Climate-Related Distribution Shifts of Migratory Songbirds and Sciurids in the White Mountain National Forest
Abstract
1. Introduction
2. Materials and Methods
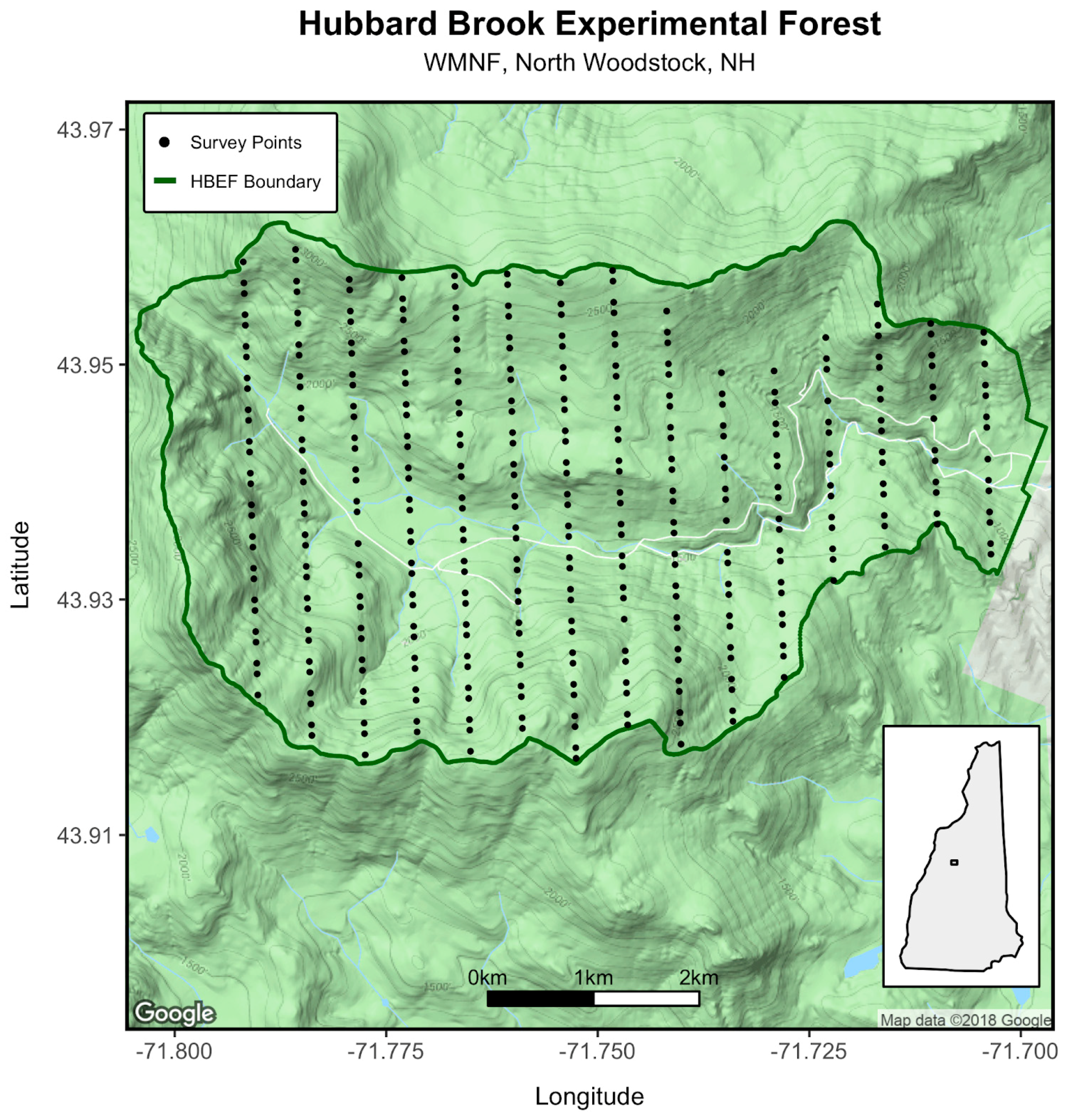
2.1. Study Site
2.2. Survey Methods
2.3. Surveyed Species
2.4. Environmental Variables
2.5. Statistical Methods
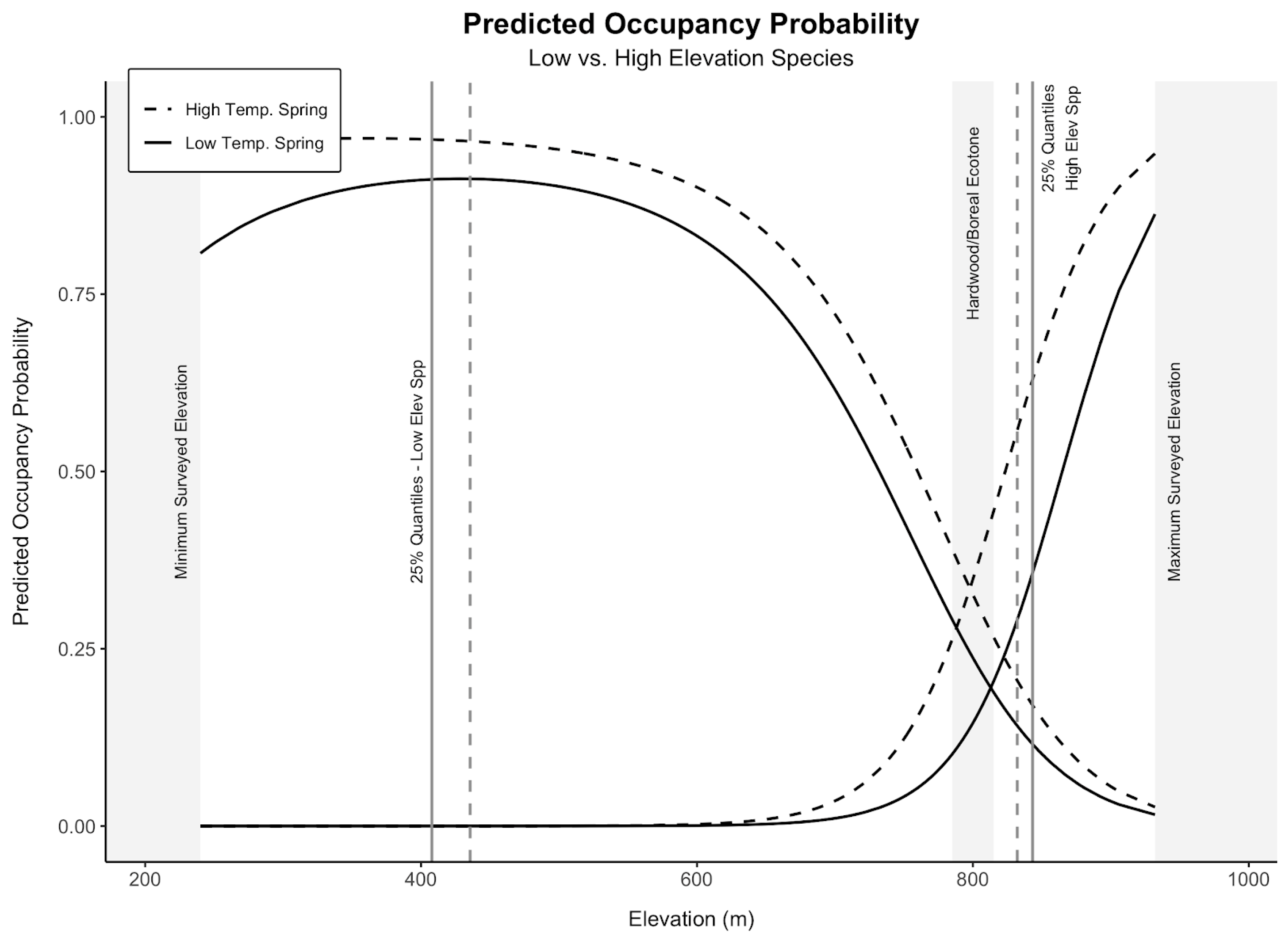
3. Results
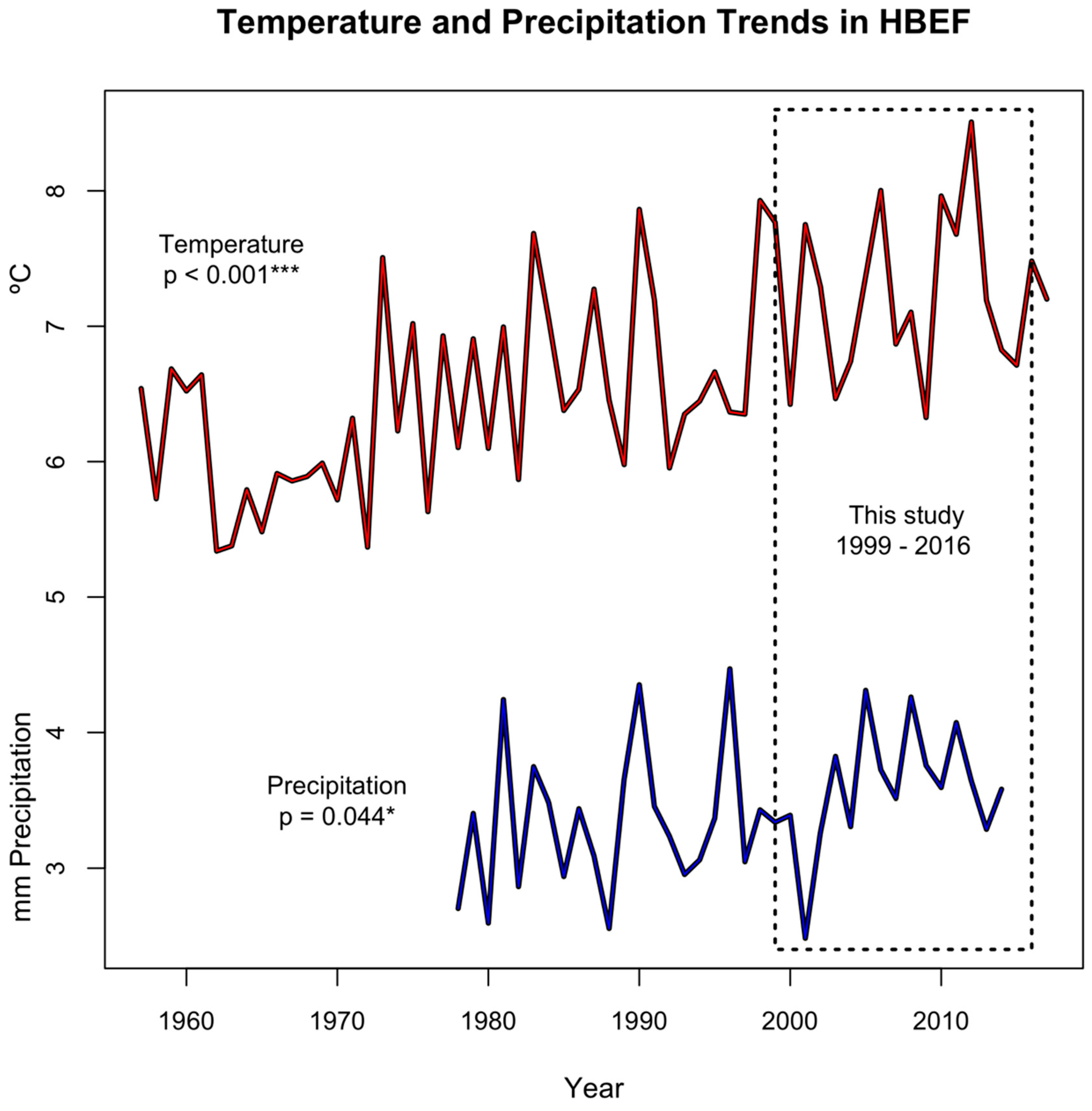
3.1. Environmental Variables
3.2. Low-Elevation Birds
3.3. High-Elevation Birds
3.4. Mammals
4. Discussion
4.1. Future Implications
4.2. Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beckage, B.; Osborne, B.; Gavin, D.G.; Pucko, C.; Siccama, T.; Perkins, T. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl. Acad. Sci. USA 2008, 105, 4197–4202. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.G.; Class Freeman, A.M. Rapid upslope shifts in New Guinean birds illustrate strong distributional responses of tropical montane species to global warming. Proc. Natl. Acad. Sci. USA 2014, 111, 4490–4494. [Google Scholar] [CrossRef] [PubMed]
- Zuckerberg, B.; Woods, A.M.; Porter, W.F. Poleward shifts in breeding bird distributions in New York State. Glob. Chang. Biol. 2009, 15, 1866–1883. [Google Scholar] [CrossRef]
- Hitch, A.T.; Leberg, P.L. Breeding Distributions of North American Bird Species Moving North as a Result of Climate Change. Conserv. Biol. 2007, 21, 534–539. [Google Scholar] [CrossRef] [PubMed]
- La Sorte, F.A.; Thompson, F.R. Poleward shifts in winter ranges of North American birds. Ecology 2007, 88, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.; Lundrigan, B.L.; Hoffman, S.M.G.; Haraminac, A.P.; Seto, S.H. Climate-induced changes in the small mammal communities of the Northern Great Lakes Region. Glob. Chang. Biol. 2009, 15, 1434–1454. [Google Scholar] [CrossRef]
- Chen, I.-C.; Shiu, H.-J.; Benedick, S.; Holloway, J.D.; Chey, V.K.; Barlow, H.S.; Hill, J.K.; Thomas, C.D. Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc. Natl. Acad. Sci. USA 2009, 106, 1479–1483. [Google Scholar] [CrossRef]
- Archaux, F. Breeding upwards when climate is becoming warmer: No bird response in the French Alps. IBIS 2004, 146, 138–144. [Google Scholar] [CrossRef]
- Lenoir, J.; Gegout, J.-C.; Guisan, A.; Vittoz, P.; Wohlgemuth, T.; Zimmermann, N.E.; Dullinger, S.; Pauli, H.; Willner, W.; Svenning, J.-C. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 2010, 33, 295–303. [Google Scholar] [CrossRef]
- McCain, C.M.; Colwell, R.K. Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate: Climate change risk for montane vertebrates. Ecol. Lett. 2011, 14, 1236–1245. [Google Scholar] [CrossRef]
- Wen, Z.; Wu, Y.; Ge, D.; Cheng, J.; Chang, Y.; Yang, Z.; Xia, L.; Yang, Q. Heterogeneous distributional responses to climate warming: Evidence from rodents along a subtropical elevational gradient. BMC Ecol. 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, W.V.; King, D.I. Montane birds shift downslope despite recent warming in the northern Appalachian Mountains. J. Ornithol. 2017, 158, 493–505. [Google Scholar] [CrossRef]
- Rodenhouse, N.L.; Matthews, S.N.; McFarland, K.P.; Lambert, J.D.; Iverson, L.R.; Prasad, A.; Sillett, T.S.; Holmes, R.T. Potential effects of climate change on birds of the Northeast. Mitig. Adapt. Strategies Glob. Chang. 2008, 13, 517–540. [Google Scholar] [CrossRef]
- Sekercioglu, C.H.; Schneider, S.H.; Fay, J.P.; Loarie, S.R. Climate Change, Elevational Range Shifts, and Bird Extinctions: Elevation, Climate Change, and Bird Extinctions. Conserv. Biol. 2008, 22, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Sabo, S.R.; Holmes, R.T. Foraging Niches and the Structure of Forest Bird Communities in Contrasting Montane Habitats. Condor 1983, 85, 121–138. [Google Scholar] [CrossRef]
- Saunders, D.A. Adirondack Mammals; College of Environmental Science and Forestry, State University of New York: New York, NY, USA, 1988; 216p. [Google Scholar]
- Crick, H.Q.P. The impact of climate change on birds: Impact of climate change on birds. IBIS 2004, 146, 48–56. [Google Scholar] [CrossRef]
- Jetz, W.; Wilcove, D.S.; Dobson, A.P. Projected Impacts of Climate and Land-Use Change on the Global Diversity of Birds. PLoS Biol. 2007, 5, e157. [Google Scholar] [CrossRef]
- IPCC Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Janowiak, M.K.; D’Amato, A.W.; Swanston, C.W.; Iverson, L.; Thompson, F.R.; Dijak, W.D.; Matthews, S.; Peters, M.P.; Prasad, A.; Fraser, J.S.; et al. New England and Northern New York Forest Ecosystem Vulnerability Assessment and Synthesis: A Report from the New England Climate Change Response Framework Project; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2018.
- Tingley, M.W.; Koo, M.S.; Moritz, C.; Rush, A.C.; Beissinger, S.R. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Glob. Chang. Biol. 2012, 18, 3279–3290. [Google Scholar] [CrossRef]
- Seidel, T.M.; Weihrauch, D.M.; Kimball, K.D.; Pszenny, A.A.P.; Soboleski, R.; Crete, E.; Murray, G. Evidence of Climate Change Declines with Elevation Based on Temperature and Snow Records from 1930s to 2006 on Mount Washington, New Hampshire, U.S.A. Arct. Antarct. Alpine Res. 2009, 41, 362–372. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Hubbard Brook Research Foundation. Chapter 01: The Hubbard Brook Ecosystem Study: Site, History, and Research Approaches; HBEF: North Woodstock, NH, USA, 2018; Available online: https://hubbardbrook.org/online-book (accessed on 31 December 2018).
- Holmes, R.T.; Sherry, T.W.; Sturges, F.W. Bird Community Dynamics in a Temperate Deciduous Forest: Long-Term Trends at Hubbard Brook. Ecol. Monogr. 1986, 56, 201–220. [Google Scholar] [CrossRef]
- Reitsma, L.R.; Holmes, R.T.; Sherry, T.W. Effects of Removal of Red Squirrels, Tamiasciurus hudsonicus, and Eastern Chipmunks, Tamias striatus, on Nest Predation in a Northern Hardwood Forest: An Artificial Nest Experiment. Oikos 1990, 57, 375. [Google Scholar] [CrossRef]
- Sherry, T.W.; Wilson, S.; Hunter, S.; Holmes, R.T. Impacts of nest predators and weather on reproductive success and population limitation in a long-distance migratory songbird. J. Avian Biol. 2015, 46, 559–569. [Google Scholar] [CrossRef]
- Rodewald, P. The Birds of North America; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2015. [Google Scholar]
- Elliott, L. Social behavior and foraging ecology of the eastern chipmunk (Tamias striatus) in the Adirondack Mountains. Smithson. Contrib. Zool. 1978, 1–107. [Google Scholar] [CrossRef]
- Rusch, D.A.; Reeder, W.G. Population Ecology of Alberta Red Squirrels. Ecology 1978, 59, 400–420. [Google Scholar] [CrossRef]
- Chandler, R.B.; Royle, J.A.; King, D.I. Inference about density and temporary emigration in unmarked populations. Ecology 2011, 92, 1429–1435. [Google Scholar] [CrossRef]
- Fiske, I.; Chandler, R. unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance. J. Stat. Softw. 2011, 43, 1–23. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference, R package Version 1.42.1; 2018. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 31 December 2018).
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Holmes, R.T.; Kaiser, S.A.; Rodenhouse, N.L.; Sillett, T.S.; Webster, M.S.; Pyle, P.; Patten, M.A. Black-Throated Blue Warbler (Setophaga caerulescens), version 3.0. In The Birds of North America; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2017. [Google Scholar] [CrossRef]
- Porneluzi, P.; Van Horn, M.A.; Donovan, T.M. Ovenbird (Seiurus aurocapilla), version 2.0. In The Birds of North America; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2011. [Google Scholar] [CrossRef]
- Cimprich, D.A.; Moore, F.R.; Guilfoyle, M.P. Red-eyed/Chivi Vireo (Vireo olivaceus/chivi), version 2.0. In The Birds of North America; Poole, A.F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2000. [Google Scholar] [CrossRef]
- Dellinger, R.; Wood, P.B.; Jones, P.W.; Donovan, T.M. Hermit Thrush (Catharus guttatus), version 2.0. In The Birds of North America; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2012. [Google Scholar] [CrossRef]
- Foote, J.R.; Mennill, D.J.; Ratcliffe, L.M.; Smith, S.M. Black-capped Chickadee (Poecile atricapillus), version 2.0. In The Birds of North America; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2010. [Google Scholar] [CrossRef]
- DeLuca, W.; Holberton, R.; Hunt, P.D.; Eliason, B.C. Blackpoll Warbler (Dendroica striata). In The Birds of North America; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2013. [Google Scholar] [CrossRef]
- Mack, D.E.; Yong, W. Swainson’s Thrush (Catharus ustulatus), version 2.0. In The Birds of North America; Poole, A.F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2000. [Google Scholar] [CrossRef]
- Dunn, E.H.; Hall, G.A. Magnolia Warbler (Setophaga magnolia), version 2.0. In The Birds of North America; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2010. [Google Scholar] [CrossRef]
- Nolan, V., Jr.; Ketterson, E.D.; Cristol, D.A.; Rogers, C.M.; Clotfelter, E.D.; Titus, R.C.; Schoech, S.J.; Snajdr, E. Dark-eyed Junco (Junco hyemalis), version 2.0. In The Birds of North America; Poole, A.F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2002. [Google Scholar] [CrossRef]
- Hejl, S.J.; Holmes, J.A.; Kroodsma, D.E. Winter Wren (Troglodytes hiemalis), version 2.0. In The Birds of North America; Poole, A.F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2002. [Google Scholar] [CrossRef]
- Wolfson, A. Day Length, Migration, and Breeding Cycles in Birds. Sci. Mon. 1952, 74, 191–200. [Google Scholar]
- Bauer, S.; Nolet, B.A.; Giske, J.; Chapman, J.W.; Åkesson, S.; Hedenström, A.; Fryxell, J.M. Cues and decision rules in animal migration. In Animal Migration; Milner-Gulland, E.J., Fryxell, J.M., Sinclair, A.R.E., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 68–87. ISBN 978-0-19-956899-4. [Google Scholar]
- Richardson, W.J. Timing and Amount of Bird Migration in Relation to Weather: A Review. Oikos 1978, 30, 224. [Google Scholar] [CrossRef]
- Loehle, C. Tree life history strategies: The role of defenses. Can. J. For. Res. 1988, 18, 209–222. [Google Scholar] [CrossRef]
- Clotfelter, E.D.; Pedersen, A.B.; Cranford, J.A.; Ram, N.; Snajdr, E.A.; Nolan, V.; Ketterson, E.D. Acorn mast drives long-term dynamics of rodent and songbird populations. Oecologia 2007, 154, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Ulateig, G. Vegetation Changes and Small Rodent Responses along Alpine Gradients in Oceanic and Continental Climate; Norwegian University of Life Sciences: Ås, Norway, 2010. [Google Scholar]
- van Doorn, N.S.; Battles, J.J.; Fahey, T.J.; Siccama, T.G.; Schwarz, P.A. Links between biomass and tree demography in a northern hardwood forest: A decade of stability and change in Hubbard Brook Valley, New Hampshire. Can. J. For. Res. 2011, 41, 1369–1379. [Google Scholar] [CrossRef]
- Smith, M.C. Red Squirrel Responses to Spruce Cone Failure in Interior Alaska. J. Wildl. Manag. 1968, 32, 305–317. [Google Scholar] [CrossRef]
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate Change and the Past, Present, and Future of Biotic Interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a Century of Climate Change on Small-Mammal Communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.E.; Marzluff, J.M. Rodents as Nest Predators: Influences on Predatory Behavior and Consequences to Nesting Birds. Auk 2003, 120, 1180–1187. [Google Scholar] [CrossRef]
| Mean Spring Temperature (1999–2016) | |||||
|---|---|---|---|---|---|
| Low-Elevation Species | |||||
| Spp. | % Occu | Intercept | R2 | p | Significance |
| BTBW | 2.5% | 8.71 | 0.30 | 0.016 | * |
| 5% | 10.29 | 0.38 | 0.007 | ** | |
| 25% | 10.40 | 0.61 | 0.000 | *** | |
| 50% | 7.06 | 0.38 | 0.007 | ** | |
| 75% | 3.77 | −0.01 | 0.370 | ||
| 95% | 3.43 | −0.04 | 0.545 | ||
| 97.5% | 4.39 | −0.03 | 0.446 | ||
| OVEN | 2.5% | −0.90 | −0.01 | 0.390 | |
| 5% | −1.00 | −0.04 | 0.534 | ||
| 25% | 0.40 | −0.07 | 0.882 | ||
| 50% | 2.78 | −0.01 | 0.361 | ||
| 75% | 5.78 | 0.10 | 0.129 | ||
| 95% | 10.12 | 0.22 | 0.040 | * | |
| 97.5% | 11.05 | 0.21 | 0.041 | * | |
| REVI | 2.5% | 0.77 | 0.14 | 0.082 | . |
| 5% | 1.62 | 0.21 | 0.040 | * | |
| 25% | 6.43 | 0.53 | 0.001 | *** | |
| 50% | 10.95 | 0.67 | 0.000 | *** | |
| 75% | 15.86 | 0.70 | 0.000 | *** | |
| 95% | 21.55 | 0.55 | 0.001 | *** | |
| 97.5% | 19.41 | 0.42 | 0.004 | ** | |
| High-Elevation Species | |||||
| BLPW | 2.5% | 14.81 | −0.06 | 0.656 | |
| 5% | 9.47 | −0.01 | 0.392 | ||
| 25% | 4.93 | 0.21 | 0.041 | * | |
| 50% | 3.47 | 0.34 | 0.011 | * | |
| 75% | 1.83 | 0.33 | 0.012 | * | |
| 95% | 0.42 | 0.47 | 0.002 | ** | |
| 97.5% | 0.16 | 0.23 | 0.034 | * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Tatenhove, A.; Filiberti, E.; Sillett, T.S.; Rodenhouse, N.; Hallworth, M. Climate-Related Distribution Shifts of Migratory Songbirds and Sciurids in the White Mountain National Forest. Forests 2019, 10, 84. https://doi.org/10.3390/f10020084
Van Tatenhove A, Filiberti E, Sillett TS, Rodenhouse N, Hallworth M. Climate-Related Distribution Shifts of Migratory Songbirds and Sciurids in the White Mountain National Forest. Forests. 2019; 10(2):84. https://doi.org/10.3390/f10020084
Chicago/Turabian StyleVan Tatenhove, Aimee, Emily Filiberti, T. Scott Sillett, Nicholas Rodenhouse, and Michael Hallworth. 2019. "Climate-Related Distribution Shifts of Migratory Songbirds and Sciurids in the White Mountain National Forest" Forests 10, no. 2: 84. https://doi.org/10.3390/f10020084
APA StyleVan Tatenhove, A., Filiberti, E., Sillett, T. S., Rodenhouse, N., & Hallworth, M. (2019). Climate-Related Distribution Shifts of Migratory Songbirds and Sciurids in the White Mountain National Forest. Forests, 10(2), 84. https://doi.org/10.3390/f10020084





