Hydraulic and Photosynthetic Traits Vary with Successional Status of Woody Plants on the Loess Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Species Selection and Sampling
2.3. Economic Traits
2.4. Hydraulic Traits
2.5. Statistical Analysis
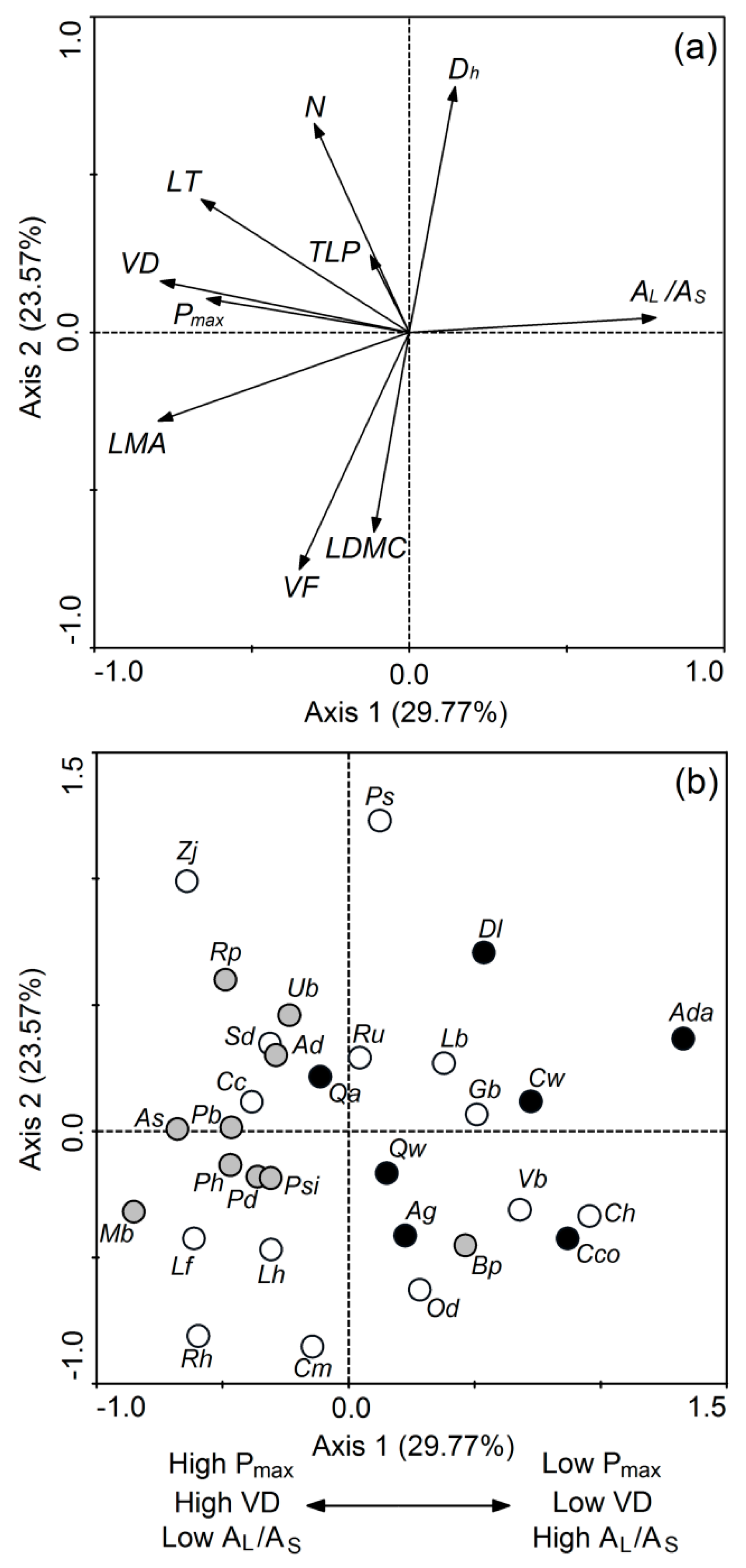
3. Results
4. Discussion
4.1. Differences of Economic and Hydraulic Traits among Different Successional Stages
4.2. Strategies of Different Successional Stages: Growth vs. Survival
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Global Forest Resources Assessment: How Are the World’s Forests Changing? Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Chai, Y.; Yue, M.; Wang, M.; Xu, J.; Liu, X.; Zhang, R.; Wan, P. Plant functional traits suggest a change in novel ecological strategies for dominant species in the stages of forest succession. Oecologia 2016, 180, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Uriarte, M.; Aide, T.M.; Erickson, D.L.; Forero-Montaña, J.; Kress, W.J.; Swenson, N.G.; Zimmerman, J.K. Functional convergence and phylogenetic divergence during secondary succession of subtropical wet forests in Puerto Rico. J. Veg. Sci. 2016, 27, 283–294. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R. Functional identity of overstorey tree height and understorey conservative traits drive aboveground biomass in a subtropical forest. Ecol. Indic. 2017, 83, 158–168. [Google Scholar] [CrossRef]
- Meiners, S.J.; Cadotte, M.W.; Fridley, J.D.; Pickett, S.T.A.; Walker, L.R. Is successional research nearing its climax? New approaches for understanding dynamic communities. Funct. Ecol. 2015, 29, 154–164. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Lavorel, S.; Landsberg, J.; Forbes, T.M.S. Plant functional classifications: From general groups to specific groups based on response to disturbance. Trends Ecol. Evol. 1997, 12, 474–478. [Google Scholar] [CrossRef]
- Targetti, S.; Messeri, A.; Staglianò, N.; Argenti, G. Leaf functional traits for the assessment of succession following management in semi-natural grasslands: A case study in the North Apennines, Italy. Appl. Veg. Sci. 2013, 16, 325–332. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavenderbares, J.; Chapin, F.S.; Cornelissen, J.H.C.; Diemer, M. World-wide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; McCormack, M.L.; Ma, C.; Kong, D.; Zhang, Q.; Chen, X.; Zeng, H.; Niinemets, Ü.; Guo, D. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett. 2015, 18, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Coley, P.D.; Bryant, J.P. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar]
- Mcgill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New. Phytol. 2009, 181, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, M.; Lasky, J.R.; Boukili, V.K.; Chazdon, R.L. A trait-mediated, neighbourhood approach to quantify climate impacts on successional dynamics of tropical rainforests. Funct. Ecol. 2016, 30, 157–167. [Google Scholar] [CrossRef]
- Kazakou, E.; Garnier, E.; Navas, M.L.; Roumet, C.; Collin, C.; Laurent, G. Components of nutrient residence time and the leaf economics spectrum in species from Mediterranean old-fields differing in successional status. Funct. Ecol. 2007, 21, 235–245. [Google Scholar] [CrossRef]
- Navas, M.L.; Ducout, B.; Roumet, C.; Richarte, J.; Garnier, J.; Garnier, E. Leaf life span, dynamics and construction cost of species from Mediterranean old-fields differing in successional status. New Phytol. 2003, 159, 213–228. [Google Scholar] [CrossRef]
- Zhu, S.D.; Song, J.J.; Li, R.H.; Ye, Q. Plant hydraulics and photosynthesis of 34 woody species from different successional stages of subtropical forests. Plant Cell Environ. 2013, 36, 879–891. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, X.; Yue, M.; Guo, J.; Wang, M.; Wan, P.; Zhang, X.; Zhang, C. Leaf traits in dominant species from different secondary successional stages of deciduous forest on the Loess Plateau of northern China. Appl. Veg. Sci. 2015, 18, 50–63. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Feild, T.S. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 2010, 13, 175–183. [Google Scholar] [CrossRef]
- Cosme, L.H.; Schietti, J.; Costa, F.R.; Oliveira, R.S. The importance of hydraulic architecture to the distribution patterns of trees in a central Amazonian forest. New Phytol. 2017, 215, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Luglio, J. Leaf hydraulic capacity and drought vulnerability: Possible trade-offs and correlations with climate across three major biomes. Funct. Ecol. 2014, 28, 810–818. [Google Scholar] [CrossRef]
- Oliveira, R.S. Can hydraulic traits be used to predict sensitivity of drought-prone forests to crown decline and tree mortality? Plant Soil 2013, 364, 1–3. [Google Scholar] [CrossRef]
- Zhu, S.D.; Chen, Y.J.; Fu, P.L.; Cao, K.F.; Goldstein, G. Different hydraulic traits of woody plants from tropical forests with contrasting soil water availability. Tree Physiol. 2017, 37, 1469–1477. [Google Scholar] [CrossRef]
- Mcculloh, K.A.; Meinzer, F.C.; Sperry, J.S.; Lachenbruch, B.; Voelker, S.L.; Woodruff, D.R.; Domec, J.C. Comparative hydraulic architecture of tropical tree species representing a range of successional stages and wood density. Oecologia 2011, 167, 27–37. [Google Scholar] [CrossRef]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, L.; Lei, M.; Dang, H.; Quan, J.; Tian, T.; Chai, Y.; Yue, M. The relationships between leaf economics and hydraulic traits of woody plants depend on water availability. Sci. Total Environ. 2018, 621, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Jiao, J.; Yin, Q.; Wang, N.; Wang, Z.; Li, Y.; Yu, W.; Wei, Y.; Yan, F.; Cao, B. Successional trajectory over 10 years of vegetation restoration of abandoned slope croplands in the Hill-Gully Region of the Loess Plateau. Land Degrad. Dev. 2016, 27, 919–932. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.D.; Chen, Y.J.; Ye, Q.; He, P.C.; Liu, H.; Li, R.H.; Fu, P.L.; Jiang, G.F.; Cao, K.F. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Cochard, H.; Mencuccini, M.; Sterck, F.; Herrero, A.; Korhonen, J.F.J.; Llorens, P.; Nikinmaa, E.; Nolè, A.; Poyatos, R.; et al. Hydraulic adjustment of Scots pine across Europe. New Phytol. 2009, 184, 353–364. [Google Scholar] [CrossRef]
- Pratt, R.B.; Jacobsen, A.L. Conflicting demands on angiosperm xylem: Tradeoffs among storage, transport and biomechanics. Plant Cell Environ. 2017, 40, 897–913. [Google Scholar] [CrossRef]
- Chai, Y.; Yue, M.; Liu, X.; Guo, Y.; Wang, M.; Xu, J.; Zhang, C.; Chen, Y.; Zhang, L.; Zhang, R. Patterns of taxonomic, phylogenetic diversity during a long-term succession of forest on the Loess Plateau, China: Insights into assembly process. Sci. Rep. 2016, 6, 27087. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.C. Recovering succession of vegetation in forest region of north Shaanxi Loess Plateau. J. Northwest For. Univ. 1993, 8, 87–94. [Google Scholar]
- Yue, M. Division of successional phase in the Platycladus orientalis forest in the South Loess Plateau of the Northern Shanxi Province. Acta Phytoecol. Sinica 1998, 22, 327–335. [Google Scholar]
- Wang, S.; Wang, X.; Hua, G.; Fan, W.; Lv, H.; Duan, R. Distinguishing the importance between habitat specialization and dispersal limitation on species turnover. Ecol. Evol. 2013, 3, 3545–3553. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Ye, Z.P. A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 2007, 45, 637–640. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Jordan, G.J. Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol. 2011, 192, 437–448. [Google Scholar] [CrossRef]
- Schulte, P.J.; Hinckley, T.M. A comparison of pressure-volume curve data analysis techniques. J. Exp. Bot. 1985, 36, 1590–1602. [Google Scholar] [CrossRef]
- Anfodillo, T.; Carraro, V.; Carrer, M.; Fior, C.; Rossi, S. Convergent tapering of xylem conduits in different woody species. New Phytol. 2006, 169, 279–290. [Google Scholar] [CrossRef]
- Carrer, M.; von Arx, G.; Castagneri, D.; Petit, G. Distilling allometric and environmental information from time series of conduit size: The standardization issue and its relationship to tree hydraulic architecture. Tree Physiol. 2015, 35, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Zimmermann, M.H. Hydraulic architecture of whole plants and plant performance. In Xylem Structure and the Ascent of Sap; Springer: Berlin/Heidelberg, Germany, 2002; pp. 175–214. [Google Scholar]
- Webb, C.O.; Donoghue, M.J. Phylomatic: Tree assembly for applied phylogenetics. Mol. Ecol. Resour. 2005, 5, 181–183. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Zhang, J.; Yang, H.; Xu, L.; Wang, Q.; Sack, L.; Wu, X.; Hou, J.; He, N. Variation in leaf chlorophyll concentration from tropical to cold-temperate forests: Association with gross primary productivity. Ecol. Indic. 2018, 85, 383–389. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Simonin, K.A.; Limm, E.B.; Dawson, T.E. Hydraulic conductance of leaves correlates with leaf lifespan: Implications for lifetime carbon gain. New Phytol. 2012, 193, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Boyce, C.K. Evolution of a unique anatomical precision in angiosperm leaf venation lifts constraints on vascular plant ecology. Proc. R. Soc. B 2014, 281, 20132829. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Gutiérrez, M.V. Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees. Plant Cell Environ. 2002, 25, 1435–1444. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J.; Yu, T.; Peng, S.; Huang, J. Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in Oryza. New Phytol. 2017, 213, 572–583. [Google Scholar] [CrossRef]
- Sack, L.; Frole, K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 2006, 87, 483–491. [Google Scholar] [CrossRef]
- Thomas, N.B.; Grace, P.J.; Scoffoni, C.; Sack, L. How does leaf anatomy influence water transport outside the xylem? Plant Physiol. 2015, 168, 1616–1635. [Google Scholar]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of Leaf Hydraulic Conductance with Dehydration: Relationship to Leaf Size and Venation Architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef]
- Ishida, A.; Nakano, T.; Yazaki, K.; Matsuki, S.; Koike, N.; Lauenstein, D.; Shimizu, M.; Yamashita, N. Coordination between leaf and stem traits related to leaf carbon gain and hydraulics across 32 drought-tolerant angiosperms. Oecologia 2008, 156, 193–202. [Google Scholar] [CrossRef]
- Khansaritoreh, E.; Schuldt, B.; Dulamsuren, C. Hydraulic traits and tree-ring width in Larix sibirica Ledeb. as affected by summer drought and forest fragmentation in the Mongolian forest steppe. Ann. For. Sci. 2018, 75, 30. [Google Scholar] [CrossRef]
- Gould, P.J.; Harrington, C.A. Extending sapwood-Leaf area relationships from stems to roots in Coast Douglas-fir. Ann. For. Sci. 2008, 65, 802. [Google Scholar] [CrossRef]
- Simonin, K.; Kolb, T.E.; Montes-Helu, M.; Koch, G.W. Restoration thinning and influence of tree size and leaf area to sapwood area ratio on water relations of Pinus ponderosa. Tree Physiol. 2006, 26, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Togashi, H.F.; Prentice, I.C.; Evans, B.J.; Forrester, D.I.; Drake, P.; Feikema, P.; Brooksbank, K.; Eamus, D.; Taylor, D. Morphological and moisture availability controls of the leaf area-to-sapwood area ratio: Analysis of measurements on Australian trees. Ecol. Evol. 2015, 5, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Sterck, F.J.; Poorter, L.; Schieving, F. Leaf traits determine the growth-survival trade-off across rain forest tree species. Am. Nat. 2006, 167, 758–765. [Google Scholar]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S.; et al. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Zwieniecki, M.A.; Holbrook, N.M. Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiol. Plantarum. 2008, 132, 446–451. [Google Scholar] [CrossRef] [PubMed]

| Successional Stages | Latin Name | Code | Family Name | Plant Functional Type | Number of Sampled Individuals | Mean Canopy (Sampling) Height |
|---|---|---|---|---|---|---|
| Shrub stage | Corylus heterophylla Fisch. | Ch | Betulaceae | shrub | 4 | 0.5 m (0.5 m) |
| Cotinus coggygria Scop. | Cc | Anacardiaceae | shrub | 4 | 1 m (1 m) | |
| Cotoneaster melanocarpus Lodd. | Cm | Rosaceae | shrub | 4 | 2.5 m (2.5 m) | |
| Grewia biloba G. Don | Gb | Tiliaceae | shrub, small tree | 4 | 1.5 m (1.5 m) | |
| Lespedeza bicolor Turcz. | Lb | Fabaceae | shrub | 4 | 1.5 m (1.5 m) | |
| Lonicera fragrantissima Lindl. & J. Paxton | Lf | Caprifoliaceae | shrub | 4 | 0.8 m (0.8 m) | |
| Lonicera hispida Pall. ex Schult. | Lh | Caprifoliaceae | shrub | 4 | 1 m (1 m) | |
| Ostryopsis davidiana Decne. | Od | Betulaceae | shrub | 4 | 1 m (1 m) | |
| Periploca sepium Bunge | Ps | Asclepiadaceae | shrub | 4 | 1.2 m (1.2 m) | |
| Rhamnus utilis Decne. | Ru | Rhamnaceae | shrub, small tree | 4 | 0.8 m (0.8 m) | |
| Rosa hugonis Hemsl. | Rh | Rosaceae | shrub | 4 | 0.6 m (0.6 m) | |
| Sophora davidii (Franch.) Skeels | Sd | Fabaceae | shrub | 4 | 1.6 m (1.6 m) | |
| Viburnum betulifolium Batalin | Vb | Caprifoliaceae | shrub | 4 | 2.3 m (2.3 m) | |
| Ziziphus jujuba Mill. | Zj | Rhamnaceae | shrub, small tree | 4 | 0.4 m (0.4 m) | |
| Pioneer tree stage | Amygdalus davidiana (Carriére) de Vos ex L. Henry | Ad | Rosaceae | tree | 4 | 2.5 m (2.5 m) |
| Armeniaca sibirica (L.) Lam. | As | Rosaceae | shrub, small tree | 4 | 3 m (3 m) | |
| Betula platyphylla Sukaczev | Bp | Betulaceae | tree | 4 | 10 m (8 m) | |
| Malus baccata (L.) Borkh. | Mb | Rosaceae | tree | 4 | 4 m (4 m) | |
| Populus davidiana Dode | Pd | Salicaceae | tree | 3 | 8 m (7 m) | |
| Populus hopeiensis Hu & H.F.Chow | Ph | Salicaceae | tree | 3 | 6 m (5 m) | |
| Populus simonii Carr. | Psi | Salicaceae | tree | 3 | 6 m (5 m) | |
| Pyrus betulifolia Bunge | Pb | Rosaceae | tree | 4 | 5 m (5 m) | |
| Rhus potaninii Maxim. | Rp | Anacardiaceae | tree | 4 | 3 m (3 m) | |
| Ulmus bergmanniana C.K. Schneid. | Ub | Ulmaceae | tree | 4 | 3 m (3 m) | |
| Late successional stage | Acer davidii Franch. | Ada | Aceraceae | tree | 4 | 4.5 m (4.5 m) |
| Acer ginnala Maxim. | Ag | Aceraceae | tree | 4 | 2.5 m (2.5 m) | |
| Carpinus cordata Blume. | Cco | Betulaceae | tree | 4 | 3 m (3 m) | |
| Cornus walteri Wangerin | Cw | Cornaceae | tree | 4 | 3.5 m (3.5 m) | |
| Diospyros lotus L. | Dl | Ebenaceae | tree | 4 | 3 m (3 m) | |
| Quercus aliena Blume | Qa | Fagaceae | tree | 3 | 10 m (8 m) | |
| Quercus wutaishanica Mayr | Qw | Fagaceae | tree | 3 | 9.5 m (8 m) |
| LT | Pmax | LDMC | LMA | N | VD | TLP | AL/AS | VF | Dh | |
|---|---|---|---|---|---|---|---|---|---|---|
| LT | −0.02 | −0.30 | 0.21 | 0.09 | 0.47 * | 0.13 | −0.13 | −0.19 | 0.05 | |
| Pmax | 0.26 | 0.18 | 0.43 * | 0.44 * | 0.40 | −0.14 | −0.28 | 0.20 | 0.18 | |
| LDMC | −0.28 | 0.05 | 0.58 ** | −0.30 | 0.31 | −0.44 * | −0.58 ** | 0.35 | −0.30 | |
| LMA | 0.41 * | 0.31 | 0.28 | −0.06 | 0.56 ** | −0.23 | −0.75 ** | 0.32 | −0.21 | |
| N | 0.33 | 0.41 * | −0.30 | 0.01 | 0.15 | 0.19 | 0.09 | −0.38 | 0.33 | |
| VD | 0.52 ** | 0.55 ** | 0.14 | 0.45 * | 0.30 | −0.16 | −0.59 ** | 0.05 | 0.11 | |
| TLP | 0.13 | 0.00 | −0.32 | −0.01 | 0.17 | −0.04 | 0.13 | 0.00 | 0.03 | |
| AL/AS | −0.47 ** | −0.24 | −0.07 | −0.72 ** | −0.04 | −0.49 ** | −0.23 | −0.14 | 0.07 | |
| VF | −0.06 | 0.31 | 0.25 | 0.34 | −0.32 | 0.09 | 0.01 | −0.20 | −0.79 ** | |
| Dh | 0.17 | 0.03 | −0.28 | −0.23 | 0.26 | 0.08 | 0.00 | 0.07 | −0.73 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Q.; He, J.; Tian, T.; Quan, J.; Zhao, P.; Chai, Y.; Wang, L.; Yue, M. Hydraulic and Photosynthetic Traits Vary with Successional Status of Woody Plants on the Loess Plateau. Forests 2019, 10, 327. https://doi.org/10.3390/f10040327
Yin Q, He J, Tian T, Quan J, Zhao P, Chai Y, Wang L, Yue M. Hydraulic and Photosynthetic Traits Vary with Successional Status of Woody Plants on the Loess Plateau. Forests. 2019; 10(4):327. https://doi.org/10.3390/f10040327
Chicago/Turabian StyleYin, Qiulong, Jingwen He, Tingting Tian, Jiaxin Quan, Peng Zhao, Yongfu Chai, Lei Wang, and Ming Yue. 2019. "Hydraulic and Photosynthetic Traits Vary with Successional Status of Woody Plants on the Loess Plateau" Forests 10, no. 4: 327. https://doi.org/10.3390/f10040327
APA StyleYin, Q., He, J., Tian, T., Quan, J., Zhao, P., Chai, Y., Wang, L., & Yue, M. (2019). Hydraulic and Photosynthetic Traits Vary with Successional Status of Woody Plants on the Loess Plateau. Forests, 10(4), 327. https://doi.org/10.3390/f10040327





