Identification of Key Genes in the Synthesis Pathway of Volatile Terpenoids in Fruit of Zanthoxylum bungeanum Maxim
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Volatile Organic Compounds Analysis by HS-SPME–GC-MS
2.3. RNA of Fruit Extraction and cDNA Synthesis
2.4. Primer Design and Real-Time Quantitative PCR
2.5. Bioinformatics Analysis of Key Genes
3. Results
3.1. Analysis of Volatile Organic Compounds in Two Cultivars of Z. bungeanum in Different Periods
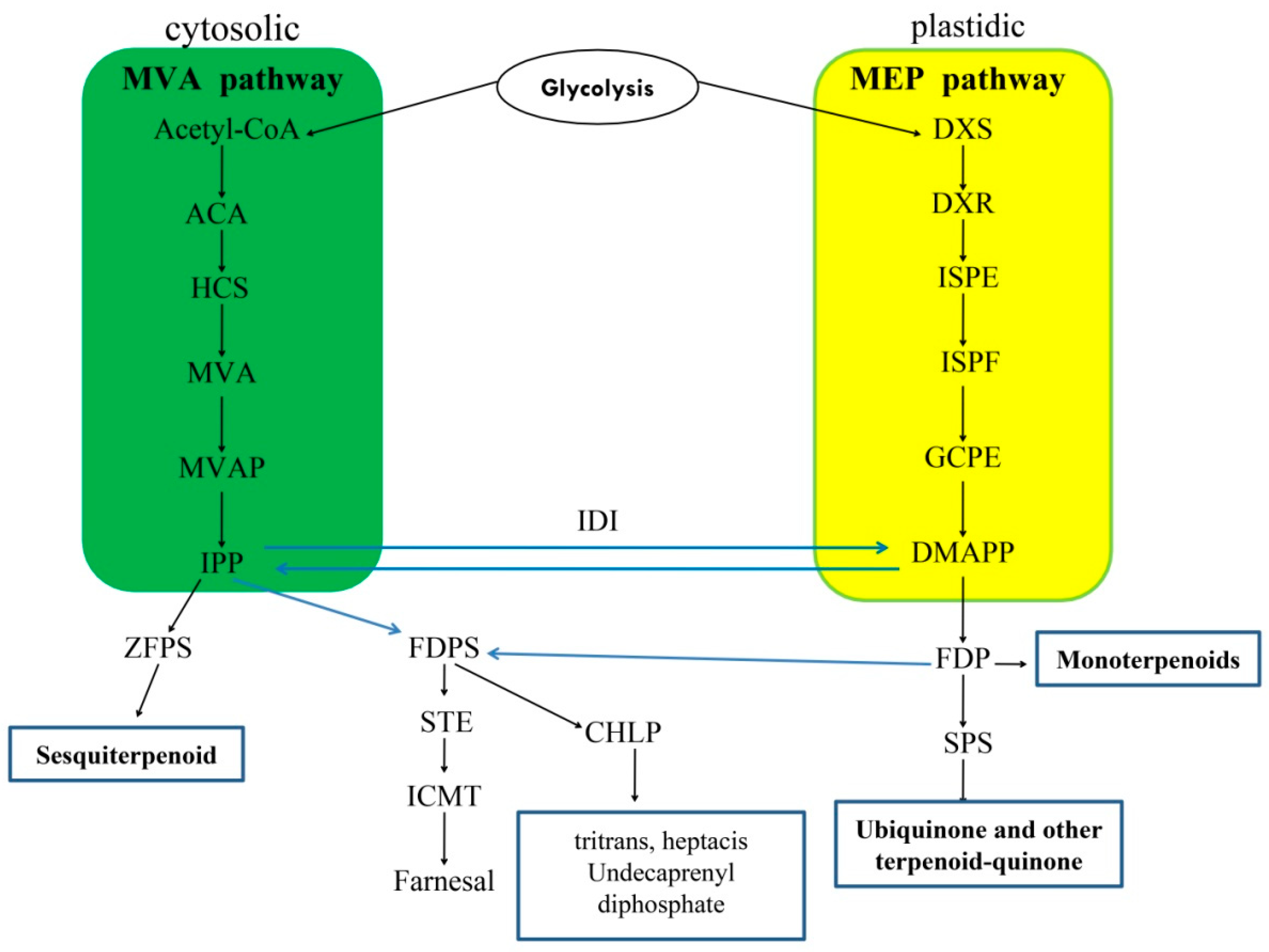
3.2. Expression Profiles of Candidate Genes in Terpenoid Biosynthetic Pathway
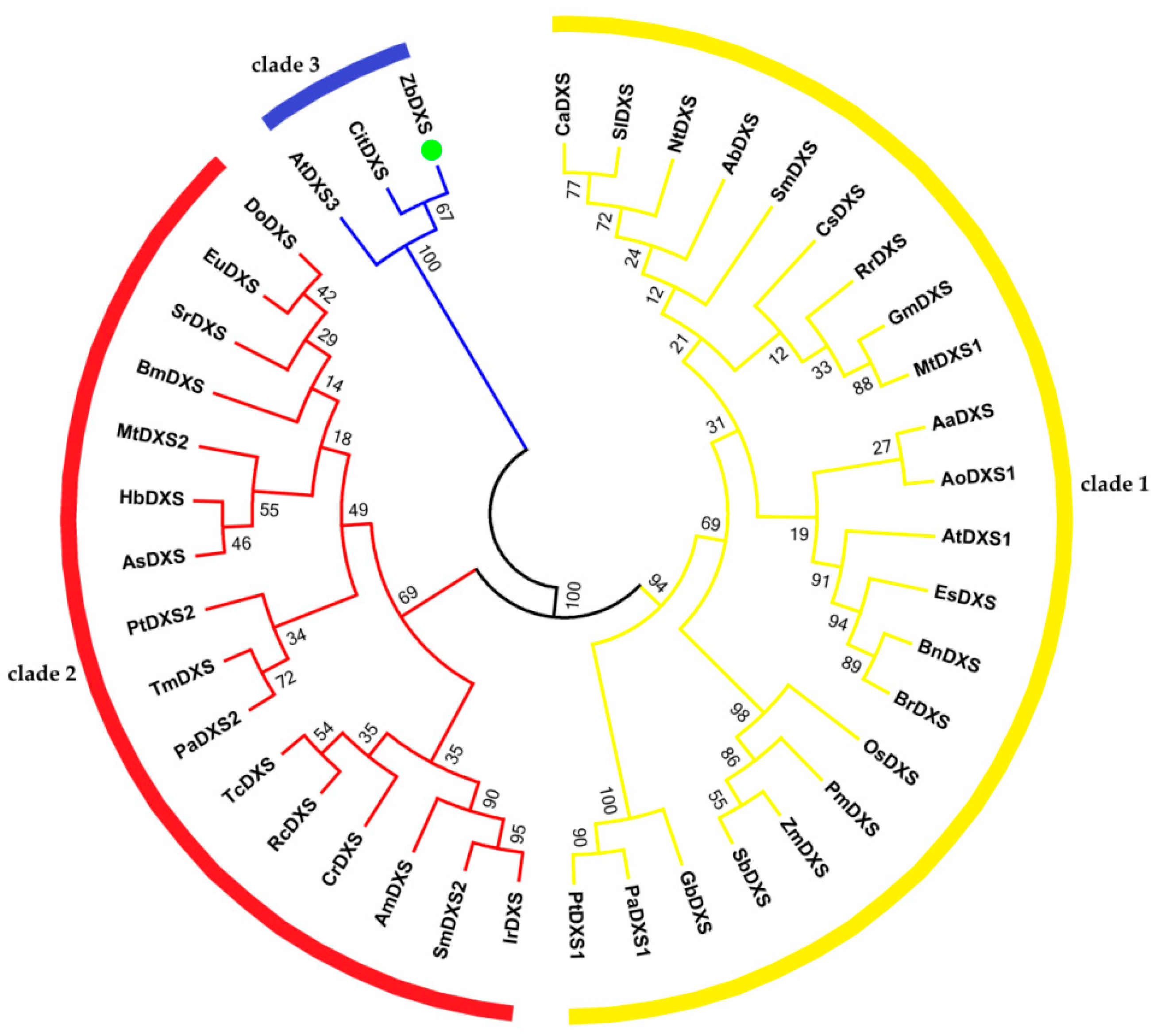
3.2.1. Phylogenetic Analysis of ZbDXS
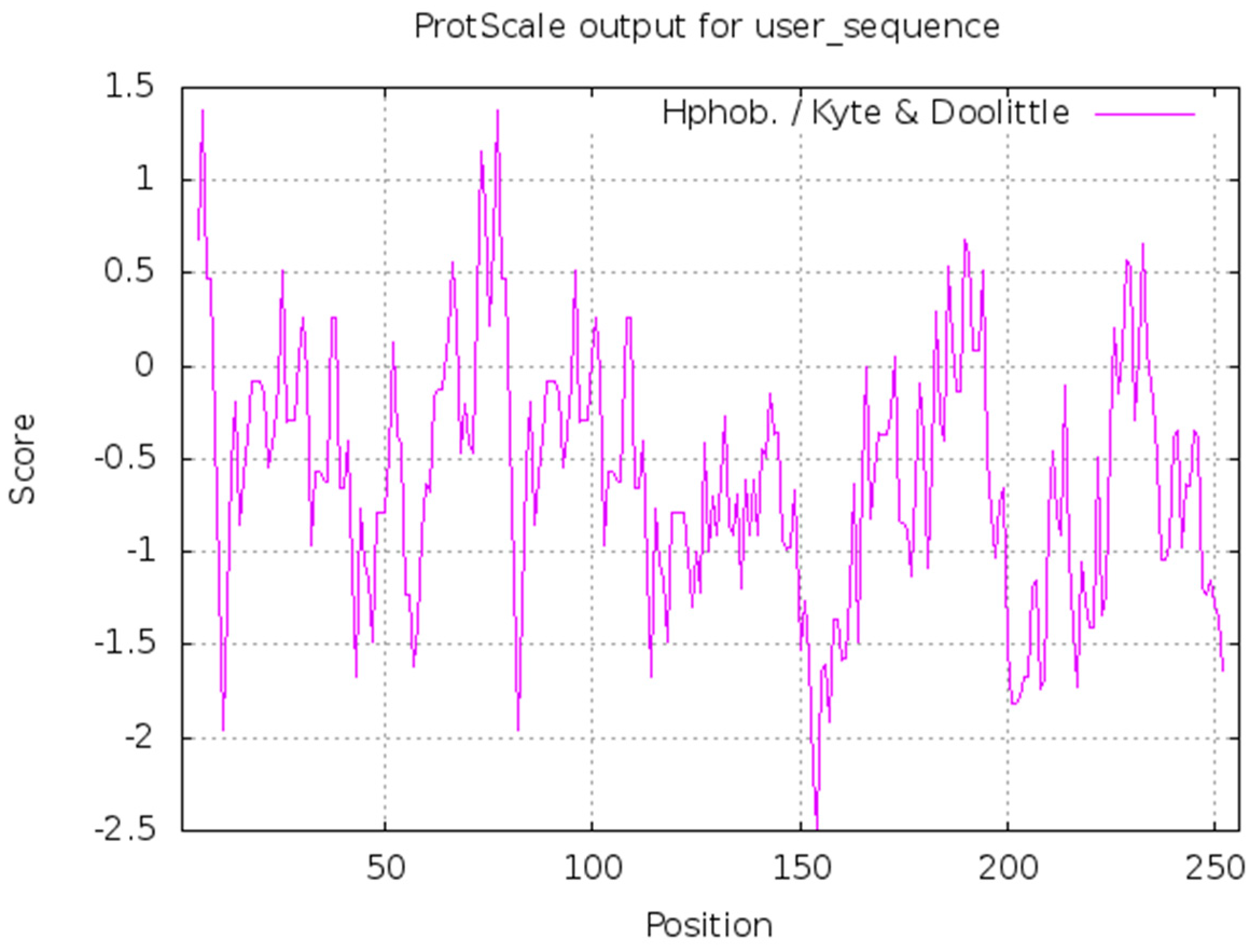
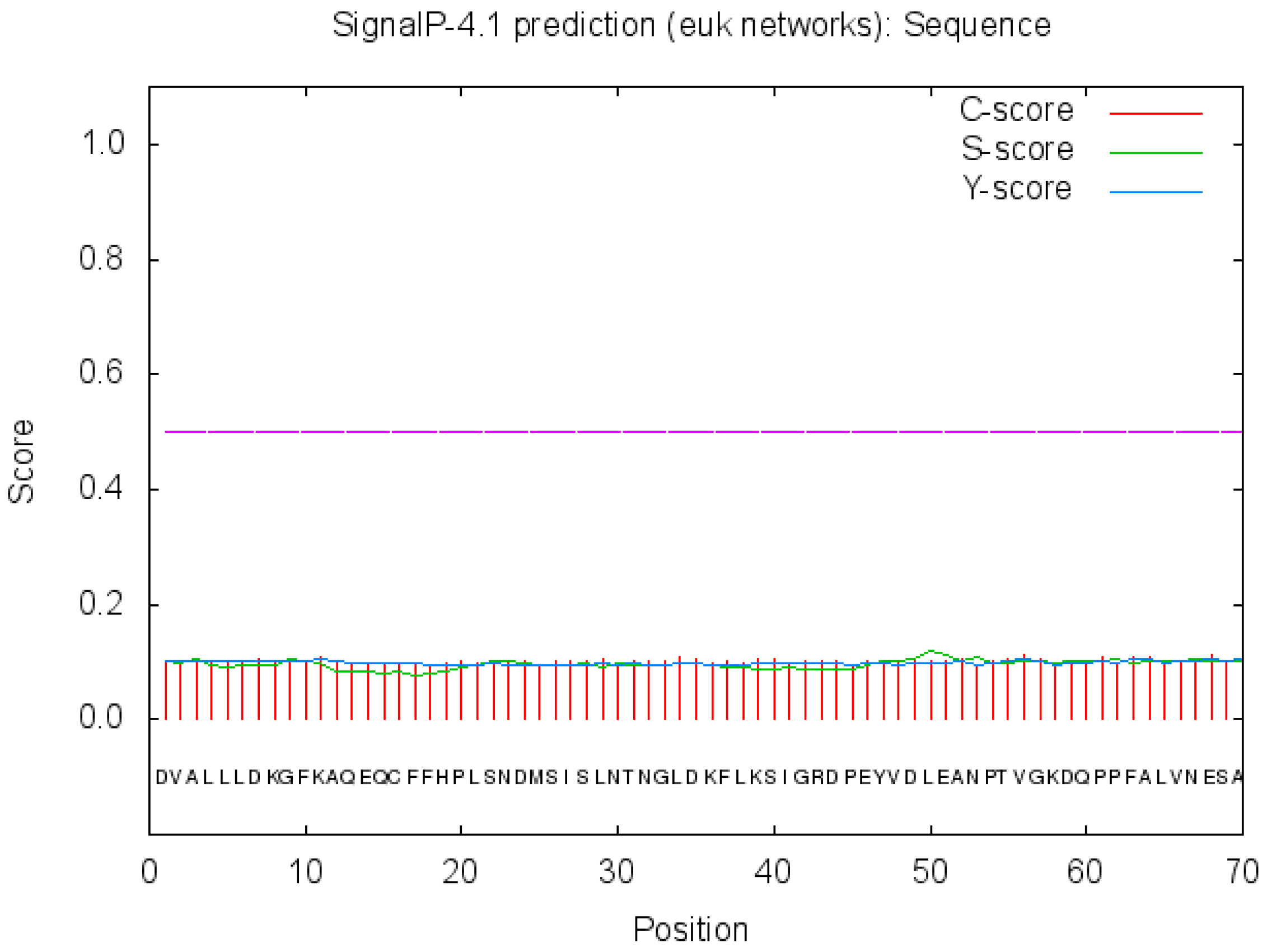
3.2.2. Bioinformatics Analysis of ZbDXS of Z. bungeanum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fei, X.; Hou, L.; Shi, J.; Yang, T.; Liu, Y.; Wei, A. Patterns of Drought Response of 38 WRKY Transcription Factors of Zanthoxylum bungeanum Maxim. Int. J. Mol. Sci. 2018, 20, 68. [Google Scholar] [CrossRef]
- Fei, X.; Shi, Q.; Yang, T.; Fei, Z.; Wei, A. Expression Stabilities of Ten Candidate Reference Genes for RT-qPCR in Zanthoxylum bungeanum Maxim. Molecules 2018, 23, 802. [Google Scholar] [CrossRef]
- Lu, D.; Lo, V. Scent and synaesthesia: The medical use of spice bags in early China. J. Ethnopharmacol. 2015, 167, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Y.; Li, X.; Hou, L.X.; Wei, A.Z. Sensory Characteristics and Antioxidant Activity of Zanthoxylum bungeanum Maxim. Pericarps. Chem. Biodivers. 2019, 16, e1800238. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.L. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Bulotta, S.; Corradino, R.; Celano, M.; D’Agostino, M.; Maiuolo, J.; Oliverio, M.; Procopio, A.; Iannone, M.; Rotiroti, D.; Russo, D. Antiproliferative and antioxidant effects on breast cancer cells of oleuropein and its semisynthetic peracetylated derivatives. Food Chem. 2011, 127, 1609–1614. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Ren, J.N.; Tai, Y.N.; Dong, M.; Shao, J.H.; Yang, S.Z.; Pan, S.Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef]
- Okamoto, G.; Liao, K.; Fushimi, T.; Hirano, K. Aromatic substances evolved from the whole berry, skin and flesh of Muscat of Alexandria grapes. Sci. Rep. Fac. Agric. 2001, 90, 21–25. [Google Scholar]
- Byers, K.J.R.P.; Bradshaw, H.D.; Riffell, J.A. Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus). J. Exp. Biol. 2014, 217, 614–623. [Google Scholar] [CrossRef]
- Manuel, R.C.; Albert, B. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar]
- Wright, L.P.; Rohwer, J.M.; Ghirardo, A.; Hammerbacher, A.; Ortiz-Alcaide, M.; Raguschke, B.; Schnitzler, J.P.; Gershenzon, J.; Phillips, M.A. Deoxyxylulose 5-Phosphate Synthase Controls Flux through the Methylerythritol 4-Phosphate Pathway in Arabidopsis. Plant Physiol. 2014, 165, 1488–1504. [Google Scholar] [CrossRef]
- Bach, T.J. Hydroxymethylglutaryl-CoA reductase, a key enzyme in phytosterol synthesis. Lipids 1986, 21, 82–88. [Google Scholar] [CrossRef]
- Laule, O.; Furholz, A.; Chang, H.S.; Zhu, T.; Wang, X.; Heifetz, P.B.; Gruissem, W.; Lange, M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef]
- Trapp, S.C.; Croteau, R.B. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001, 158, 811–832. [Google Scholar]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 69, 1621–1637. [Google Scholar] [CrossRef]
- Igual, J.C.; Gonzalez-Bosch, C.; Dopazo, J.; Perez-Ortin, J.E. Phylogenetic analysis of the thiolase family. Implications for the evolutionary origin of peroxisomes. J. Mol. Evol. 1992, 35, 147–155. [Google Scholar] [CrossRef]
- Luskey, K.L.; Stevens, B. Human 3-hydroxy-3-methylglutaryl coenzyme A reductase. Conserved domains responsible for catalytic activity and sterol-regulated degradation. J. Biol. Chem. 1985, 260, 10271–10277. [Google Scholar]
- Basson, M.E.; Thorsness, M.; Finer-Moore, J.; Stroud, R.M.; Rine, J. Structural and functional conservation between yeast and human 3-hydroxy-3-methylglutaryl coenzyme A reductases, the rate-limiting enzyme of sterol biosynthesis. Mol. Cell. Biol. 1988, 8, 3797–3808. [Google Scholar] [CrossRef]
- Dhe-Paganon, S.; Magrath, J.; Abeles, R.H. Mechanism of mevalonate pyrophosphate decarboxylase: Evidence for a carbocationic transition state. Biochemistry 1994, 33, 13355–13362. [Google Scholar] [CrossRef]
- Sprenger, G.A.; Schorken, U.; Wiegert, T.; Grolle, S.; de Graaf, A.A.; Taylor, S.V.; Begley, T.P.; Bringer-Meyer, S.; Sahm, H. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin andpyridoxol. Proc. Natl. Acad. Sci. USA 1997, 94, 12857–12862. [Google Scholar] [CrossRef]
- Takahashi, S.; Kuzuyama, T.; Watanabe, H.; Seto, H.A. 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 9879–9884. [Google Scholar] [CrossRef]
- Baker, J.; Franklin, D.B.; Parker, J. Sequence and characterization of the gcpE gene of Escherichia coli. Fems Microbiol. Lett. 1992, 73, 175–180. [Google Scholar] [CrossRef]
- Florence, N.; Kish, C.M.; Jennifer, B.; Beverly, U.; Kenichi, S.; Conrad, W.; Clark, D.G.; Natalia, D. Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. Plant Cell. 2003, 15, 2992. [Google Scholar]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell. 2012, 24, 2635–2648. [Google Scholar] [CrossRef]
- Magnard, J.L.; Roccia, A.; Caissard, J.C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Hibrandsaint, O.L.; Jullien, F.; Nicolè, F. PLANT VOLATILES. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and Characterization of Terpene Synthase Genes Accounting for the Volatile Terpene Emissions in Flowers of Freesia hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Xia, J.; Zeng, J.; Xiang, L.; Zhu, S.; Zheng, Q.; Xie, H.; Yang, C.; Chen, M.; et al. Molecular Characterization of the 1-Deoxy-d-Xylulose 5-Phosphate Synthase Gene Family in Artemisia annua. Front. Plant Sci. 2018, 9, 952. [Google Scholar] [CrossRef]
- Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α- and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef]
- Carretero-Paulet, L.; Cairo, A.; Talavera, D.; Saura, A.; Imperial, S.; Rodriguez-Concepcion, M.; Campos, N.; Boronat, A. Functional and evolutionary analysis of DXL1, a non-essential gene encoding a 1-deoxy-d-xylulose 5-phosphate synthase like protein in Arabidopsis thaliana. Gene 2013, 524, 40–53. [Google Scholar] [CrossRef]
- Cordoba, E.; Porta, H.; Arroyo, A.; San Román, C.; Medina, L.; Rodríguez-Concepción, M.; León, P. Functional characterization of the three genes encoding 1-deoxy-D-xylulose 5-phosphate synthase in maize. J. Exp. Bot. 2011, 62, 2023–2038. [Google Scholar] [CrossRef]
- Eisenreich, W.; Bacher, A.; Arigoni, D.; Rohdich, F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell. Mol. Life Sci. 2004, 61, 1401–1406. [Google Scholar] [CrossRef]
- Isabelle, M.; Michel, J.; Christophe, A. Changes in physicochemical characteristics and volatile constituents of strawberry (Cv. Cigaline) during maturation. J. Agric. Food Chem. 2004, 52, 1248–1254. [Google Scholar]
- Skinkis, P.A.; Bordelon, B.P.; Butz, E.M. Effects of sunlight exposure on berry and wine monoterpenes and sensory characteristics of traminette. Am. J. Enol. Viticult. 2010, 61, 147–156. [Google Scholar]
- Toselli, M.; Baldi, E.; Marangoni, B.; Noferini, M.; Fiori, G.; Bregoli, A.M.; Ndagijimana, M.; Pestana, M.; Correia, P.J. Effects of mineral and organic fertilization and ripening stage on the emission of volatile organic compounds and antioxidant activity of ‘Stark RedGold’ nectarine. Acta Horticult. 2010, 868, 381–388. [Google Scholar] [CrossRef]
- Gachons, C.P.D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L. cv Sauvignon blanc in field conditions. J. Sci. Food Agric. 2010, 85, 73–85. [Google Scholar] [CrossRef]
- Zhu, H.L.; Zhu, B.Z.; Fu, D.Q.; Xie, Y.H.; Hao, Y.L.; Luo, Y.B. Role of Ethylene in the Biosynthetic Pathways of Aroma Volatiles in Ripening Fruit. Russ. J. Plant Physiol. 2005, 52, 691–695. [Google Scholar] [CrossRef]

| Gene | Description | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| UBQ | ubiquitin extension protein | TCGAAGATGGCCGTACATTG | TCCTCTAAGCCTCAGCACCA |
| TIF | Translation initiation factor | TTCCTCCCATTACGTTGCT | GCTGGTTACGGACTCTTTG |
| DXS | 1-deoxy-D-xylulose-5-phosphate synthase | GAGATTGGGAAGGGAAGAAT | CATCAGCCACAGTCACAGAA |
| HCS | hydroxymethylglutaryl-CoA synthase | AATGTTGCTGGGAAGTTGAA | GGCTACTGTCTTTGCTCGTTA |
| HMGR | hydroxymethylglutaryl-CoA reductase | GTTGACTCCTTGTACCGAAGAT | GCAACTTCTGGCGATACTGA |
| ZFPS | (2Z,6Z)-farnesyl diphosphate synthase | TGGACTTACACTTGCCC | CGAGAGTTTCTTCTTTGG |
| SPS | all-trans-nonaprenyl-diphosphate synthase | CAAAGCATCGTCGTTTAGCC | TTTCCCTCTTCGCATGTCAC |
| ICMT | protein-S-isoprenylcysteine O-methyltransferase | TAATGCTGTGTAACCCCA | CGTAACCCAAAAAACTCTCT |
| IDI | isopentenyl-diphosphate Delta-isomerase | AACACTAACCCAAACCCTG | GCTTCAAACCTTCTTCTCCA |
| FDP | farnesyl diphosphate synthase | TGGACTTACACTTGCCC | CGAGAGTTTCTTCTTTGG |
| ISPE | 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase | GTTCATTTTAGGAGGACCC | CCCATCTTCTCTCTTGTTT |
| ISPF | 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase | TCCATCCAACCGTCCAAA | CCCCAATGCTCCCAAAAT |
| FDPS | farnesyl diphosphate synthase | GGAAGCCGACGAACCACA | GCTCTAACTACCCGCGCA |
| GCPE | (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase | CGCAGAGATACGAGAGAAAA | CTCCACCAACATACCCAAAG |
| DXR | 1-deoxy-D-xylulose-5-phosphate reductoisomerase | AGATTATGGCTGGGGAAC | AGAGGAAGGACAAAAGGA |
| STE | STE24 endopeptidase | CGTGCTGGTCTTGTGAAA | GGGCGGATGAGAATAGTG |
| CHLP | geranylgeranyl diphosphate/geranylgeranyl-bacteriochlorophyllide a reductase | GTTGATGGGTATTGATAGGGC | CGTGAAGATGTTTTAGGGTTTT |
| ACA | acetyl-CoA C-acetyltransferase | GCAGCATTGGTTCTAGTGAGTG | GCATCGGCATATCCTGTGAT |
| Compounds | H1 | H2 | H3 | H4 | H5 | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Monoterpene | ||||||||||
| α-Pinene | 11.20 ± 0.08 | 12.35 ± 0.12 | 14.31 ± 0.03 | 10.68 ± 0.07 | 9.84 ± 0.03 | 0.96 ± 0.02 | 11.89 ± 0.02 | 13.92 ± 0.02 | 10.75 ± 0.31 | 8.70 ± 0.25 |
| Bicyclo[3.1.0]hexane | UD | UD | 3.36 ± 0.03 | 3.96 ± 0.02 | 1.23 ± 0.02 | 3.27 ± 0.04 | 5.64 ± 0.03 | 7.36 ± 0.01 | 6.75 ± 0.03 | 5.43 ± 0.03 |
| Bicyclo[2.2.1]heptane | UD | UD | 3.94 ± 0.05 | 1.54 ± 0.01 | 1.21 ± 0.01 | UD | UD | UD | UD | UD |
| Bicyclo[3.1.0]hex-2-ene | UD | UD | 1.66 ± 0.02 | 1.75 ± 0.04 | 2.64 ± 0.03 | UD | UD | UD | 0.76 ± 0.04 | 0.93 ± 0.01 |
| Bicyclo[3.1.1]hept-2-ene | UD | UD | 7.11 ± 0.02 | 6.58 ± 0.01 | 2.28 ± 0.03 | 1.06 ± 0.03 | 1.80 ± 0.04 | 8.14 ± 0.02 | 3.48 ± 0.01 | 2.20 ± 0.04 |
| α-Ocimene | 8.20 ± 0.16 | 8.68 ± 0.05 | 8.84 ± 0.01 | 7.21 ± 0.03 | 7.08 ± 0.07 | 6.77 ± 0.04 | 8.85 ± 0.05 | 9.92 ± 0.02 | 8.36 ± 0.42 | 7.18 ± 0.03 |
| Linalool | 1.19 ± 0.01 | 5.38 ± 0.02 | 5.95 ± 0.03 | 2.64 ± 0.03 | 1.78 ± 0.02 | 5.45 ± 0.03 | 6.84 ± 0.03 | 6.92 ± 0.04 | 6.63 ± 0.01 | 6.25 ± 0.07 |
| 2,4,6-Octatriene | UD | UD | UD | 2.38 ± 0.01 | 1.95 ± 0.03 | UD | UD | 1.54 ± 0.03 | 2.28 ± 0.18 | 1.15 ± 0.22 |
| 1,5-Cyclodecadiene | UD | UD | UD | 3.74 ± 0.04 | 1.27 ± 0.03 | UD | UD | 2.78 ± 0.09 | 2.96 ± 0.04 | 0.76 ± 0.01 |
| Terpineol | UD | 2.38 ± 0.02 | 5.45 ± 0.02 | 3.56 ± 0.04 | 1.64 ± 0.02 | UD | UD | 1.04 ± 0.02 | 0.85 ± 0.01 | 0.66 ± 0.01 |
| 1,3-Cyclohexadiene | UD | UD | UD | UD | 0.96 ± 0.01 | UD | UD | UD | UD | 3.11 ± 0.03 |
| D-Limonene | UD | 0.64 ± 0.02 | 3.97 ± 0.01 | 2.48 ± 0.01 | 1.03 ± 0.02 | UD | UD | UD | UD | UD |
| Cyclohexene | UD | UD | UD | 4.17 ± 0.01 | 6.17 ± 0.02 | UD | UD | UD | UD | UD |
| Eucalyptol | 1.25 ± 0.01 | 2.14 ± 0.02 | 2.38 ± 0.02 | 2.48 ± 0.03 | 1.29 ± 0.01 | UD | UD | UD | UD | UD |
| α-Myrcene | UD | UD | 6.29 ± 0.02 | 5.27 ± 0.01 | 1.77 ± 0.02 | UD | UD | UD | UD | UD |
| 3-Cyclohexen-1-one | UD | UD | 3.90 ± 0.04 | 3.22 ± 0.03 | 1.78 ± 0.01 | UD | UD | UD | UD | UD |
| 2-Cyclohexen-1-one | UD | UD | UD | UD | 2.87 ± 0.01 | UD | UD | UD | UD | UD |
| 6-Octenal | UD | UD | UD | UD | 1.51 ± 0.02 | UD | UD | UD | UD | UD |
| Sesquiterpene | ||||||||||
| Caryophyllene | UD | 1.67 ± 0.08 | 2.66 ± 0.08 | 2.23 ± 0.01 | 0.86 ± 0.04 | 2.55 ± 0.05 | 2.80 ± 0.03 | 1.30 ± 0.03 | 0.94 ± 0.02 | 0.74 ± 0.06 |
| ç-Elemene | UD | UD | UD | UD | 1.04 ± 0.01 | UD | UD | UD | 0.69 ± 0.04 | 0.70 ± 0.07 |
| Humulene | UD | UD | UD | UD | 1.45 ± 0.01 | UD | UD | UD | UD | 0.69 ± 0.02 |
| (1S,2E,6E,10R)-3,7,11,11-Tetramethylbicyclo[8.1.0]undeca-2,6-diene | 1.33 ± 0.04 | 3.13 ± 0.42 | 2.37 ± 0.09 | 2.01 ± 0.03 | 1.35 ± 0.07 | UD | 2.24 ± 0.01 | 7.28 ± 0.02 | 3.28 ± 0.02 | 2.91 ± 0.01 |
| (S,1Z,6Z)-8-Isopropyl-1-methyl-5-methylenecyclodeca-1,6-diene | UD | UD | UD | UD | UD | UD | UD | 0.99 ± 0.02 | 1.03 ± 0.02 | 0.53 ± 0.03 |
| Germacrene D | UD | UD | 3.18 ± 0.01 | 2.14 ± 0.02 | 0.30 ± 0.04 | UD | UD | UD | UD | UD |
| 1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphthalene | UD | UD | UD | UD | 1.03 ± 0.02 | UD | UD | 1.11 ± 0.02 | 1.09 ± 0.01 | 0.63 ± 0.01 |
| (1S,4aR,8aS)-1,4a-Isopropyl-7-methyl-4-methylene-1,2,3,4,5,6,8a-octahydronaphthalene | UD | UD | UD | UD | UD | UD | UD | UD | UD | 0.64 ± 0.04 |
| Alcohols | ||||||||||
| (3E,5E)-2,6-Dimethylocta-3,5,7-trien-2-ol | UD | UD | UD | UD | UD | UD | UD | UD | 0.77 ± 0.01 | 0.58 ± 0.03 |
| (2E,4S,7E)-4-Isopropyl-1,7-dimethylcyclodeca-2,7-dienol | UD | UD | UD | UD | UD | UD | UD | UD | UD | 1.19 ± 0.05 |
| 1,6,10-Dodecatrien-3-ol | UD | UD | 2.77 ± 0.05 | 5.86 ± 0.02 | 1.07 ± 0.03 | UD | UD | UD | 0.94 ± 0.02 | 0.8 ± 0.03 |
| (2E,4S,7E)-4-Isopropyl-1,7-dimethylcyclodeca-2,7-dienol | UD | UD | UD | 0.57 ± 0.07 | 1.50 ± 0.08 | UD | UD | UD | UD | 1.46 ± 0.03 |
| Geraniol | UD | UD | UD | UD | 2.90 ± 0.04 | UD | UD | UD | UD | UD |
| Cyclohexanol | UD | UD | UD | 2.61 ± 0.01 | 3.21 ± 0.02 | UD | UD | UD | UD | UD |
| 5-Isopropyl-2-methylbicyclo[3.1.0]hexan-2-ol | UD | UD | UD | UD | 5.15 ± 0.04 | UD | UD | UD | UD | UD |
| 3-Cyclohexen-1-ol | UD | UD | UD | UD | 7.39 ± 0.10 | UD | UD | UD | UD | UD |
| 6-Octen-1-ol | UD | UD | UD | UD | 1.27 ± 0.01 | UD | UD | UD | UD | UD |
| Esters | ||||||||||
| Geranyl acetate | UD | 2.33 ± 0.12 | 4.93 ± 0.01 | 4.67 ± 0.01 | 1.03 ± 0.04 | UD | UD | UD | UD | 1.39 ± 0.02 |
| Linalyl acetate | UD | 2.83 ± 0.02 | 5.50 ± 0.08 | 4.54 ± 0.02 | 0.60 ± 0.02 | UD | UD | UD | UD | UD |
| Myrtenyl acetate | UD | UD | UD | UD | 1.59 ± 0.02 | UD | UD | UD | UD | UD |
| α-Terpinyl acetate | UD | UD | 2.95 ± 0.01 | 4.30 ± 0.04 | 3.67 ± 0.02 | UD | UD | UD | UD | UD |
| Protein | Protein ID in NCBI | NOTE |
|---|---|---|
| CitDXS | XP_006483309.1 | Citrus sinensis |
| AtDXS1 | AEE76517.1 | Arabidopsis thaliana |
| RrDXS | AEZ53173.1 | Rosa rugosa |
| GmDXS | NP_001236070.1 | Glycine max |
| ZmDXS | ACT32136.1 | Zea mays |
| AsDXS | AHI62962.1 | Aquilaria sinensis |
| IrDXS | AMM72794.1 | Isodonrubescens |
| NtDXS | CBA12009.1 | Nicotiana tabacum |
| SmDXS | ACF21004.1 | Salvia miltiorrhiza |
| AbDXS | AGG54706.1 | Aconitum balfourii |
| BnDXS | AHN09416.1 | Brassica napus |
| OsDXS | XP_015640505.1 | Oryza sativa |
| CrDXS | ABI35993.1 | Catharanthus roseus |
| GbDXS | AAS89341.1 | Ginkgo biloba |
| AaDXS | AAD56390.2 | Artemisia annua |
| EuDXS | AFU93069.1 | Eucommia ulmoides) |
| CaDXS | PHT95029.1 | Capsicum annuum |
| SlDXS | CAZ66648.1 | Solanum lycopersicum |
| SbDXS | XP_002441088.1 | Sorghum bicolor |
| EsDXS | XP_006414485.1 | Eutremasalsugineum |
| AoDXS1 | AEK69518.1 | Alpiniaofficinarum |
| SmDXS2 | ACQ66107.1 | Salvia miltiorrhiza |
| AmDXS | AAW28999.1 | Antirrhinum majus |
| AtDXS3 | AED91669.1 | Arabidopsis thaliana |
| BrDXS | ABE60813.1 | Brassica rapa |
| HbDXS | ABF18929.1 | Heveabrasiliensis |
| MtDXS1 | CAD22530.1 | Medicago truncatula |
| MtDXS2 | CAN89181.1 | Medicago truncatula |
| PaDXS1 | ABS50518.1 | Piceaabies |
| PaDXS2 | ABS50520.1 | Piceaabies |
| PtDXS1 | ACJ67021.1 | Pinus taeda |
| PtDXS2 | ACJ67020.1 | Pinus taeda |
| RcDXS | XP_002533688.1 | Ricinus communis |
| SrDXS | ACI43010.1 | Stevia rebaudiana |
| TmDXS | AAS89342.1 | Taxusmedia |
| DoDXS | AHF22384.1 | Dendrobium officinale |
| CsDXS | BAF75640.1 | Croton stellatopilosus |
| TcDXS | EOY31729.1 | Theobroma cacao |
| BmDXS | ACP30544.2 | Bacopa monnieri |
| PmDXS | RLN29936.1 | Panicum miliaceum |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Fei, X.; Hu, Y.; Liu, Y.; Wei, A. Identification of Key Genes in the Synthesis Pathway of Volatile Terpenoids in Fruit of Zanthoxylum bungeanum Maxim. Forests 2019, 10, 328. https://doi.org/10.3390/f10040328
Shi J, Fei X, Hu Y, Liu Y, Wei A. Identification of Key Genes in the Synthesis Pathway of Volatile Terpenoids in Fruit of Zanthoxylum bungeanum Maxim. Forests. 2019; 10(4):328. https://doi.org/10.3390/f10040328
Chicago/Turabian StyleShi, Jingwei, Xitong Fei, Yang Hu, Yulin Liu, and Anzhi Wei. 2019. "Identification of Key Genes in the Synthesis Pathway of Volatile Terpenoids in Fruit of Zanthoxylum bungeanum Maxim" Forests 10, no. 4: 328. https://doi.org/10.3390/f10040328
APA StyleShi, J., Fei, X., Hu, Y., Liu, Y., & Wei, A. (2019). Identification of Key Genes in the Synthesis Pathway of Volatile Terpenoids in Fruit of Zanthoxylum bungeanum Maxim. Forests, 10(4), 328. https://doi.org/10.3390/f10040328




