Propagule Dispersal Determines Mangrove Zonation at Intertidal and Estuarine Scales
Abstract
:1. Introduction
2. Materials and Methods
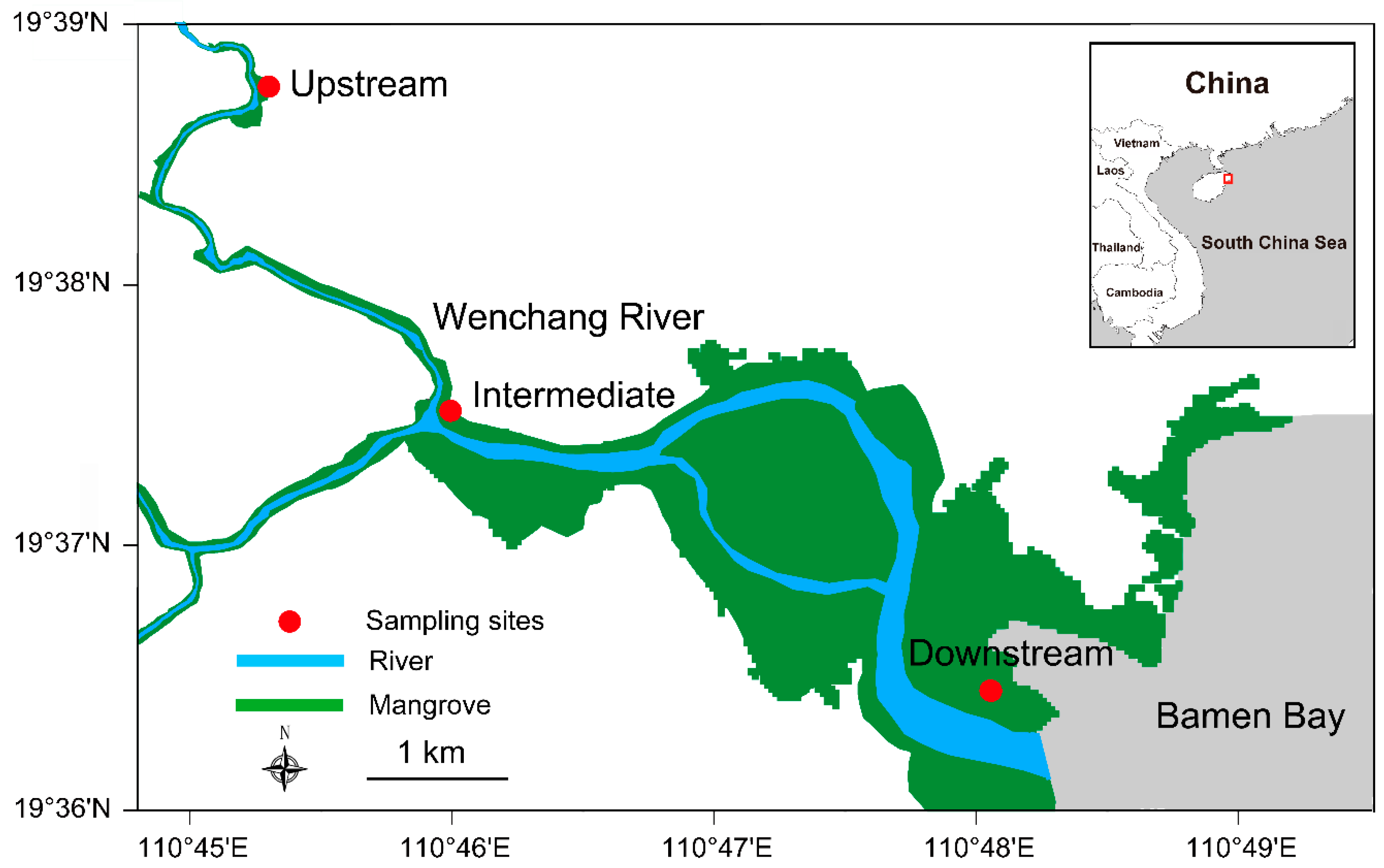
2.1. Research Site
2.2. Measurement of Surface Elevation
- i: code number of each survey station
- K: code number of the landward station of a transect
- Hi: surface elevation of station i
- Ni: individual number of mangrove species at station i
2.3. Collection of Mature Propagules
2.4. Observation of Specific Gravity, Buoyancy and Root Initiation during Propagule Dispersal
2.5. Measurements of Propagule Characteristics
2.6. Statistics
3. Results
3.1. Static Densities of Mangrove Species
3.2. Changes in Propagule Specific Gravity during Floating
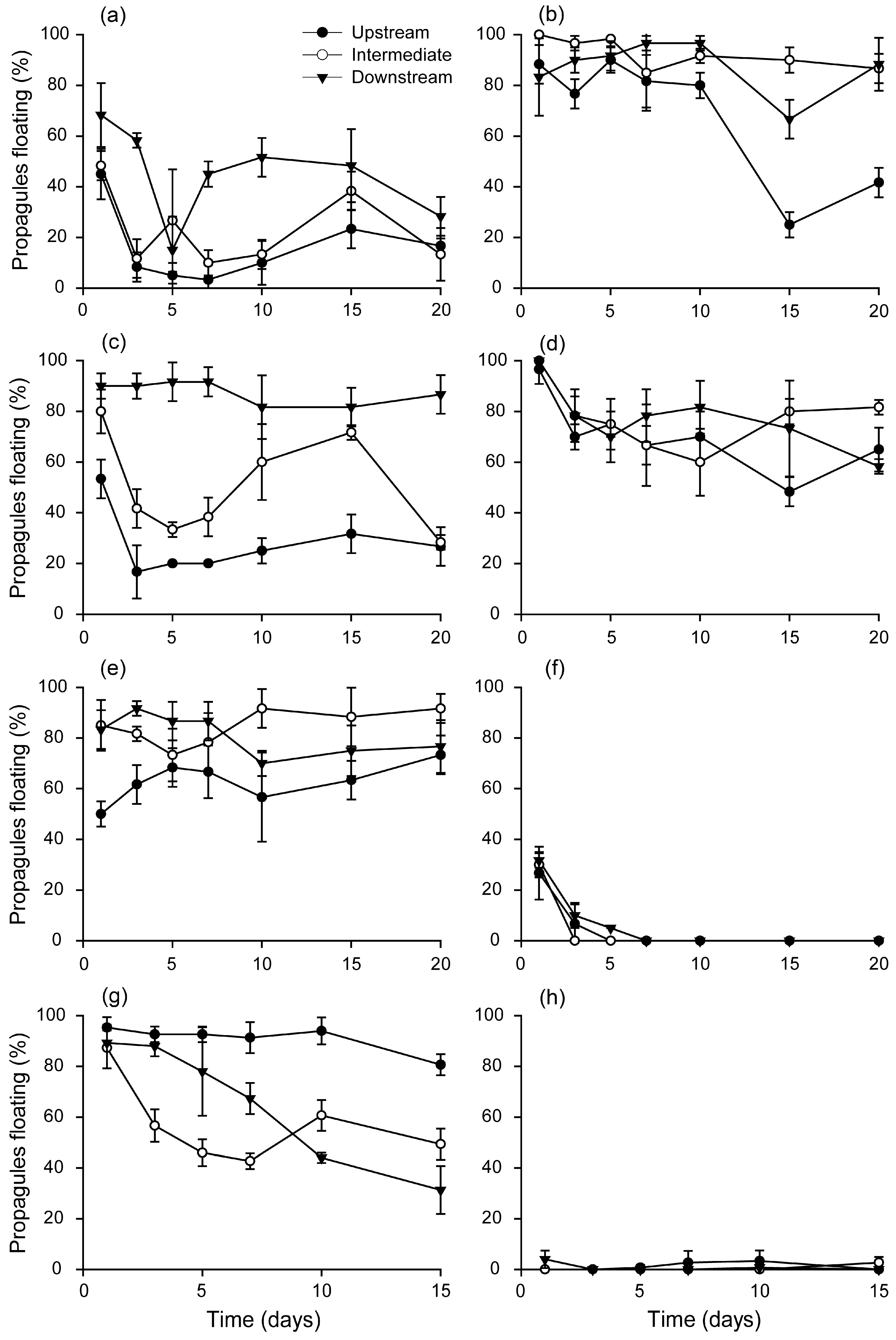
3.3. Changes in Propagule Buoyancy during Floating
3.4. Root Initiation during Dispersal
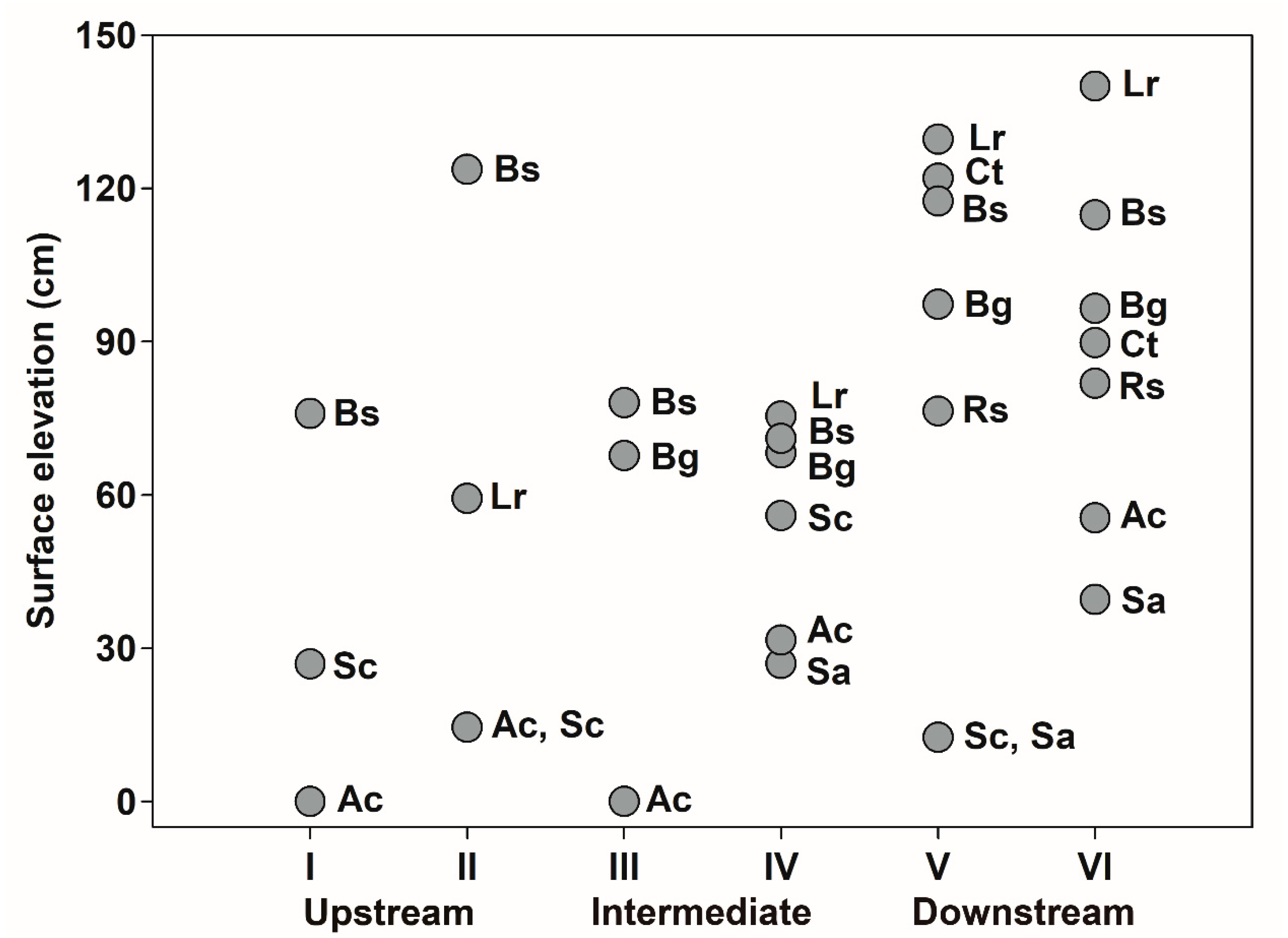
3.5. Intertidal and Estuarine Distribution of Mangrove Species
3.6. Propagule Dispersal Attributes and Tidal Distribution Patterns
4. Discussion
4.1. Effect of Water Salinity on Propagule Buoyancy and Propagule Changes during Dispersal
4.2. Propagule Specific Gravity and Root Initiation Determine Establishment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sousa, W.P.; Kennedy, P.G.; Mitchell, B.J.; Ordóñez, B.M. Supply-side ecology in mangroves: Do propagule dispersal and seedling establishment explain forest structure? Ecol. Monogr. 2007, 77, 53–76. [Google Scholar] [CrossRef]
- Clobert, J.; Baguette, M.; Benton, T.G.; Bullock, J.M. Dispersal Ecology and Evolution, 1st ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Bonte, D.; Dahirel, M. Dispersal: A central and independent trait in life history. Oikos 2017, 126, 472–479. [Google Scholar] [CrossRef]
- Beckman, N.G.; Bullock, J.M.; Salguero-Gómez, R. High dispersal ability is related to fast life-history strategies. J. Ecol. 2018, 106, 1349–1362. [Google Scholar] [CrossRef]
- Connell, J.H. The consequences of variation in initial settlement vs. post-settlement mortality in rocky intertidal communities. J. Exp. Mar. Biol. Ecol. 1985, 93, 11–45. [Google Scholar] [CrossRef]
- Young, C.M. Novelty of “supply-side ecology”. Science 1987, 235, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Mapstone, B.D.; Fowler, A.J. Recruitment and the structure of assemblages of fish on coral reefs. Trends Ecol. Evol. 1988, 3, 72–77. [Google Scholar] [CrossRef]
- Underwood, A.J.; Fairweather, P.G. Supply-side ecology and benthic marine assemblages. Trends Ecol. Evol. 1989, 4, 16–20. [Google Scholar] [CrossRef]
- Booth, D.J.; Brosnan, D.M. The role of recruitment dynamics in rocky shore and coral reef fish communities. Adv. Ecol. Res. 1995, 26, 309–385. [Google Scholar]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Wright, S.J.; Calderón, O.; Condit, R.; Hubbell, S.P. Interspecific variation in primary seed dispersal in a tropical forest. J. Ecol. 2008, 96, 653–667. [Google Scholar] [CrossRef]
- Caughlin, T.T.; Ferguson, J.M.; Lichstein, J.W.; Bunyavejchewin, S.; Levey, D.J. The importance of long-distance seed dispersal for the demography and distribution of a canopy tree species. Ecology 2014, 95, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Cetina-Heredia, P.; Roughan, M.; van Sebille, E.; Feng, M.; Coleman, M.A. Strengthened currents override the effect of warming on lobster larval dispersal and survival. Glob. Chang. Biol. 2015, 21, 4377–4386. [Google Scholar] [CrossRef] [PubMed]
- Van der Stocken, T.; Carroll, D.; Menemenlis, D.; Simard, M.; Koedam, N. Global-scale dispersal and connectivity in mangroves. Proc. Natl. Acad. Sci. USA 2019, 116, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Van der Pijl, L. Principles of Dispersal in Higher Plants, 3rd ed.; Springer: New York, NY, USA, 1982. [Google Scholar]
- Nathan, R.; Safriel, U.N.; Noy-Meir, I. Field validation and sensitivity analysis of a mechanistic model for tree seed dispersal by wind. Ecology 2001, 82, 374–388. [Google Scholar] [CrossRef]
- Levin, S.A.; Muller-Landau, H.C.; Nathan, R.; Chave, J. The ecology and evolution of seed dispersal: A theoretical perspective. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 575–604. [Google Scholar] [CrossRef]
- Van der Stocken, T.; Vanschoenwinkel, B.; De Ryck, D.; Koedam, N. Caught in transit: Offshore interception of seafaring propagules from seven mangrove species. Ecosphere 2018, 9, e02208. [Google Scholar] [CrossRef]
- Duke, N.C. Australia’s Mangroves: The Authoritative Guide to Australia’s Mangrove Plants, 1st ed.; University of Queensland Press: Brisbane, Australia, 2006. [Google Scholar]
- Chapman, S.K.; Feller, I.C. Away-field advantage: Mangrove seedlings grow best in litter from other mangrove species. Oikos 2011, 120, 1880–1888. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves, 2nd ed.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Duke, N.C.; Ball, M.C.; Ellison, J.C. Factors influencing biodiversity and distributional gradients in mangroves. Glob. Ecol. Biogeogr. Lett. 1998, 7, 27–47. [Google Scholar] [CrossRef]
- Clarke, P.J.; Kerrigan, R.A.; Westphal, C.J. Dispersal potential and early growth in 14 tropical mangroves: Do early life history traits correlate with patterns of adult distribution? J. Ecol. 2001, 89, 648–659. [Google Scholar] [CrossRef]
- Crase, B.; Liedloff, A.; Vesk, P.A.; Burgman, M.A.; Wintle, B.A. Hydroperiod is the main driver of the spatial pattern of dominance in mangrove communities. Glob. Ecol. Biogeogr. 2013, 22, 806–817. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wang, M. The Mangroves of China; Science Press: Beijing, China, 2007. [Google Scholar]
- Ellison, A.M. Macroecology of mangroves: Large-scale patterns and processes in tropical coastal forests. Trees 2002, 16, 181–194. [Google Scholar] [CrossRef]
- McKee, K.L. Interspecific variation in growth, biomass partitioning, and defensive characteristics of neotropical mangrove seedlings: Response to light and nutrient availability. Am. J. Bot. 1995, 82, 299–307. [Google Scholar] [CrossRef]
- Sousa, W.P.; Mitchell, B.J. The effect of seed predators on plant distributions: Is there a general pattern in mangroves? Oikos 1999, 86, 55–66. [Google Scholar] [CrossRef]
- Allen, J.A.; Krauss, K.W. Influence of propagule flotation longevity and light availability on establishment of introduced mangrove species in Hawai’i. Pac. Sci. 2006, 60, 367–376. [Google Scholar] [CrossRef]
- De Ryck, D.J.R.; Robert, E.M.R.; Schmitz, N.; Van der Stocken, T.; Nitto, D.D.; Dahdouh-Guebas, F.; Koedam, N. Size does matter, but not only size: Two alternative dispersal strategies for viviparous mangrove propagules. Aquat. Bot. 2012, 103, 66–73. [Google Scholar] [CrossRef]
- Van der Stocken, T.; De Ryck, D.J.R.; Vanschoenwinkel, B.; Deboelpaep, E.; Bouma, T.J.; Dahdouh-Guebas, F.; Koedam, N. Impact of landscape structure on propagule dispersal in mangrove forests. Mar. Ecol. Prog. Ser. 2015, 524, 95–106. [Google Scholar] [CrossRef]
- Steinke, T.D.; Ward, C.J. Use of plastic drift cards as indicators of possible dispersal of propagules of the mangrove Avicennia marina by ocean currents. Afr. J. Mar. Sci. 2003, 25, 169–176. [Google Scholar] [CrossRef]
- Wang, W.Q.; Ke, L.; Tam, N.F.Y.; Wong, Y.S. Changes in the main osmotica during the development of Kandelia candel hypocotyls and after mature hypocotyls were transplanted in solutions with different salinities. Mar. Biol. 2002, 141, 1029–1034. [Google Scholar]
- Robert, E.M.R.; Oste, J.; Van der Stocken, T.; De Ryck, D.J.R.; Quisthoudt, K.; Kairo, J.G.; Dahdouh-Guebas, F.; Koedam, N.; Schmitz, N. Viviparous mangrove propagules of Ceriops tagal and Rhizophora mucronata, where both Rhizophoraceae show different dispersal and establishment strategies. J. Exp. Mar. Biol. Ecol. 2015, 468, 45–54. [Google Scholar] [CrossRef]
- Tonné, N.; Beeckman, H.; Robert, E.M.R.; Koedam, N. Towards an unknown fate: The floating behaviour of recently abscised propagules from wide ranging Rhizophoraceae mangrove species. Aquat. Bot. 2017, 140, 23–33. [Google Scholar] [CrossRef]
- Clarke, P.J. The population dynamics of the mangrove shrub Aegiceras corniculatum (Myrsinaceae): Fecundity, dispersal, establishment and population structure. Proc. Linn. Soc. N. S. W. 1995, 115, 35–44. [Google Scholar]
- Kadoya, T.; Inoue, T. Spatio-temporal pattern of specific gravity of mangrove diaspore: Implications for upstream dispersal. Ecography 2015, 38, 472–479. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Sauter, K. Structure and dynamics of mangrove forests along a flooding gradient. Estuaries 1991, 14, 49–56. [Google Scholar] [CrossRef]
- Ye, Y.; Cao, C.Q. Factors influencing mangroves intertidal zonation. Chin. J. Ecol. 2008, 27, 615–618. [Google Scholar]
- Ye, Y.; Lu, C.Y.; Hu, H.Y.; Tam, N.F.Y. Comparisons of tolerances to salt stress among three salt-secreting mangrove species. Acta Ecol. Sin. 2004, 24, 2444–2450. [Google Scholar]
- Fang, J.Y.; Wang, X.P.; Shen, Z.H.; Tang, Z.Y.; He, J.S.; Yu, D.; Jiang, Y.; Wang, Z.H.; Zheng, C.Y.; Zhu, J.L.; et al. Methods and protocols for plant community inventory. Biodivers. Sci. 2009, 17, 533–548. [Google Scholar]
- Oh, R.R.Y.; Friess, D.A.; Brown, B.M. The role of surface elevation in the rehabilitation of abandoned aquaculture ponds to mangrove forests, Sulawesi, Indonesia. Ecol. Eng. 2017, 100, 325–334. [Google Scholar] [CrossRef]
- Leong, R.C.; Friess, D.A.; Crase, B.; Lee, W.K.; Webb, E.L. High-resolution pattern of mangrove species distribution is controlled by surface elevation. Estuar. Coast. Shelf Sci. 2018, 202, 185–192. [Google Scholar] [CrossRef]
- Rabinowitz, D. Dispersal properties of mangrove propagules. Biotropica 1978, 10, 47–57. [Google Scholar] [CrossRef]
- Hughes, S.W. Archimedes revisited: A faster, better, cheaper method of accurately measuring the volume of small objects. Phys. Educ. 2005, 40, 468–474. [Google Scholar] [CrossRef]
- Clarke, P.J.; Myerscough, P.J. Buoyancy of Avicennia marina propagules in south-eastern Australia. Aust. J. Bot. 1991, 39, 77–83. [Google Scholar] [CrossRef]
- Rabinowitz, D. Early growth of mangrove seedlings in Panamá, and an hypothesis concerning the relationship of dispersal and zonation. J. Biogeogr. 1978, 5, 113–133. [Google Scholar] [CrossRef]
- Alleman, L.K.; Hester, M.W. Reproductive ecology of black mangrove (Avicennia germinans) along the Louisiana coast: Propagule production cycles, dispersal limitations, and establishment elevations. Estuar. Coast. 2011, 34, 1068–1077. [Google Scholar] [CrossRef]
- Nakanishi, H. Dispersal ecology of the maritime plants in the Ryukyu Islands, Japan. Ecol. Res. 1988, 3, 163–173. [Google Scholar] [CrossRef]
- Johansson, M.E.; Nilsson, C.; Nilsson, E. Do rivers function as corridors for plant dispersal? J. Veg. Sci. 1996, 7, 593–598. [Google Scholar] [CrossRef]
- Balke, T.; Bouma, T.J.; Horstman, E.M.; Webb, E.L.; Erftemeijer, P.L.A.; Herman, P.M.J. Windows of opportunity: Thresholds to mangrove seedling establishment on tidal flats. Mar. Ecol. Prog. Ser. 2011, 440, 1–9. [Google Scholar] [CrossRef]
- Smith, T.J., III. Forest structure. In Tropical Mangrove Ecosystems; Coastal and Estuarine Series 41; Robertson, A.I., Alongi, D.M., Eds.; American Geophysical Union: Washington, DC, USA, 1992; pp. 101–136. [Google Scholar]
- Saenger, P. Morphological, anatomical and reproductive adaptations of Australian mangroves. In Mangrove Ecosystems in Australia: Structure, Function and Management; Clough, B.F., Ed.; Australian Institute of Marine Science in Association with Australian National University Press: Canberra, Australia, 1982; pp. 153–191. [Google Scholar]
- McIlroy, R.; Desvoyes, M. Volume 4 Beach Combing. In The Seashore Life of the Brunei Heart of Borneo, 1st ed.; Brunei Press Commercial Printing Services: Panaga, Brunei Darussalam, 2009. [Google Scholar]
- Saheb, S.B.; Kishore, I.V.; Babu, K.; Rosaiah, G.; Mallikarjuna, K. Diversity of seed germination in Xylocarpus species. Int. J. Pharm. Biol. Sci. 2015, 6, B846–B854. [Google Scholar]
- Zhang, L.; Ye, Y.; Lu, C.Y. Relationship between diaspore traits and inter-tidal zonation of non-viviparous mangrove species. J. Xiamen Univ. 2009, 48, 905–909. [Google Scholar]
- Yang, Y.; Zhong, C.R.; Li, Y.H.; Zhang, Y. The morphological structure and germination characters of seed of endangered mangrove Lumnitzera littorea (Jack.) Voigt. Mol. Plant Breed. 2016, 14, 2851–2858. [Google Scholar]
- Delgado, P.; Hensel, P.F.; Jiménez, J.A.; Day, J.W. The importance of propagule establishment and physical factors in mangrove distributional patterns in a Costa Rican estuary. Aquat. Bot. 2001, 71, 157–178. [Google Scholar] [CrossRef]
- Osborne, K.; Smith, T.J. Differential predation on mangrove propagules in open and closed canopy forest habitats. Vegetatio 1990, 89, 1–6. [Google Scholar] [CrossRef]
- Farnsworth, E.J.; Ellison, A.M. Global patterns of pre-dispersal propagule predation in mangrove forests. Biotropica 1997, 29, 318–330. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Friess, D.A.; Krauss, K.W.; Horstman, E.M.; Balke, T.; Bouma, T.J.; Galli, D.; Webb, E.L. Are all intertidal wetlands naturally created equal? Bottlenecks, thresholds and knowledge gaps to mangrove and saltmarsh ecosystems. Biol. Rev. 2012, 87, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Massel, S.R.; Furukawa, K.; Brinkman, R.M. Surface wave propagation in mangrove forests. Fluid Dyn. Res. 1999, 24, 219–249. [Google Scholar] [CrossRef]
- Jayatissa, L.P.; Wickramasinghe, W.A.A.D.L.; Dahdouh-Guebas, F.; Huxham, M. Interspecific variations in responses of mangrove seedlings to two contrasting salinities. Int. Rev. Hydrobiol. 2008, 93, 700–710. [Google Scholar] [CrossRef]
- Duke, N.C.; Meynecke, J.O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E.; Cahoon, D.R.; Friess, D.A.; Guntenspergen, G.R.; Krauss, K.W.; Reef, R.; Rogers, K.; Saunders, M.L.; Sidik, F.; Swales, A.; et al. The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 2015, 526, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Sandi, S.G.; Rodríguez, J.F.; Saintilan, N.; Riccardi, G.; Saco, P.M. Rising tides, rising gates: The complex ecogeomorphic response of coastal wetlands to sea-level rise and human interventions. Adv. Water Resour. 2018, 114, 135–148. [Google Scholar] [CrossRef]


| Species | Propagule Type | Size (Fresh Weight) (g) | Initial Specific Gravity (g/cm3) | Final Dynamic Specific Gravity (g/cm3) | ||
|---|---|---|---|---|---|---|
| Upstream | Intermediate | Downstream | ||||
| Sonneratia caseolaris (L.) Engl. * | Seed | 0.006 ± 0.017 | 0.810 ± 0.060 a | 0.985 ± 0.024 b | 1.031 ± 0.038 c | 1.080 ± 0.048 c |
| Lumnitzera racemosa Willd. | One-seeded fruit | 0.186 ± 0.028 | 0.868 ± 0.043 a | 0.963 ± 0.014 b | 0.916 ± 0.021 c | 0.948 ± 0.010 d |
| Sonneratia alba J. Smith. * | Seed | 0.072 ± 0.017 | 0.913 ± 0.043 a | 1.113 ± 0.031 b | 1.116 ± 0.020 b | 1.124 ± 0.025 c |
| Aegiceras corniculatum (L.) Blanco | Fruit/Cryptic hypocotyl | 0.907 ± 0.130 | 0.966 ± 0.026 a | 1.077 ± 0.009 b | 1.070 ± 0.010 b | 1.105 ± 0.019 c |
| Ceriops tagal (Perr.) C.B. Rob. | Seedling/Hypocotyl | 7.378 ± 1.277 | 0.975 ± 0.010 a | 0.978 ± 0.007 a | 0.991 ± 0.009 b | 1.004 ± 0.002 b |
| Bruguiera sexangula (Lour.) Poir. | Seedling/Hypocotyl | 9.252 ± 1.133 | 0.978 ± 0.027 a | 1.000 ± 0.005 b | 1.005 ± 0.005 b | 1.008 ± 0.001 c |
| Bruguiera gymnorhiza (L.) Lam. | Seedling/Hypocotyl | 23.742 ± 2.101 | 0.994 ± 0.011 a | 1.014 ± 0.008 b | 1.008 ± 0.002 b | 1.025 ± 0.004 c |
| Rhizophora stylosa Griff. | Seedling/Hypocotyl | 16.984 ± 3.075 | 1.015 ± 0.063 a | 0.992 ± 0.001 a | 0.987 ± 0.002 b | 1.009 ± 0.007 a |
| Average | 0.891 | 1.015 | 1.014 | 1.038 | ||
| Species | Site (S) | Floating Time (F) | S × F | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Sonneratia caseolaris | 3.899 | 0.049 * | 43.440 | 0.001 ** | 1.739 | 0.188 |
| Lumnitzera racemosa | 0.510 | 0.476 | 4.599 | 0.033 ** | 2.678 | 0.103 |
| Sonneratia alba | 0.690 | 0.407 | 36.112 | 0.001 ** | 0.289 | 0.591 |
| Aegiceras corniculatum | 10.696 | 0.001 ** | 57.247 | 0.001 ** | 1.396 | 0.239 |
| Ceriops tagal | 0.437 | 0.510 | 32.421 | 0.001 ** | 0.792 | 0.375 |
| Bruguiera sexangula | 0.255 | 0.614 | 1.416 | 0.236 | 1.844 | 0.176 |
| Bruguiera gymnorhiza | 18.351 | 0.001 ** | 0.064 | 0.801 | 3.498 | 0.063 |
| Rhizophora stylosa | 55.180 | 0.001 ** | 7.908 | 0.005 ** | 13.918 | 0.001 ** |
| Species | Root Initiation Time (day) | Rooting Rate after 20 Days of Floating (%) | ||||
|---|---|---|---|---|---|---|
| Upstream | Intermediate | Downstream | Upstream | Intermediate | Downstream | |
| Bruguiera sexangula | 3 | 3 | 5 | 98.3 ± 2.9 a | 95.0 ± 5.0 a | 90.0 ± 5.0 a |
| Bruguiera gymnorhiza | 7 | 7 | 7 | 91.7 ± 7.6 a | 95.0 ± 8.6 a | 95.0 ± 5.0 a |
| Rhizophora stylosa | 7 | 7 | 7 | 25.0 ± 8.7 a | 21.7 ± 7.6 a | 76.6 ± 16.0 b |
| Aegiceras corniculatum | 7 | 10 | - | 70.0 ± 21.8 a | 30.0 ± 18.0 b | 0 |
| Ceriops tagal | - | - | - | 0 | 0 | 0 |
| Lumnitzera racemosa | - | - | - | 0 | 0 | 0 |
| Sonneratia caseolaris * | 3 | 3 | 7 | 60.7 ± 3.1 a | 54.7 ± 6.4 a | 15.3 ± 3.1 b |
| Sonneratia alba * | 1 | 1 | 3 | 84.0 ± 2.0 a | 86.0 ± 5.3 a | 84.7 ± 4.2 a |
| Size (Fresh Weight) | Initial Specific Gravity | Final Dynamic Specific Gravity * | Final Buoyancy Rate * | Final Rooting Rate * | |
|---|---|---|---|---|---|
| Initial specific gravity | 0.659 | ||||
| Final dynamic specific gravity | −0.294 | −0.022 | |||
| Final buoyancy rate | 0.129 | −0.099 | −0.833 ** | ||
| Final rooting rate | 0.373 | 0.268 | 0.455 | −0.543 | |
| Surface elevation | 0.470 | 0.407 | −0.849 *** | 0.680 | −0.217 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, X.; Wang, M. Propagule Dispersal Determines Mangrove Zonation at Intertidal and Estuarine Scales. Forests 2019, 10, 245. https://doi.org/10.3390/f10030245
Wang W, Li X, Wang M. Propagule Dispersal Determines Mangrove Zonation at Intertidal and Estuarine Scales. Forests. 2019; 10(3):245. https://doi.org/10.3390/f10030245
Chicago/Turabian StyleWang, Wenqing, Xiaofei Li, and Mao Wang. 2019. "Propagule Dispersal Determines Mangrove Zonation at Intertidal and Estuarine Scales" Forests 10, no. 3: 245. https://doi.org/10.3390/f10030245
APA StyleWang, W., Li, X., & Wang, M. (2019). Propagule Dispersal Determines Mangrove Zonation at Intertidal and Estuarine Scales. Forests, 10(3), 245. https://doi.org/10.3390/f10030245




