Leaf Enzyme and Plant Productivity Responses to Environmental Stress Associated with Sea Level Rise in Two Asian Mangrove Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental System Settings
2.2.2. Measurements Method
2.2.3. Data analysis method
3. Results
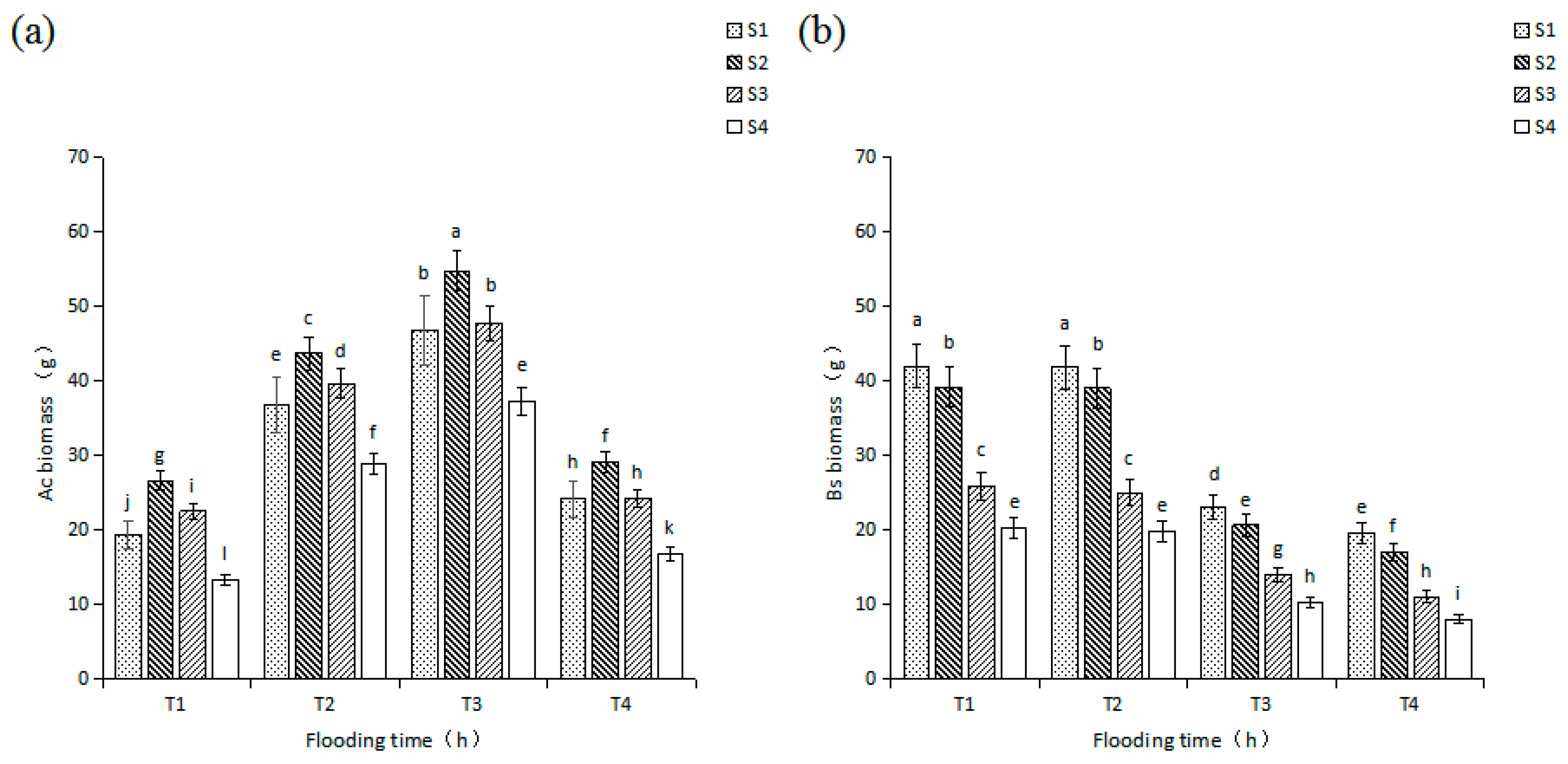
3.1. Effects of Salinity and Flooding Time on the Biomass of Seedlings
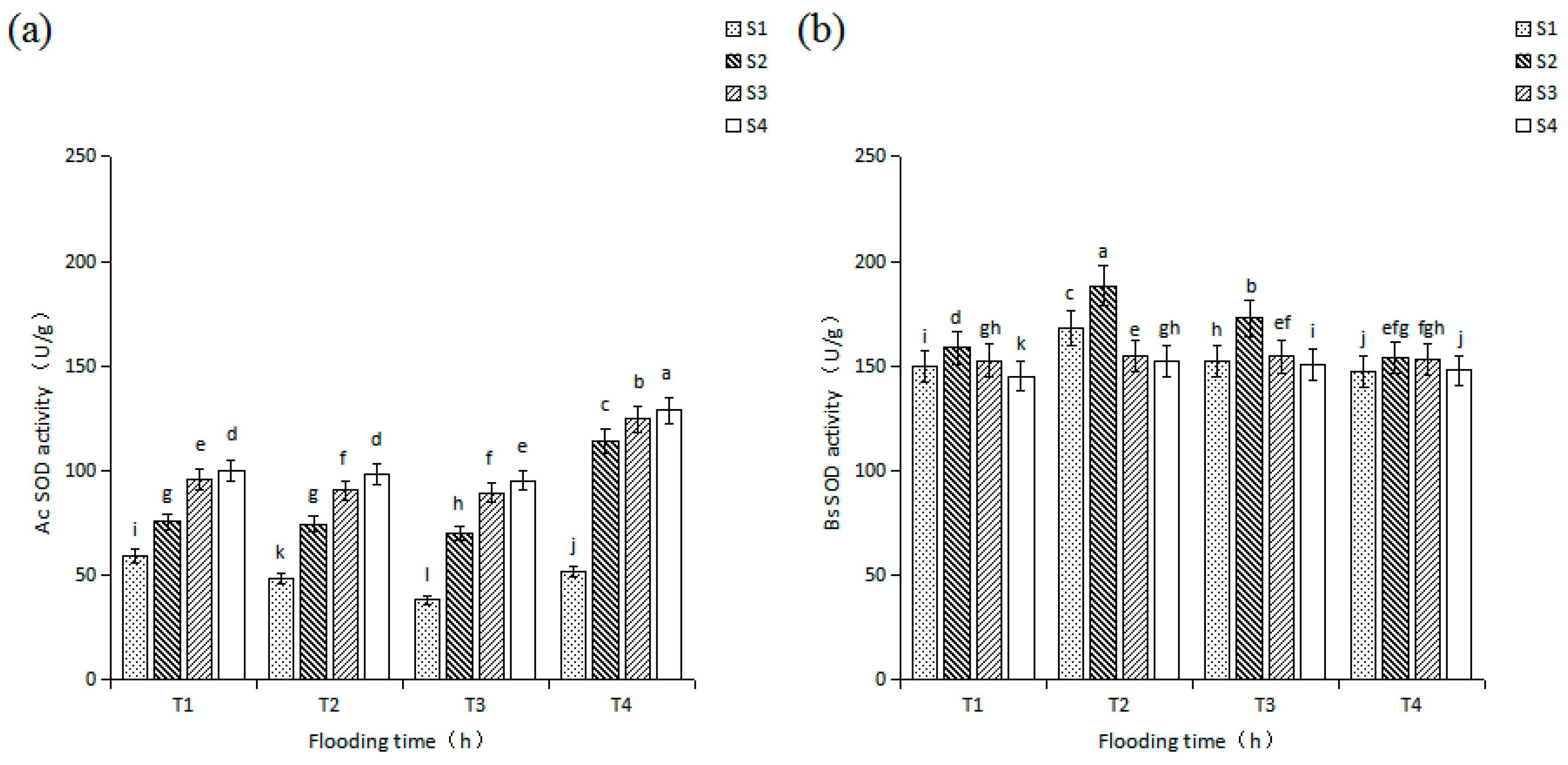
3.2. Effects of Salinity and Flooding Time on SOD Activity in Seedlings Leaves
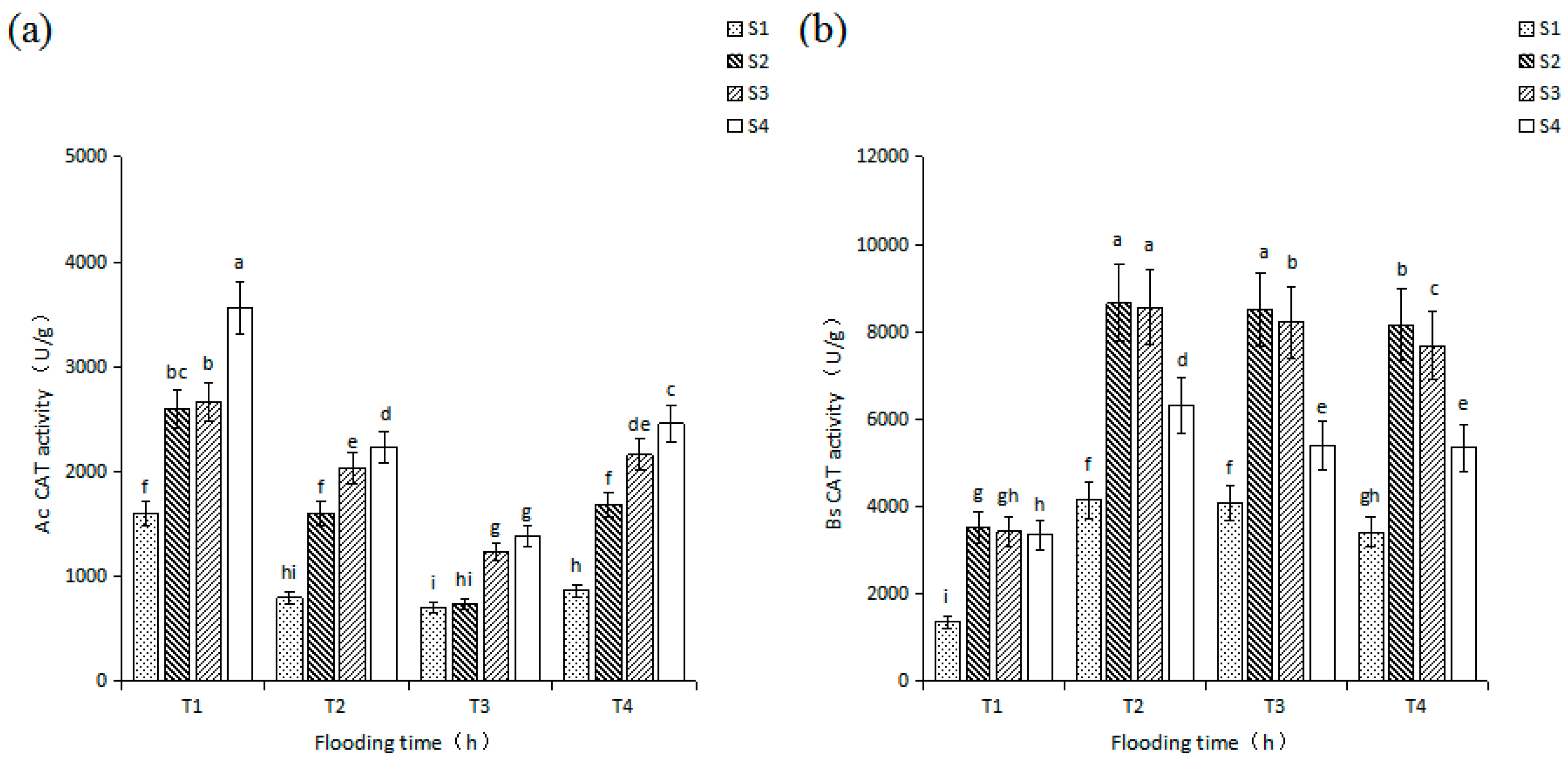
3.3. Effects of Salinity and Flooding Time on CAT Activity in Seedlings Leaves
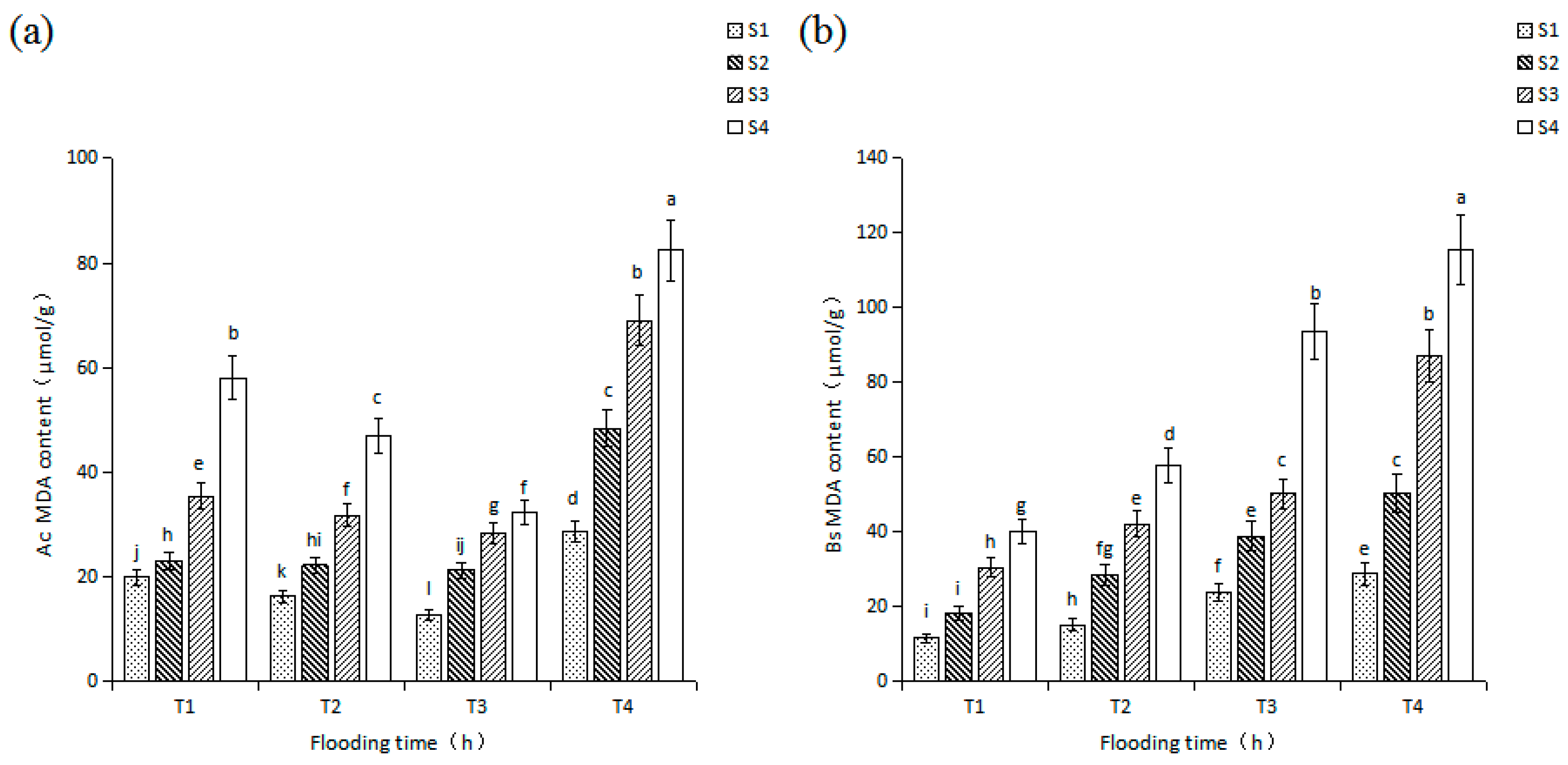
3.4. Effects of Salinity and Flooding Time on MDA Level in Seedlings Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.Q.; Wang, M. The Mangroves of China; Science Press: Beijing, China, 2007; pp. 10–19. [Google Scholar]
- Ken, W.K.; Karen, L.M.; Catherine, E.L.; Donald, R.C.; Neil, S.; Ruth, R.; Luzhen, C. How mangrove forests adjust to rising sea level. New Phytol. 2014, 202, 19–34. [Google Scholar]
- Semeniuk, V. Predicting the Effect of Sea-Level Rise on Mangroves in Northwestern Australia. J. Coast. Res. 1994, 10, 1050–1076. [Google Scholar]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Gopal. Future of wetlands in tropical and subtropical Asia, especially in the face of climate change. Aquat. Sci. 2013, 75, 39–61. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Peng, L. Photosynthetic and physiological responses of Kandelia candel L. Druce seedlings to duration of tidal immersion in artificial seawater. Environ. Exp. Bot. 2005, 54, 256–266. [Google Scholar]
- He, B.; Lai, T.; Fan, H.; Wang, W.; Zheng, H. Comparison of flooding-tolerance in four mangrove species in a diurnal tidal zone in the Beibu Gulf. Estuar. Coast. Shelf Sci. 2007, 74, 254–262. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W. Ecophysiological Responses of Viviparous Mangrove Rhizophora stylosa Seedlings to Simulated Sea-Level Rise. J. Coast. Res. 2016, 336. [Google Scholar] [CrossRef]
- Chen, L.Z.; Tam, N.F.Y.; Wang, W.Q.; Zhang, Y.H.; Lin, G.H. Significant niche overlap between native and exotic Sonneratia mangrove species along a continuum of varying inundation periods. Estuar. Coast. Shelf Sci. 2013, 117, 22–28. [Google Scholar] [CrossRef]
- Ellison, A.M.; Farnsworth, E.J. Simulated sea level change alters anatomy, physiology, growth, and reproduction of red mangrove (Rhizophora mangle L.). Oecologia 1997, 112, 435–446. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.L. Soil Physicochemical Patterns and Mangrove Species Distribution--Reciprocal Effects? J. Ecol. 1993, 81, 477–487. [Google Scholar] [CrossRef]
- Ye, Y.; Tam, N.F.Y.; Wong, Y.S.; Lu, C.Y. Growth and physiological responses of two mangrove species (Bruguiera gymnorrhiza and Kandelia candel) to waterlogging. Environ. Exp. Bot. 2003, 49, 209–221. [Google Scholar] [CrossRef]
- Satyanarayana, B.; Idris, I.F.; Mohamad, K.A.; Husain, M.L.; Shazili, N.A.M.; Dahdouh-Guebas, F. Mangrove species distribution and abundance in relation to local environmental settings: A case-study at Tumpat, Kelantan Delta, east coast of peninsular Malaysia. Bot. Marina 2010, 53, 79–88. [Google Scholar] [CrossRef]
- Knight, J.M.; Dale, P.E.R.; Dunn, R.J.K.; Broadbent, G.J.; Lemckert, C.J. Patterns of tidal flooding within a mangrove forest: Coombabah Lake, Southeast Queensland, Australia. Estuar. Coast. Shelf Sci. 2008, 76, 580–593. [Google Scholar] [CrossRef]
- Lugo, A.E.; Snedaker, S.C. The Ecology of Mangroves. Aunu. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Piou, C.; Feller, I.C.; Berger, U.; Chi, F. Zonation Patterns of Belizean Offshore Mangrove Forests 41 Years After a Catastrophic Hurricane. Biotropica 2010, 38, 365–374. [Google Scholar] [CrossRef]
- Watson, J.G. Mangrove forests of the Malay Peninsula. Malayan For. Records 1928, 6, 1–275. [Google Scholar]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Wang, B.S. Biological Free Radicals and Membrane Damage of Plants. Plant Physiol. Commun. 1988, 2, 12–16. [Google Scholar]
- Hodgson, R.A.J.; Orr, G.R.; Raison, J.K. Inhibition of photosynthesis by chilling in the light. Plant Sci. 1987, 49, 75–79. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.F.Y.; Yao, M.W.Y. Concentrations of PCBs in coastal mangrove sediments of Hong Kong. Mar. Poll. Bull. 2002, 44, 642–651. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J.; Ibrahim, M.S.; Osman, H. Antioxidant activities of mangrove Rhizophora apiculata bark extracts. Food Chem. 2008, 107, 200–207. [Google Scholar] [CrossRef]
- Rychter, A.M. Antioxidants and reactive oxygen species in plants. Ann. Bot. 2006, 98, 1114. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. A re-evaluation of the ATP:NADPH budget during C3 photosynthesis: A contribution from nitrate assimilation and its associated respiratory activity? J. Exp. Bot. 1998, 49, 1895–1908. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, P. Study on the membrane lipid peroxidation of the leaves of Kandelia candel seedlings to long-term and short-term salinity. Acta Oceanol. Sinica, 2000. [Google Scholar]
- Modarresi, M.; Moradian, F.; Nematzadeh, G.A. Antioxidant responses of halophyte plant Aeluropus littoralis under long-term salinity stress. Biologia 2014, 69, 478–483. [Google Scholar] [CrossRef]
- Ben Amor, N.; Jimenez, A.; Megdiche, W.; Lundqvist, M.; Sevilla, F.C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol. Plant. 2010, 126, 446–457. [Google Scholar] [CrossRef]
- Pang, C.H.; Zhang, S.J.; Gong, Z.Z.; Wang, B.S. NaCl treatment markedly enhances H2O2 - scavenging system in leaves of halophyte Suaeda salsa. Physiol. Plant. 2010, 125, 490–499. [Google Scholar]
- Zhang, Z.L.; Qu, W.J. Experimental Guidance for Plant Physiology; Higher Educated Press: Beijing, China, 2003; pp. 40–45. [Google Scholar]
- Qi, Z. Experimental Instruction of Plant Physiology; Agricultural Press of China: Beijing, China, 2004; pp. 26–56. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2015, 20, 154–159. [Google Scholar] [CrossRef]
- Ellison, J.C. Vulnerability assessment of mangroves to climate change and sea-level rise impacts. Wetl. Ecol. Manag. 2015, 23, 115–137. [Google Scholar] [CrossRef]
- Yáñez-Espinosa, L.; Terrazas, T.; López-Mata, L. Effects of flooding on wood and bark anatomy of four species in a mangrove forest community. Trees 2001, 15, 91–97. [Google Scholar] [CrossRef]
- Youssef, T.; Saenger, P. Anatomical adaptive strategies to flooding and rhizosphere oxidation in mangrove seedlings. Aust. J. Bot. 1996, 44, 297–313. [Google Scholar] [CrossRef]
- Krauss, K.W.; Twilley, R.R.; Doyle, T.W.; Gardiner, E.S. Leaf gas exchange characteristics of three neotropical mangrove species in response to varying hydroperiod. Tree Physiol. 2006, 26, 959–968. [Google Scholar] [CrossRef]
- Liao, Y.; Lan, Z. The effect of salt stress on membrane protection system for roots, stems and leaves of Rhizophora stylosa seedlings. Ecol. Environ. 2007, 16, 1449–1454. [Google Scholar]
- Ye, Y.; Gu, Y.T.; Gao, H.Y.; Lu, C.Y. Combined effects of simulated tidal sea-level rise and salinity on seedlings of a mangrove species, Kandelia candel (L.) Druce. Hydrobiologia 2010, 641, 287–300. [Google Scholar] [CrossRef]
- Hoppe-Speer, S.C.L.; Adams, J.B.; Rajkaran, A.; Bailey, D. The response of the red mangrove Rhizophora mucronata Lam. to salinity and inundation in South Africa. Aquat. Bot. 2011, 95, 71–76. [Google Scholar] [CrossRef]
- Baowen, L. The Adaptability of Seedlings of Three Mangrove Species to Tide-Flooding and Water Salinity. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2010. [Google Scholar]
- Meijuan, L. Studies on the Aegiceras Corniculation Seedings in Response to Simulated Tidal Flooding Stress. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2012. [Google Scholar]
- Ball, M.C. Interactive effects of salinity and irradiance on growth: Implications for mangrove forest structure along salinity gradients. Trees 2002, 16, 126–139. [Google Scholar] [CrossRef]
- Burchett, M.D.; Field, C.D.; Pulkownik, A. Salinity, growth and root respiration in the grey mangrove, Avicennia marina. Physiol. Plant. 2010, 60, 113–118. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, R.C.; Dong, S.S.; Su, H. Adaptation to salinity in mangroves: Implication on the evolution of salt-tolerance. Chin. Sci. Bull. 2008, 53, 1708–1715. [Google Scholar] [CrossRef]
- Doyle, T.W.; Krauss, K.W.; Conner, W.H.; From, A.S. Predicting the retreat and migration of tidal forests along the northern Gulf of Mexico under sea-level rise. For. Ecol. Manag. 2010, 259, 770–777. [Google Scholar] [CrossRef]
| Treatment | Salinity (ppt) | Flooding Time (h) | Treatment | Salinity (ppt) | Flooding Time (h) |
|---|---|---|---|---|---|
| S1T1 | 10 | 2 | S3T1 | 30 | 6 |
| S1T2 | 10 | 2 | S3T2 | 30 | 6 |
| S1T3 | 10 | 2 | S3T3 | 30 | 6 |
| S1T4 | 10 | 2 | S3T4 | 30 | 6 |
| S2T1 | 20 | 4 | S4T1 | 40 | 8 |
| S2T2 | 20 | 4 | S4T2 | 40 | 8 |
| S2T3 | 20 | 4 | S4T3 | 40 | 8 |
| S2T4 | 20 | 4 | S4T5 | 40 | 8 |
| Flooding Time (h) | Injection Water Time | Discharge Water Time |
|---|---|---|
| 2 (T1) | 07:00; 19:00 | 09:00: 21:00 |
| 4 (T2) | 07:00; 19:00 | 11:00; 23:00 |
| 6 (T3) | 07:00; 19:00 | 13:00; 01:00 |
| 8 (T4) | 07:00; 19:00 | 15:00; 03:00 |
| Species | Factor | Indices | df | F Value | Partial eta Squared | Sig. |
|---|---|---|---|---|---|---|
| S | Biomass | 3 | 432.099 | 0.976 | *** | |
| SOD | 3 | 7686.618 | 0.999 | *** | ||
| CAT | 3 | 437.385 | 0.976 | *** | ||
| MDA | 3 | 2853.114 | 0.996 | *** | ||
| Ac | T | Biomass | 3 | 1784.257 | 0.994 | *** |
| SOD | 3 | 2330.502 | 0.995 | *** | ||
| CAT | 3 | 515.113 | 0.98 | *** | ||
| MDA | 3 | 2582.568 | 0.996 | *** | ||
| S*T | Biomass | 9 | 3.824 | 0.518 | *** | |
| SOD | 9 | 227.149 | 0.985 | *** | ||
| CAT | 9 | 19.496 | 0.846 | *** | ||
| MDA | 9 | 158.578 | 0.978 | *** | ||
| S | Biomass | 3 | 779.386 | 0.986 | *** | |
| SOD | 3 | 846.407 | 0.988 | *** | ||
| CAT | 3 | 4091.513 | 0.997 | *** | ||
| MDA | 3 | 7272.151 | 0.999 | *** | ||
| Bs | T | Biomass | 3 | 1063.382 | 0.99 | *** |
| SOD | 3 | 599.598 | 0.983 | *** | ||
| CAT | 3 | 4063.503 | 0.997 | *** | ||
| MDA | 3 | 4639.692 | 0.998 | *** | ||
| S*T | Biomass | 9 | 25.034 | 0.876 | *** | |
| SOD | 9 | 139.975 | 0.975 | *** | ||
| CAT | 9 | 152.487 | 0.977 | *** | ||
| MDA | 9 | 452.477 | 0.992 | *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Li, D.; Yang, X.; Zhang, M.; Deng, Q. Leaf Enzyme and Plant Productivity Responses to Environmental Stress Associated with Sea Level Rise in Two Asian Mangrove Species. Forests 2019, 10, 250. https://doi.org/10.3390/f10030250
Lv X, Li D, Yang X, Zhang M, Deng Q. Leaf Enzyme and Plant Productivity Responses to Environmental Stress Associated with Sea Level Rise in Two Asian Mangrove Species. Forests. 2019; 10(3):250. https://doi.org/10.3390/f10030250
Chicago/Turabian StyleLv, Xiaobo, Donghai Li, Xiaobo Yang, Mengwen Zhang, and Qin Deng. 2019. "Leaf Enzyme and Plant Productivity Responses to Environmental Stress Associated with Sea Level Rise in Two Asian Mangrove Species" Forests 10, no. 3: 250. https://doi.org/10.3390/f10030250
APA StyleLv, X., Li, D., Yang, X., Zhang, M., & Deng, Q. (2019). Leaf Enzyme and Plant Productivity Responses to Environmental Stress Associated with Sea Level Rise in Two Asian Mangrove Species. Forests, 10(3), 250. https://doi.org/10.3390/f10030250






