Are Wildfires a Threat to Fungi in European Pinus Forests? A Case Study of Boreal and Mediterranean Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Design
2.3. Taxa Identification and Classification
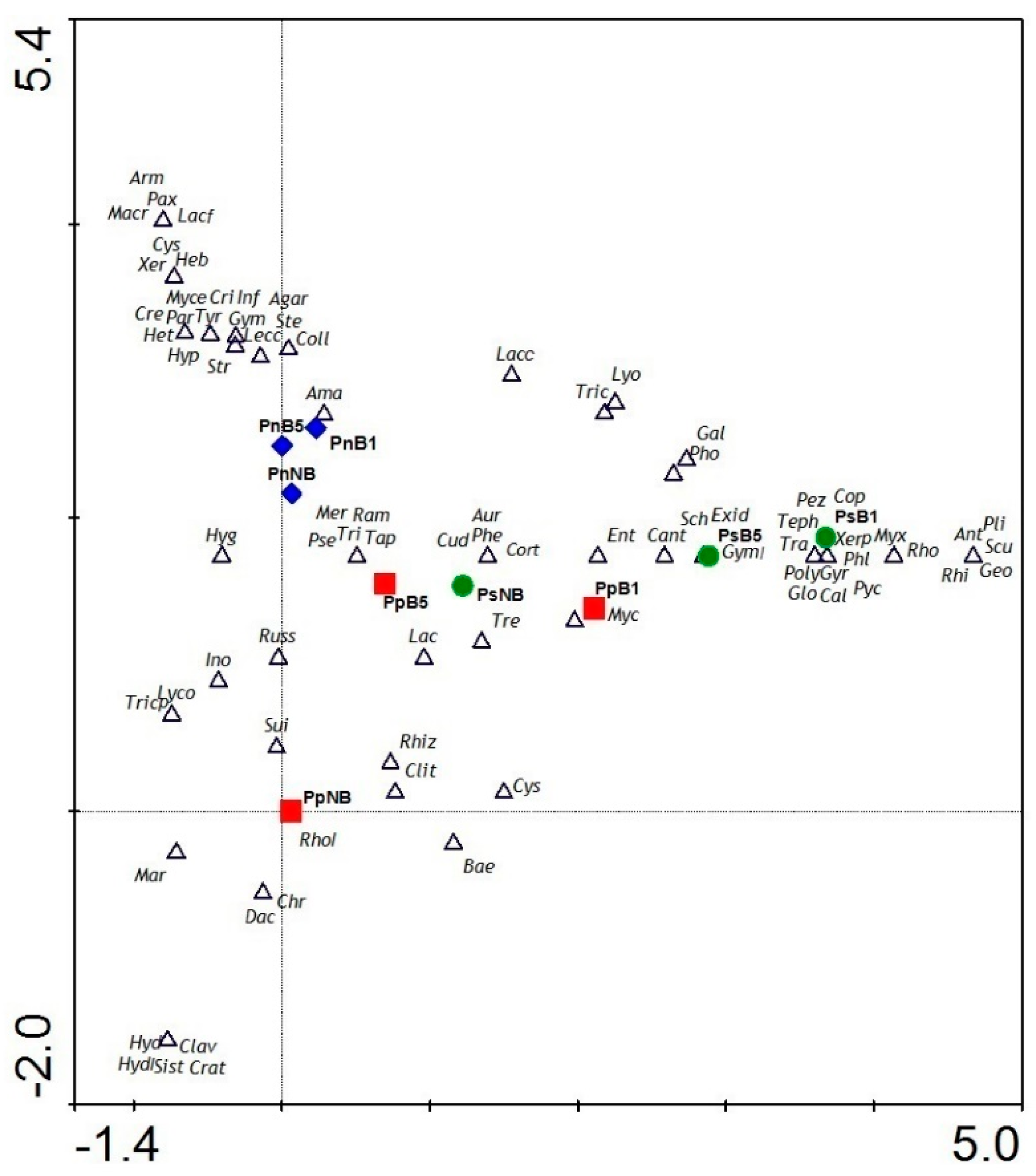
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Fungal Richness Succession after Fire
3.3. Taxa Composition
4. Discussion
4.1. General Data
4.2. Effect of Fire on Richness and Succession after Fire
4.3. Taxa Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Espelta, J.M.; Retana, J.; Habrouk, A. An Economic and Ecological Multi-Criteria Evaluation of Reforestation Methods to Recover Burned Pinus Nigra Forests in NE Spain. For. Ecol. Manag. 2003, 180, 185–198. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.; Nabuurs, G.; Zimmermann, N.E. Climate Change May Cause Severe Loss in the Economic Value of European Forest Land. Nat. Clim. Chang. 2012, 3, 203–207. [Google Scholar] [CrossRef]
- Miura, S.; Amacher, M.; Hofer, T.; San-Miguel-Ayanz, J.; Ernawati; Thackway, R. Protective Functions and Ecosystem Services of Global Forests in the Past Quarter-Century. For. Ecol. Manag. 2015, 352, 35–46. [Google Scholar] [CrossRef]
- Ramos-Diez, I.; Navarro-Hevia, J.; San Martín Fernández, R.; Díaz-Gutiérrez, V.; Mongil-Manso, J. Analysis of Methods to Determine the Sediment Retained by Check Dams and to Estimate Erosion Rates in Badlands. Environ. Monit. Assess. 2016, 188, 405. [Google Scholar] [CrossRef] [PubMed]
- Roces-Díaz, J.V.; Vayreda, J.; Banqué-Casanovas, M.; Díaz-Varela, E.; Bonet, J.A.; Brotons, L.; de-Miguel, S.; Herrando, S.; Martínez-Vilalta, J. The Spatial Level of Analysis Affects the Patterns of Forest Ecosystem Services Supply and Their Relationships. Sci. Total Environ. 2018, 626, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Pardos, M.; Pérez, S.; Calama, R.; Alonso, R.; Lexer, M.J. Ecosystem Service Provision, Management Systems and Climate Change in Valsaín Forest, Central Spain. Reg. Environ. Chang. 2017, 17, 17–32. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forest. Science 2013, 339, 819–824. [Google Scholar] [CrossRef]
- Watkinson, S.; Bebber, D.; Darrah, P.; Fricker, M.; Boddy, L. The Role of Wood Decay Fungi in the Carbon and Nitrogen Dynamics of the Forest Floor; Gadd, G.M., Ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Garcia, C.; Hernandez, T.; Roldan, A.; Albaladejo, J.; Castillo, V. Organic Amendment and Mycorrhizal Inoculation as a Practice in Afforestation of Soils with Pinus Halepensis Miller: Effect on Their Microbial Activity. Soil Biol. Biochem. 2000, 32, 1173–1181. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and Litter Decomposition in Terrestial Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Kilronomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal Fungal Diversity Determines Plant Biodiversity, Ecosystem Variability and Productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Bonet, J.A.; González-olabarria, J.R.; Martínez-de-Aragón, J. Mushroom Production as an Alternative for Rural Development in a Forested Mountainous Area. J. Mt. Sci. 2014, 11, 535–543. [Google Scholar] [CrossRef]
- Büntgen, U.; Latorre, J.; Egli, S.; Martínez-Peña, F. Socio-Economic, Scientific, and Political Benefits of Mycotourism. Ecosphere 2017, 8, e01870. [Google Scholar] [CrossRef]
- Pausas, J.G.; Llovet, J.; Anselm, R.; Vallejo, R. Are Wildfires a Disaster in the Mediterranean Basin?—A Review Vegetation Changes Shrublands Dominated by Resprouting Species. Int. J. Wildl. Fire 2008, 17, 713–723. [Google Scholar] [CrossRef]
- Wallenius, T.H.; Kauhanen, H.; Herva, H.; Pennanen, J. Long Fire Cycle in Northern Boreal Pinus Forests in Finnish Lapland. Can. J. For. Res. 2010, 40, 2027–2035. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Vega, J.A.; Jiménez, E.; Rigolot, E. Fire Resistance of European Pines. For. Ecol. Manag. 2008, 256, 246–255. [Google Scholar] [CrossRef]
- Angelsam, P.; Kuuluvainen, T. Boreal Forest Disturbance Regimes, Successional Dynamics and Landscape Structures: A European Perspective. Ecol. Bull. 2004, 51, 117–136. [Google Scholar]
- Vernet, J.-L. History of the Pinus Sylvestris and Pinus Nigra Ssp. Salzmanni Forest in the Sub-Mediterranean Mountains (Grands Causses, Pinus Saint-Guilhem-Le-Désert, Southern Massif Central, France) Based on Charcoal from Limestone and Dolomitic Deposits. Veg. Hist. Archaeobot. 2006, 16, 23–42. [Google Scholar] [CrossRef]
- Pimont, F.; Prodon, R.; Rigolot, E. Comparison of Postfire Mortality in Endemic Corsican Black Pine (Pinus Nigra Ssp. Laricio) and Its Direct Competitor (Pinus Pinaster). Ann. For. Sci. 2011, 68, 425–432. [Google Scholar] [CrossRef]
- Peay, K.G.; Garbelotto, M.; Bruns, T.D. Spore Heat Resistance Plays an Important Role in Disturbance-Mediated Assemblage Shift of Ectomycorrhizal Fungi Colonizing Pinus Muricata Seedlings. J. Ecol. 2009, 97, 537–547. [Google Scholar] [CrossRef]
- Dahlberg, A.; Schimmel, J.; Taylor, A.F.S.; Johannesson, H. Post-fire Legacy of Ectomycorrhizal Fungal Communities in the Swedish Boreal Forest in Relation to fire Severity and Logging Intensity. 2001, 100, 151–161. [Google Scholar] [CrossRef]
- Kropp, B.R.; Albee, S. The effects of silvicultural treatments on ocurrence of mycorrhizal sporocarps in a Pinus contorta forest: A preliminary study. Biol. Conserv. 1996, 70, 313–318. [Google Scholar] [CrossRef]
- Martínez de Aragón, J.; Bonet, J.A.; Fischer, C.R.; Colinas, C. Productivity of Ectomycorrhizal and Selected Edible Saprotrophic Fungi in Pine Forests of the Pre-Pyrenees Mountains, Spain: Predictive Equations for Forest Management of Mycological Resources. For. Ecol. Manag. 2007, 252, 239–256. [Google Scholar] [CrossRef]
- Cairney, J.W.G.; Bastias, B.A. Influences of Fire on Forest Soil Fungal Communities. Can. J. For. Res. 2007, 37, 207–215. [Google Scholar] [CrossRef]
- Gassibe, P.V.; Fabero, R.F.; Hernández-Rodríguez, M.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Fungal Community Succession Following Wildfire in a Mediterranean Vegetation Type Dominated by Pinus Pinaster in Northwest Spain. For. Ecol. Manag. 2011, 262, 655–662. [Google Scholar] [CrossRef]
- Mediavilla, O.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Changes in Sporocarp Production and Vegetation Following Wildfire in a Mediterranean Forest Ecosystem Dominated by Pinus Nigra in Northern Spain. For. Ecol. Manag. 2014, 331, 85–92. [Google Scholar] [CrossRef]
- Gassibe, P.V.; Fabero, R.F.; Hernández-Rodríguez, M.; Oria-de-Rueda, J.A.; Oviedo, F.B.; Martín-Pinto, P. Post-Fire Production of Mushrooms in Pinus Pinaster Forests Using Classificatory Models. J. For. Res. 2014, 19, 348–356. [Google Scholar] [CrossRef]
- Claridge, A.W.; Trappe, J.M.; Hansen, K. Do Fungi Have a Role as Soil Stabilizers and Remediators after Forest Fire? For. Ecol. Manag. 2009, 257, 1063–1069. [Google Scholar] [CrossRef]
- Lindahl, B.O.; Taylor, F.S.; Finlay, R.D. Defining Nutritunial Constraints on Carbon Cycling in Boreal Forests-towards a Less “Phytocentric” Perspective. Plant Soil 2002, 242, 123–135. [Google Scholar] [CrossRef]
- Motiejunaite, J.; Adamonyte, G.; Iršenaite, R.; Juzenas, S.; Kasparavičius, J.; Kutorga, E.; Markovskaja, S. Early Fungal Community Succession Following Crown Fire in Pinus Mugo Stands and Surface Fire in Pinus Sylvestris Stands. Eur. J. For. Res. 2014, 133, 745–756. [Google Scholar] [CrossRef]
- Liu, Y.; Goodrick, S.L.; Stanturf, J.A. Future U.S. Wildfire Potential Trends Projected Using a Dynamically Downscaled Climate Change Scenario. For. Ecol. Manag. 2013, 294, 120–135. [Google Scholar] [CrossRef]
- Salo, K.; Kouki, J. Severity of Forest Wildfire Had a Major Influence on Early Successional Ectomycorrhizal Macrofungi Assemblages, Including Edible Mushrooms. For. Ecol. Manag. 2018, 415–416, 70–84. [Google Scholar] [CrossRef]
- Rincón, A.; Pueyo, J.J. Effect of Fire Severity and Site Slope on Diversity and Structure of the Ectomycorrhizal Fungal Community Associated with Post-Fire Regenerated Pinus Pinaster Ait. Seedlings. For. Ecol. Manag. 2010, 260, 361–369. [Google Scholar] [CrossRef]
- Bonet, J.A.; Fischer, C.R.; Colinas, C. The Relationship between Forest Age and Aspect on the Production of Sporocarps of Ectomycorrhizal Fungi in Pinus Sylvestris Forests of the Central Pyrenees. For. Ecol. Manag. 2004, 203, 157–175. [Google Scholar] [CrossRef]
- Martín-Pinto, P.; Vaquerizo, H.; Peñalver, F.; Olaizola, J.; Oria-De-Rueda, J.A. Early Effects of a Wildfire on the Diversity and Production of Fungal Communities in Mediterranean Vegetation Types Dominated by Cistus Ladanifer and Pinus Pinaster in Spain. For. Ecol. Manag. 2006, 225, 296–305. [Google Scholar] [CrossRef]
- Knudsen, H.; Vesterholt, J. Funga Nordica: Agaricoid, Boletoid and Cyphelloid Genera; Nordsvamp: Copenhagen, Denmark, 2008. [Google Scholar]
- Von Bonsdorff, T.; Hopsu-Neuvonen, A.; Huhtinen, S.; Korhonen, J.; Kosonen, L.; Moisio, S.; Palmén, J. Sienimetsästä markkinoille; Opetushallitus: Helsinki, Finland, 2013. [Google Scholar]
- Ter Braak, C.J.F.; Prentice, I.C. A Theory of Gradient Analysis. Adv. Ecol. Res. 2013, 34, 271–317. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Oria-de-Rueda, J.A.; Hernández-Rodriguez, M.; Martín-Pinto, P.; Pando, V.; Olaizola, J. Could Artificial Reforestations Provide as Much Production and Diversity of Fungal Species as Natural Forest Stands in Marginal Mediterranean Areas? For. Ecol. Manag. 2010, 260, 171–180. [Google Scholar] [CrossRef]
- Dejene, T.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Fungal Diversity and Succession Following Stand Development in Pinus Patula Schiede Ex Schltdl. & Cham. Plantations in Ethiopia. For. Ecol. Manag. 2017, 395, 9–18. [Google Scholar]
- Buscardo, E.; Rodríguez-Echeverría, S.; De Angelis, P.; Freitas, H. Ectomycorrhizal Communities in Fire Prone Environments: Essential Partners for Pinetrees Re-Establishment. Ecosistemas 2009, 18, 55–63. [Google Scholar]
- Sun, H.; Santalahti, M.; Pumpanen, J.; Köster, K.; Berninger, F.; Raffaello, T.; Jumpponen, A.; Asiegbu, F.O.; Heinonsalo, J. Fungal Community Shifts in Structure and Function across a Boreal Forest Fire Chronosequence. Appl. Environ. Microbiol. 2015, 81, 7869–7880. [Google Scholar] [CrossRef] [PubMed]
- Castaño, C.; Lindahl, B.D.; Alday, J.G.; Hagenbo, A.; Martínez-de-Aragón, J.; Parladé, J.; Pera, J.; Bonet, J.A. Soil Microclimate Changes Affect Soil Fungal Communities in a Mediterranean Pine Forest. New Phytol. 2018, 220, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, A. Effects of Fire on Ectomycorrhizal Fungi in Fennoscandian Boreal Forests. Silva Fenn. 2002, 36, 69–80. [Google Scholar] [CrossRef]
- Treseder, K.; Mack, M.C.; Cross, A. Relationships among Fires, Fungi, and Soil Dynamics in Alaskan Boreal Forests. Ecol. Appl. 2004, 14, 1826–1838. [Google Scholar] [CrossRef]
- Dejene, T.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Fungal Community Succession and Sporocarp Production Following Fire Occurrence in Dry Afromontane Forests of Ethiopia. For. Ecol. Manag. 2017, 398, 37–47. [Google Scholar] [CrossRef]
- Visser, S. Ectomycorrhizal Fungal Succession in Jack Pine Stands Following Wildfire. New Phytol. 1995, 129, 389–401. [Google Scholar] [CrossRef]
- Buscardo, E.; Freitas, H.; Pereira, J.S.; de Angelis, P. Common Environmental Factors Explain Both Ectomycorrhizal Species Diversity and Pine Regeneration Variability in a Post-Fire Mediterranean Forest. Mycorrhiza 2011, 21, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Baar, J.; Horton, T.; Kretzer, A.; Bruns, T. Mycorrhizal Colonization of Pinus Muricata from Resistant Propagules after a Stand-replacing Wildfire. Aquat. Ecol. 1999, 143, 409–418. [Google Scholar]
- Hernández-Rodríguez, M.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Post-Fire Fungal Succession in a Mediterranean Ecosystem Dominated by Cistus Ladanifer L. For. Ecol. Manag. 2013, 289, 48–57. [Google Scholar] [CrossRef]
- Ortega-Martínez, P.; Águeda, B.; Fernández-Toirán, L.M.; Martínez-Peña, F. Tree Age Influences on the Development of Edible Ectomycorrhizal Fungi Sporocarps in Pinus Sylvestris Stands. Mycorrhiza 2011, 21, 65–70. [Google Scholar] [CrossRef]
- Jonsson, L.M.; Nilsson, M.C.; Wardle, D.A.; Zackrisson, O. Context Dependent Effects of Ectomycorrhizal Species Richness on Tree Seedling Productivity. Oikos 2001, 93, 353–364. [Google Scholar] [CrossRef]
- Baxter, J.W.; Dighton, J. Phosphorus Source Alters Host Plant Response to Ectomycorrhizal Diversity. Mycorrhiza 2005, 15, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Buée, M.; Courty, P.E.; Mignot, D.; Garbaye, J. Soil Niche Effect on Species Diversity and Catabolic Activities in an Ectomycorrhizal Fungal Community. Soil Biol. Biochem. 2007, 39, 1947–1955. [Google Scholar] [CrossRef]
- Oliver, A.K.; Callaham, M.A.; Jumpponen, A. Soil Fungal Communities Respond Compositionally to Recurring Frequent Prescribed Burning in a Managed Southeastern US Forest Ecosystem. For. Ecol. Manag. 2015, 345, 1–9. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Smith, J.E.; Horton, T.R.; Weber, N.S.; Spatafora, J.W. Pezizalean Mycorrhizas and Sporocarps in Ponderosa Pine (Pinus Ponderosa) after Prescribed Fires in Eastern Oregon, USA. Mycorrhiza 2005, 15, 79–86. [Google Scholar] [CrossRef]
- Agerer, R. Exploration Types of Ectomycorrhizae: A Proposal to Classify Ectomycorrhizal Mycelial Systems According to Their Patterns of Differentiation and Putative Ecological Importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Torres, P.; Honrubia, M. Changes and Effects of a Natural Fire on Ectomycorrhizal Inoculum Potential of Soil in a Pinus Halepensis Forest. For. Ecol. Manag. 1997, 96, 189–196. [Google Scholar] [CrossRef]
- Santos-Silva, C.; Louro, R. Assessment of the Diversity of Epigeous Basidiomycota under Different Soil-Management Systems in a Montado Ecosystem: A Case Study Conducted in Alentejo. Agrofor. Syst. 2016, 90, 117–126. [Google Scholar] [CrossRef]

| Characteristics | Pinus pinaster | Pinus nigra | Pinus sylvestris |
|---|---|---|---|
| Location | Northwestern Spain (0706439 Longitude-UTM, 4632901 Latitude-UTM) | Northcentral Spain (376090 Longitude-UTM, 4711724 Latitude-UTM) | Eastern Finland (304500 Longitude-UTM, 631600-Latitude-UTM) |
| Altitude | 750–780 m.a.s.l. | 950-960 m.a.s.l | 180 m.a.s.l. |
| Mean annual rainfall (mm) | 744.0 mm | 528.1 mm | 601.0 mm |
| Variation of the precipitation of the sampling years with respect to the mean rainfall (%) | NB: +19 B1: +19 B5: +37 | NB: −18 B1: −18 B5: −18 | NB: −12 B1: −12 B5: −15 |
| Mean Ta (°C) | 14.5 °C | 10.5 °C | 2.1 °C |
| Variation of the temperature of the sampling years with respect to the mean temperature (%) | NB: +18 B1: +18 B5: +18 | NB: +18 B1: +18 B5: +18 | NB: −20 B1: −20 B5: 0 |
| Soil characteristics | Paleozoic metamorphic rocks, dominated by Ordovician and Silurian shales | Cluster, siliceous sand and shales | Nutrient-poor mineral soil, i.e., podzol soil, acidic and oligotrophic |
| Shrub and secondary species communities | Quercus ilex subsp. ballota (Desf.) Samp., Q. pyrenaica Willd and Cistus ladanifer L. | Q. pyrenaica Willd and C. laurifolius L. | Picea abies (L.) H. Karst, Vaccinium myrtillus L., V. vitis-idaea L., Betula pendula Roth and B. pubescens Ehrh. |
| Study Sites | Wet Mediterranean Pinus pinaster | Dry Mediterranean Pinus nigra | Boreal Pinus sylvestris |
|---|---|---|---|
| Wet Mediterranean Pinus pinaster | 0.886 | 0.231 | |
| Dry Mediterranean Pinus nigra | 39 | 0.215 | |
| Boreal Pinus sylvestris | 15 | 14 |
| Study Sites | PpNB | PpB1 | PpB5 | PnNB | PnB1 | PnB5 | PsNB | PsB1 | PsB5 |
|---|---|---|---|---|---|---|---|---|---|
| PpNB | 0.172 | 0.306 | 0.317 | 0.067 | 0.250 | 0.161 | 0.029 | 0.103 | |
| PpB1 | 5 | 0.194 | 0.100 | 0.100 | 0.129 | 0.040 | 0.182 | 0.138 | |
| PpB5 | 11 | 6 | 0.239 | 0.207 | 0..333 | 0.147 | 0.114 | 0.150 | |
| PnNB | 13 | 4 | 11 | 0.235 | 0.410 | 0.211 | 0.045 | 0.152 | |
| PnB1 | 2 | 2 | 6 | 8 | 0.179 | 0.143 | 0.091 | 0.148 | |
| PnB5 | 9 | 4 | 12 | 16 | 5 | 0.057 | 0.028 | 0.048 | |
| PsNB | 5 | 1 | 5 | 8 | 3 | 2 | 0.120 | 0.167 | |
| PsB1 | 1 | 4 | 4 | 2 | 2 | 1 | 3 | 0.207 | |
| PsB5 | 4 | 4 | 6 | 7 | 4 | 2 | 5 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Manchón, I.; Salo, K.; Oria-de-Rueda, J.A.; Bonet, J.A.; Martín-Pinto, P. Are Wildfires a Threat to Fungi in European Pinus Forests? A Case Study of Boreal and Mediterranean Forests. Forests 2019, 10, 309. https://doi.org/10.3390/f10040309
Franco-Manchón I, Salo K, Oria-de-Rueda JA, Bonet JA, Martín-Pinto P. Are Wildfires a Threat to Fungi in European Pinus Forests? A Case Study of Boreal and Mediterranean Forests. Forests. 2019; 10(4):309. https://doi.org/10.3390/f10040309
Chicago/Turabian StyleFranco-Manchón, Iván, Kauko Salo, Juan Andrés Oria-de-Rueda, José Antonio Bonet, and Pablo Martín-Pinto. 2019. "Are Wildfires a Threat to Fungi in European Pinus Forests? A Case Study of Boreal and Mediterranean Forests" Forests 10, no. 4: 309. https://doi.org/10.3390/f10040309
APA StyleFranco-Manchón, I., Salo, K., Oria-de-Rueda, J. A., Bonet, J. A., & Martín-Pinto, P. (2019). Are Wildfires a Threat to Fungi in European Pinus Forests? A Case Study of Boreal and Mediterranean Forests. Forests, 10(4), 309. https://doi.org/10.3390/f10040309





