P, Chen X-L, Qu Y-Q. Heterodichogamy, Pollen Viability, and Seed Set in a Population of Polyploidy Cyclocarya Paliurus (Batal) Iljinskaja (Juglandaceae). Forests. 2019; 10(4):347. https://doi.org/10.3390/f10040347
Chicago/Turabian StyleMao, Xia, Xiang-Xiang Fu, Peng Huang, Xiao-Ling Chen, and Yin-Quan Qu. 2019. "Heterodichogamy, Pollen Viability, and Seed Set in a Population of Polyploidy Cyclocarya Paliurus (Batal) Iljinskaja (Juglandaceae)" Forests 10, no. 4: 347. https://doi.org/10.3390/f10040347
APA StyleMao, X., Fu, X.-X., Huang, P., Chen, X.-L., & Qu, Y.-Q. (2019). Heterodichogamy, Pollen Viability, and Seed Set in a Population of Polyploidy Cyclocarya Paliurus (Batal) Iljinskaja (Juglandaceae). Forests, 10(4), 347. https://doi.org/10.3390/f10040347




