Herbaceous Encroachment from Mountain Birch Forests to Alpine Tundra Plant Communities Through Above- and Belowground Competition
Abstract
1. Introduction
2. Materials and Methods
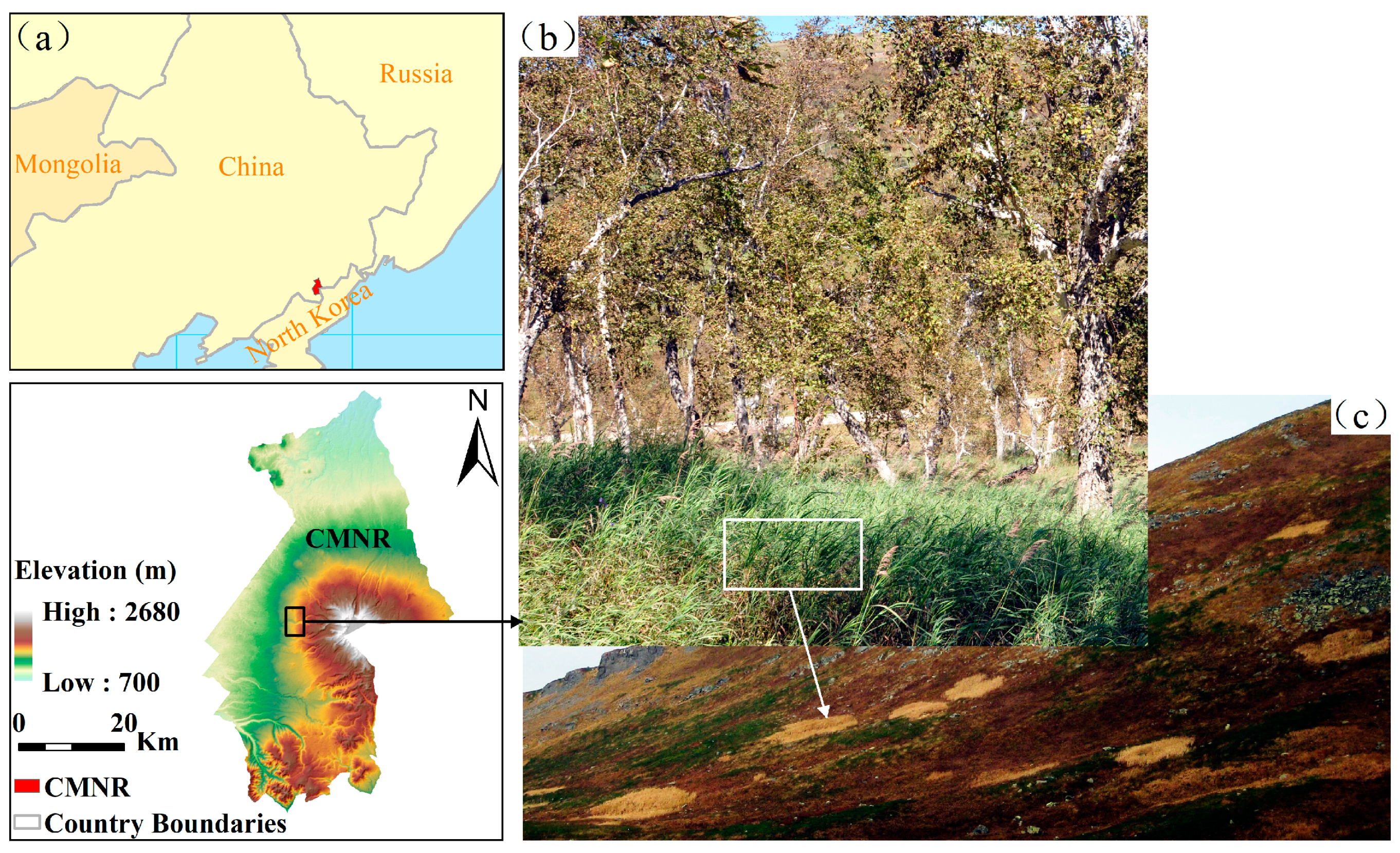
2.1. Study Area
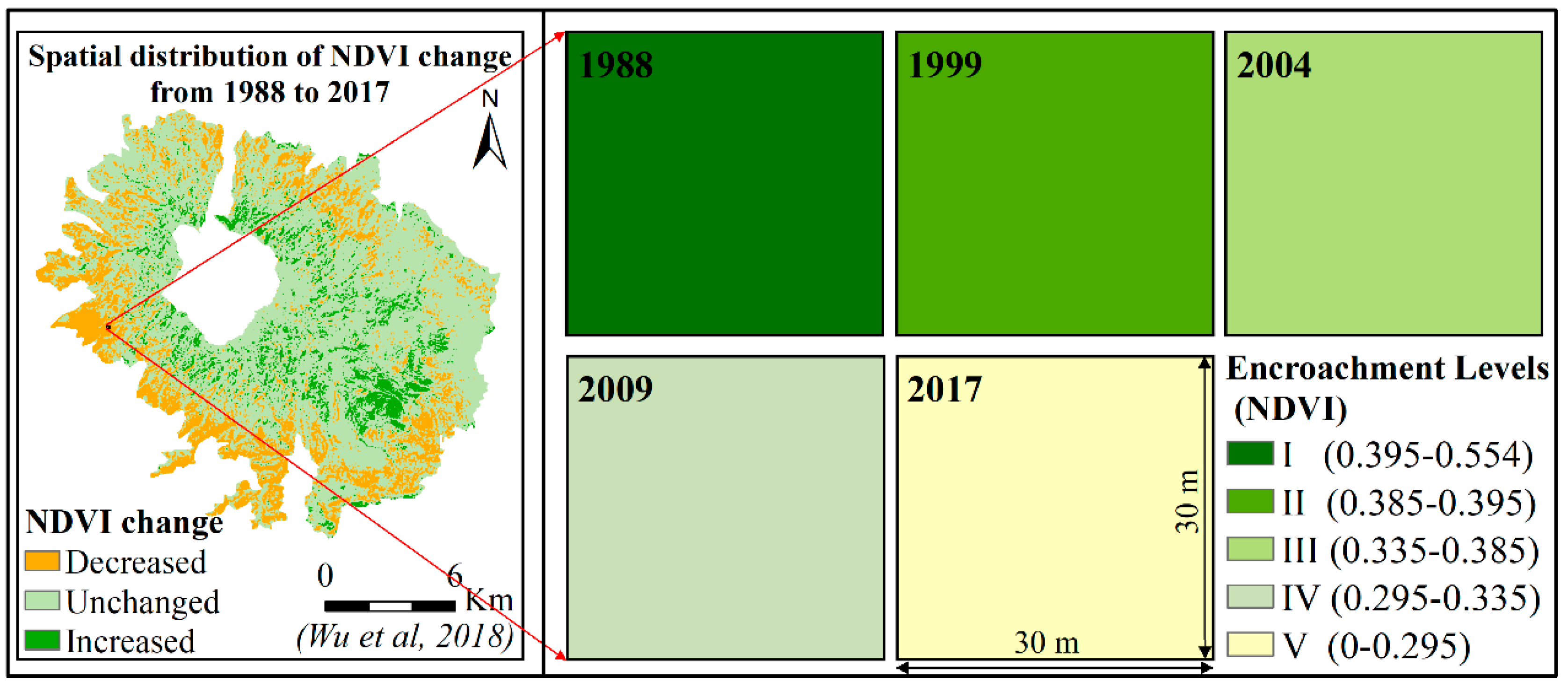
2.2. Sampling Design
2.3. Data Analysis
3. Results
3.1. Changes in Plant Morphology
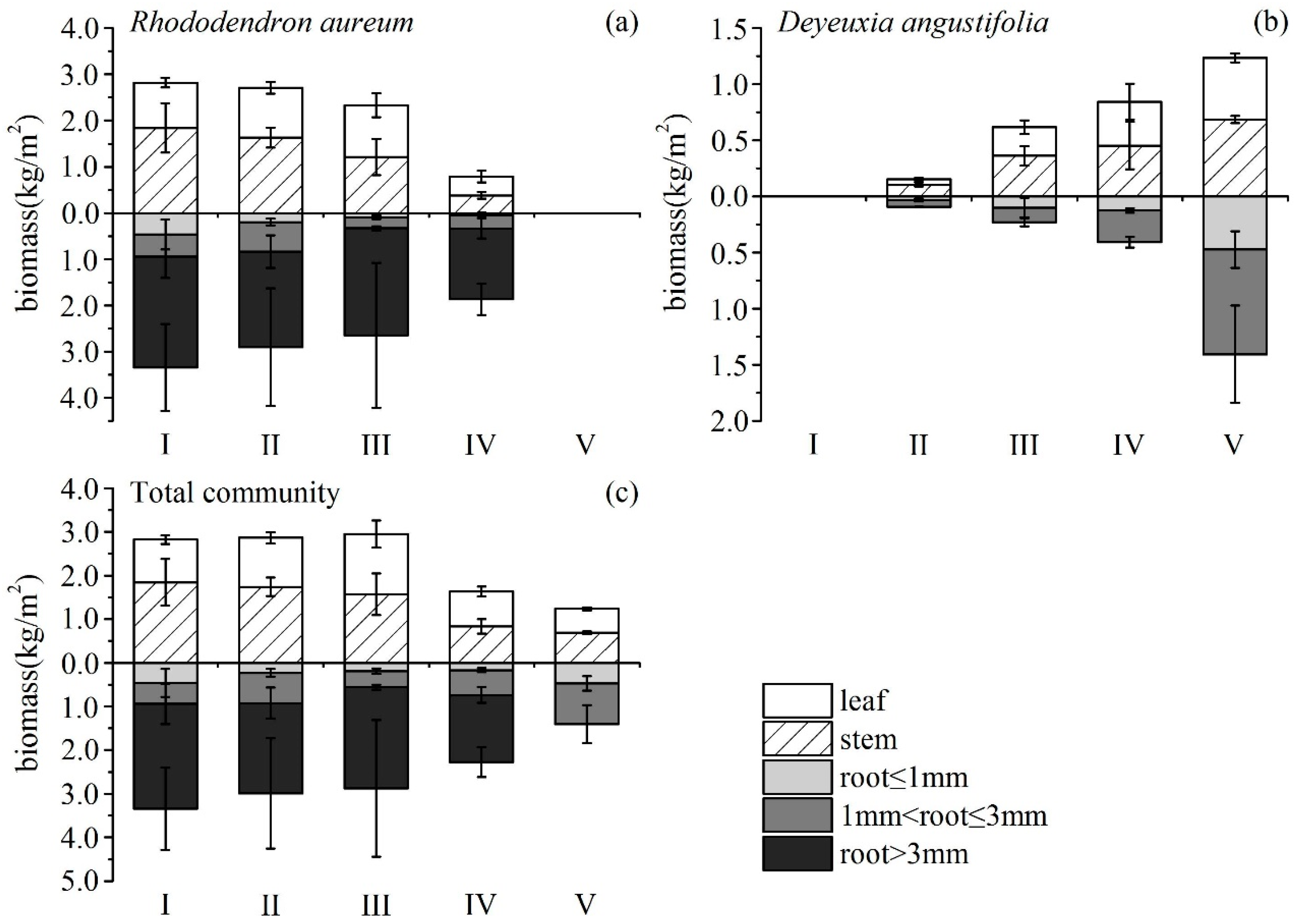
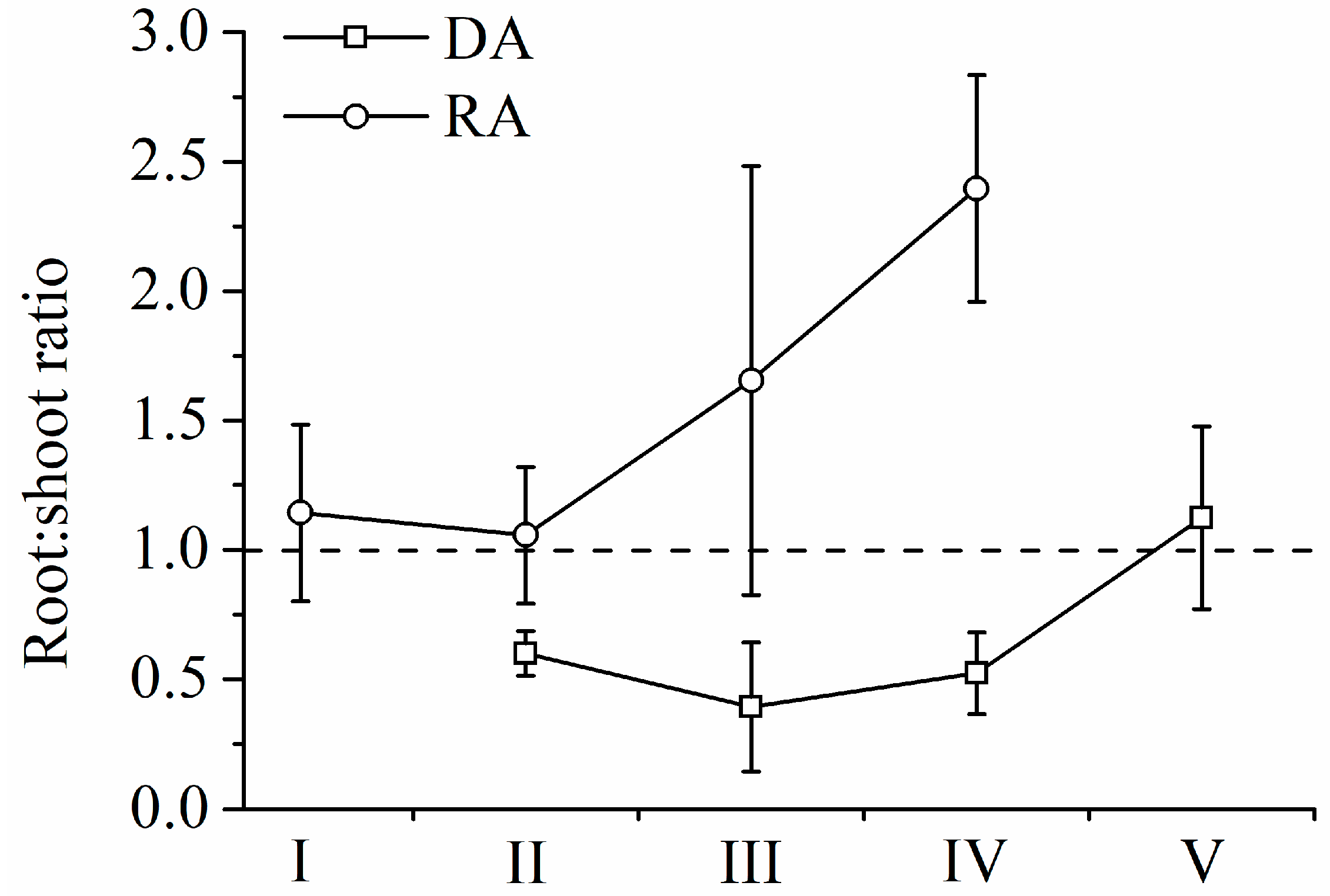
3.2. Changes in Biomass and Allocation
4. Discussion
4.1. Aboveground Competition
4.2. Belowground Competition
4.3. Implications for Vegetation Carbon Stock in Alpine Tundra
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Alexander, J.M.; Lembrechts, J.J.; Cavieres, L.A.; Daehler, C.; Haider, S.; Kueffer, C.; Liu, G.; McDougall, K.; Milbau, A.; Pauchard, A.; et al. Plant invasions into mountains and alpine ecosystems: current status and future challenges. Alp. Botany 2016, 126, 89–103. [Google Scholar] [CrossRef]
- Barros, A.; Pickering, C.M. Non-native Plant Invasion in Relation to Tourism Use of Aconcagua Park, Argentina, the Highest Protected Area in the Southern Hemisphere. Mt. Res. Dev. 2014, 34, 13–26. [Google Scholar] [CrossRef]
- Fernández-Murillo, M.P.; Rico, A.; Kindlmann, P.; Fernández-Murillo, M.P. Exotic plants along roads near La Paz, Bolivia. Weed Res. 2015, 55, 565–573. [Google Scholar] [CrossRef]
- Seipel, T.; Kueffer, C.; Naylor, B.J.; Jakobs, G.; McDougall, K.; Walsh, N.; Rew, L.J.; Daehler, C.C.; Pauchard, A.; Alexander, J.M.; et al. Processes at multiple scales affect richness and similarity of non-native plant species in mountains around the world. Global Ecol. Biogeogr. 2011, 21, 236–246. [Google Scholar] [CrossRef]
- Kalwij, J.M.; Robertson, M.P.; Van Rensburg, B.J. Annual monitoring reveals rapid upward movement of exotic plants in a montane ecosystem. Biol. Invasions 2015, 17, 3517–3529. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob. Change Biol. 2007, 13, 147–156. [Google Scholar] [CrossRef]
- Walther, G.-R.; Beißner, S.; Burga, C.A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Elmendorf, S.C.; Henry, G.H.R.; Hollister, R.D.; Björk, R.G.; Boulanger-Lapointe, N.; Cooper, E.J.; Cornelissen, J.H.C.; Day, T.A.; Dorrepaal, E.; Elumeeva, T.G.; et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nature Clim. Change 2012, 2, 453–457. [Google Scholar] [CrossRef]
- Hudson, J.M.G.; Henry, G.H.R.; Cornwell, W.K. Taller and larger: shifts in Arctic tundra leaf traits after 16 years of experimental warming. Glob. Change Biol. 2011, 17, 1013–1021. [Google Scholar] [CrossRef]
- Nie, Y.-Q.; Kong, G.-Q.; Liu, X.-S.; Luo, T.-X. Contrasting changes in above- and below-ground biomass allocation across treeline ecotones in southeast Tibet. J. Mt. Sci. 2016, 13, 2036–2045. [Google Scholar]
- Muñoz, A.A.; Cavieres, L.A. The presence of a showy invasive plant disrupts pollinator service and reproductive output in native alpine species only at high densities. J. Ecol. 2008, 96, 459–467. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the Existence of Three Primary Strategies in Plants and Its Relevance to Ecological and Evolutionary Theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Godoy, O.; Levine, J.M. Phenology effects on invasion success: insights from coupling field experiments to coexistence theory. Ecology 2014, 95, 726–736. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, T.; Zhou, D.; Zhang, H. A literature review on the above- and below-ground competition. Acta Eco. Sinica 2007, 27, 3489–3499. [Google Scholar]
- Bloom, A.J.; Chapin, F.S.; A Mooney, H. Resource Limitation in Plants-An Economic Analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Gargaglione, V.; Peri, P.L.; Rubio, G. Allometric relations for biomass partitioning of Nothofagus antarctica trees of different crown classes over a site quality gradient. For. Ecol. Manag. 2010, 259, 1118–1126. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Harpole, W.S.; Reichman, O.J.; Tilman, D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc. Natl. Acad. Sci. 2003, 100, 13384–13389. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.; Woodin, S.J. Climate change in the Arctic: using plant functional types in a meta-analysis of field experiments. Funct. Ecol. 2002, 16, 4–17. [Google Scholar] [CrossRef]
- Henry, G.; Freedman, B.; Svoboda, J. Survey of Vegetated Areas and Muskox Populations in East-Central Ellesmere Island. ARCTIC 1986, 39, 78–81. [Google Scholar] [CrossRef]
- Rejmánek, M.; Richardson, D.M. What Attributes Make Some Plant Species More Invasive? Ecology 1996, 77, 1655–1661. [Google Scholar]
- Rudak, A.; Wódkiewicz, M.; Znój, A.; Chwedorzewska, K.J.; Galera, H. Plastic biomass allocation as a trait increasing the invasiveness of annual bluegrass (Poa annua L.) in Antarctica. Polar Biol. 2018, 42, 149–157. [Google Scholar] [CrossRef]
- Zong, S.; Xu, J.; Wu, Z. Investigation and mechanism analysis on the invasion of deyeuxia. Angustifolia to tundra zone in western slope of changbai mountain. J, Mt. Sci. 2013, 31, 448–455. [Google Scholar]
- Williamson, M.H.; Fitter, A. The characters of successful invaders. Biol. Conserv. 1996, 78, 163–170. [Google Scholar] [CrossRef]
- Qian, J.J.; Zhang, W.Z. Vertical Plant List of the Changbai Mountains; Northeast Normal University Press: Changchun, China, 1979; pp. 90–95. [Google Scholar]
- Qian, H. Alpine tundra vegetation on the Changbai Mountains. For. Res. Eco. 1992, 6, 72–93. [Google Scholar]
- Zong, S. Mechanism research on the vegetation changes of the sub-alpine tundra, Changbai Mountains. Ph.D. Thesis, Northeast normal university, Changchun, China, 2014. [Google Scholar]
- Zong, S.; Jin, Y.; Xu, J.; Wu, Z.; He, H.; Du, H.; Wang, L. Nitrogen deposition but not climate warming promotes Deyeuxia angustifolia encroachment in alpine tundra of the Changbai Mountains, Northeast China. Sci. Total Environ. 2016, 544, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Wu, Z.; Du, H. Study on climate change in alpine tundra of the changbai mountain in growing season in recent 52 years. Arid Zone Res. 2013, 30, 41–49. [Google Scholar]
- Wang, L. Research on the spatial distribution pattern of plant communities in tundra in the west slope of changbai mountain besed on water and heat balance. Ph.D. Thesis, Northeast normal university, Changchun, China, 2017. [Google Scholar]
- Wu, M.; He, H.S.; Zong, S.; Tan, X.; Du, H.; Zhao, D.; Liu, K.; Liang, Y. Topographic Controls on Vegetation Changes in Alpine Tundra of the Changbai Mountains. Forests 2018, 9, 756. [Google Scholar] [CrossRef]
- Dunne, J.A.; Saleska, S.R.; Fischer, M.L.; Harte, J. INTEGRATING EXPERIMENTAL AND GRADIENT METHODS IN ECOLOGICAL CLIMATE CHANGE RESEARCH. Ecology 2004, 85, 904–916. [Google Scholar] [CrossRef]
- Ma, W.; Shi, P.; Li, W.; He, Y.; Zhang, X.; Shen, Z.; Chai, S. Changes in individual plant traits and biomass allocation in alpine meadow with elevation variation on the Qinghai-Tibetan Plateau. Sci. China Life Sci. 2010, 53, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, G.; Kang, Y.; Zhang, S.; Wu, Y.; Wang, Y. Responses of leaf morphological and anatomical structure to elevation in an alpine plant meconopsis integrifolia. Chin. J. Ecol. 2018, 37, 35–42. [Google Scholar]
- Wang, P.; Mommer, L.; Van Ruijven, J.; Berendse, F.; Maximov, T.C.; Heijmans, M.M.P.D. Seasonal changes and vertical distribution of root standing biomass of graminoids and shrubs at a Siberian tundra site. Plant Soil 2016, 407, 55–65. [Google Scholar] [CrossRef]
- Niu, K.; Luo, Y.; Choler, P.; Du, G. The role of biomass allocation strategy in diversity loss due to fertilization. Basic Appl. Ecol. 2008, 9, 485–493. [Google Scholar] [CrossRef]
- Wu, J.-S.; Shen, Z.-X.; Zhang, X.-Z.; Shi, P.-L. Biomass allocation patterns of alpine grassland species and functional groups along a precipitation gradient on the Northern Tibetan Plateau. J. Mt. Sci. 2013, 10, 1097–1108. [Google Scholar] [CrossRef]
- Heer, C.; Körner, C. High elevation pioneer plants are sensitive to mineral nutrient addition. Basic Appl. Ecol. 2002, 3, 39–47. [Google Scholar] [CrossRef]
- Connell, J.H. On the Prevalence and Relative Importance of Interspecific Competition: Evidence from Field Experiments. Am. Nat. 1983, 122, 661–696. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, Q.; Zhang, G. Impact of alpine snowpacks on primary productivity in rhododendron aureum community in changbai mountain, China. Acta Ecol. Sinica 2009, 29, 4035–4044. [Google Scholar]
- McDougall, K.L.; Morgan, J.W.; Walsh, N.G.; Williams, R.J. Plant invasions in treeless vegetation of the Australian Alps. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 159–171. [Google Scholar] [CrossRef]
- Gaur, U.N.; Raturi, G.; Bhatt, A. Quantitative Response of Vegetation in Glacial Moraine of Central Himalaya. Environmentalist 2003, 23, 237–247. [Google Scholar] [CrossRef]
- Beckage, B.; Osborne, B.; Gavin, D.; Pucko, C.; Siccama, T.; Perkins, T. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl. Acad. Sci. 2008, 105, 4197–4202. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhang, R.; Wang, P.; Yuan, Z.; Du, J. Species interactions occurring in the alpine meadow from the eastern Qinghai-Tibet plateau of China. J. Lanzhou Univ. (Nat. Sci.) 2011, 47, 79–89. [Google Scholar]
- Saccone, P.; Hoikka, K.; Virtanen, R. What if plant functional types conceal species-specific responses to environment? Study on arctic shrub communities. Ecology 2017, 98, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.A.; Hoch, G.; Cortés, A.J.; Sedlacek, J.; Wipf, S.; Rixen, C. Increased spring freezing vulnerability for alpine shrubs under early snowmelt. Oecologia 2014, 175, 219–229. [Google Scholar] [CrossRef]
- Vo, S.T.K.; Johnson, E.A. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Mt. Res. Dev. 2001, 21. [Google Scholar] [CrossRef]
- Larcher, W.; Siegwolf, R. Development of acute frost drought in Rhododendron ferrugineum at the alpine timberline. Oecologia 1985, 67, 298–300. [Google Scholar] [CrossRef]
- Iversen, C.M.; Sloan, V.L.; Sullivan, P.F.; Euskirchen, E.S.; McGuire, A.D.; Norby, R.J.; Walker, A.P.; Warren, J.M.; Wullschleger, S.D. The unseen iceberg: plant roots in arctic tundra. New Phytol. 2014, 205, 34–58. [Google Scholar] [CrossRef]
- Björk, R.G.; Majdi, H.; Klemedtsson, L.; Molau, U.; Lewis-Jonsson, L.; Lewis-Jonsson, L. Long-term warming effects on root morphology, root mass distribution, and microbial activity in two dry tundra plant communities in northern Sweden. New Phytol. 2007, 176, 862–873. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, L.; Zhang, Y.; Jiang, Y.; Tao, J.; Tian, L.; Zhang, T.; Xi, Y. Below-ground competition drives the self-thinning process of Stipa purpurea populations in northern Tibet. J. Veg. Sci. 2014, 26, 166–174. [Google Scholar] [CrossRef]
- Van Ruijven, J.; Schaepman-Strub, G.; Wang, P.; Limpens, J.; Mommer, L.; Nauta, A.L.; Berendse, F.; Blok, D.; Maximov, T.C.; Heijmans, M.M.; et al. Above- and below-ground responses of four tundra plant functional types to deep soil heating and surface soil fertilization. J. Ecol. 2017, 105, 947–957. [Google Scholar]
- Mallen-Cooper, J. Introduced plants in the high-altitude environments of Kosciusko National Park, South-Eastern Australia. Ph.D. Thesis, Australian National University, Canberra, Australian, 1990. [Google Scholar]
- Li, L. Study on Soil Microorganism of subalpine tundra in Changbai Mountain. Ph.D. Thesis, Jilin University, Changchun, China, 2017. [Google Scholar]
- Wearne, L.J.; Morgan, J.W. Shrub invasion into subalpine vegetation: implications for restoration of the native ecosystem. Plant Ecol. 2006, 183, 361–376. [Google Scholar] [CrossRef]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, M.; Ravenek, J.M.; Smit-Tiekstra, A.E.; Van Der Paauw, J.W.; De Caluwe, H.; Van Der Putten, W.H.; De Kroon, H.; Mommer, L.; Smit-Tiekstra, A.E. Spatial heterogeneity of plant-soil feedback affects root interactions and interspecific competition. New Phytol. 2015, 207, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the plant-soil system. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef]
- Yu, X. A new mechanism of invader success: Exotic plant inhibits natural vegetation restoration by changing soil microbe com-munity. Chin. Sci. Bull. 2005, 50. [Google Scholar] [CrossRef]
- Li, W.-H.; Zhang, C.-B.; Jiang, H.-B.; Xin, G.-R.; Yang, Z.-Y. Changes in Soil Microbial Community Associated with Invasion of the Exotic Weed, Mikania micrantha H.B.K. Plant Soil 2006, 281, 309–324. [Google Scholar] [CrossRef]
- Li, L.; Xing, M.; Lv, J.; Wang, X.; Chen, X. Response of rhizosphere soil microbial to Deyeuxia angustifolia encroaching in two different vegetation communities in alpine tundra. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Oechel, W.C.; Hastings, S.J.; Vourlrtis, G.; Jenkins, M.; Riechers, G.; Grulke, N. Recent change of Arctic tundra ecosystems from a net carbon dioxide sink to a source. Nature 1993, 361, 520–523. [Google Scholar] [CrossRef]
- Speed, J.D.M.; Martinsen, V.; Hester, A.J.; Holand, Ø.; Mulder, J.; Mysterud, A.; Austrheim, G. Continuous and discontinuous variation in ecosystem carbon stocks with elevation across a treeline ecotone. Biogeosciences 2015, 12, 1615–1627. [Google Scholar] [CrossRef]
- Sjögersten, S.; Wookey, P.A. The Impact of Climate Change on Ecosystem Carbon Dynamics at the Scandinavian Mountain Birch Forest–Tundra Heath Ecotone. AMBIO 2009, 38, 2–10. [Google Scholar]
- Körner, C. Treelines Will be Understood Once the Functional Difference Between a Tree and a Shrub Is. AMBIO 2012, 41, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Grafius, D.R.; Malanson, G.P. Biomass distributions in dwarf tree, krummholz, and tundra vegetation in the alpine treeline ecotone. Phys. Geogr. 2015, 36, 337–352. [Google Scholar] [CrossRef]


| Variables | Formula | Descriptions |
|---|---|---|
| Leaf biomass allocation (LBA) | Leaf biomass/total biomass × 100% | The percentage of leaf biomass of the total biomass of the plant |
| Stem biomass allocation (SBA) | Stem biomass/total biomass × 100% | The percentage of stem biomass of the total biomass of the plant |
| Fine root biomass allocation (FRBA) | Fine root biomass/total biomass × 100% | The percentage of fine root biomass of the total biomass of the plant |
| Medium root biomass allocation (MRBA) | Medium root biomass/total biomass × 100% | The percentage of medium root biomass of the total biomass of the plant |
| Coarse root biomass allocation (CRBA) | Coarse root biomass/total biomass × 100% | The percentage of coarse root biomass of the total biomass of the plant |
| Root/shoot ratio (R/S) | Belowground biomass /aboveground biomass | The relationship between aboveground and belowground biomass |
| Dependent Variable | Plot | Encroachment Level | |||||
|---|---|---|---|---|---|---|---|
| df | F | Sig. | df | F | Sig. | ||
| RLB | Contrast | 5 | 0.095 | 0.993 | 3 | 52.642 | 0.000 |
| Error | 63 | 63 | |||||
| RSB | Contrast | 5 | 0.219 | 0.953 | 3 | 44.168 | 0.000 |
| Error | 63 | 63 | |||||
| RFRB | Contrast | 5 | 2.418 | 0.050 | 3 | 29.148 | 0.000 |
| Error | 63 | 63 | |||||
| RMRB | Contrast | 5 | 0.408 | 0.841 | 3 | 2.552 | 0.076 |
| Error | 63 | 63 | |||||
| RCRB | Contrast | 5 | 0.311 | 0.895 | 3 | 3.003 | 0.058 |
| Error | 63 | 63 | |||||
| RH | Contrast | 5 | 0.408 | 0.841 | 3 | 199.027 | 0.000 |
| Error | 63 | 63 | |||||
| RAR | Contrast | 5 | 2.698 | 0.028 | 3 | 71.157 | 0.000 |
| Error | 63 | 63 | |||||
| Dependent Variable | Plot | Encroachment Level | |||||
|---|---|---|---|---|---|---|---|
| df | F | Sig. | df | F | Sig. | ||
| DLB | Contrast | 5 | 0.789 | 0.561 | 3 | 120.950 | 0.000 |
| Error | 63 | 63 | |||||
| DSB | Contrast | 5 | 0.970 | 0.443 | 3 | 97.236 | 0.000 |
| Error | 63 | 63 | |||||
| DFRB | Contrast | 5 | 0.480 | 0.790 | 3 | 42.122 | 0.000 |
| Error | 63 | 63 | |||||
| DMRB | Contrast | 5 | 0.886 | 0.496 | 3 | 89.355 | 0.000 |
| Error | 63 | 63 | |||||
| DAR | Contrast | 5 | 1.276 | 0.286 | 3 | 40.698 | 0.000 |
| Error | 63 | 63 | |||||
| DH | Contrast | 5 | 0.407 | 0.842 | 3 | 39.976 | 0.000 |
| Error | 63 | 63 | |||||
| Plant Type | Level of Encroachment | LBA | SBA | FRBA | MRBA | CRBA |
|---|---|---|---|---|---|---|
| RA | I | 17.11 ± 0.06a | 30.46 ± 0.03a | 7.30 ± 0.04a | 6.78 ± 0.05a | 38.35±0.03ab |
| II | 19.52 ± 0.02a | 29.64 ± 0.04a | 3.49 ± 0.01ab | 12.17 ± 0.07a | 35.18 ± 0.14b | |
| III | 27.70 ± 0.15a | 29.19 ± 0.14a | 2.19 ± 0.01b | 5.84 ± 0.03a | 52.01 ± 0.20ab | |
| IV | 14.98 ± 0.02a | 14.80 ± 0.04b | 1.48 ± 0.02b | 10.39 ± 0.08a | 58.35 ± 0.12a | |
| V | ||||||
| DA | I | |||||
| II | 19.64 ± 0.06b | 43.12 ± 0.06a | 13.38 ± 0.05ab | 23.87 ± 0.02b | ||
| III | 30.21 ± 0.05a | 42.96 ± 0.07a | 6.00 ± 0.01b | 15.48 ± 0.03b | ||
| IV | 30.79 ± 0.03a | 35.32 ± 0.06ab | 10.64 ± 0.04ab | 23.25 ± 0.04b | ||
| V | 21.21 ± 0.03b | 26.65 ± 0.04b | 17.94 ± 0.06a | 34.20 ± 0.09a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; He, H.S.; Zong, S.; Wu, M.; Liu, K.; Zhao, D. Herbaceous Encroachment from Mountain Birch Forests to Alpine Tundra Plant Communities Through Above- and Belowground Competition. Forests 2019, 10, 170. https://doi.org/10.3390/f10020170
Tan X, He HS, Zong S, Wu M, Liu K, Zhao D. Herbaceous Encroachment from Mountain Birch Forests to Alpine Tundra Plant Communities Through Above- and Belowground Competition. Forests. 2019; 10(2):170. https://doi.org/10.3390/f10020170
Chicago/Turabian StyleTan, Xinyuan, Hong S. He, Shengwei Zong, Miaomiao Wu, Kai Liu, and Dandan Zhao. 2019. "Herbaceous Encroachment from Mountain Birch Forests to Alpine Tundra Plant Communities Through Above- and Belowground Competition" Forests 10, no. 2: 170. https://doi.org/10.3390/f10020170
APA StyleTan, X., He, H. S., Zong, S., Wu, M., Liu, K., & Zhao, D. (2019). Herbaceous Encroachment from Mountain Birch Forests to Alpine Tundra Plant Communities Through Above- and Belowground Competition. Forests, 10(2), 170. https://doi.org/10.3390/f10020170




