Effects of Invasive Spartina alterniflora Loisel. and Subsequent Ecological Replacement by Sonneratia apetala Buch.-Ham. on Soil Organic Carbon Fractions and Stock
Abstract
1. Introduction
2. Materials and Methods
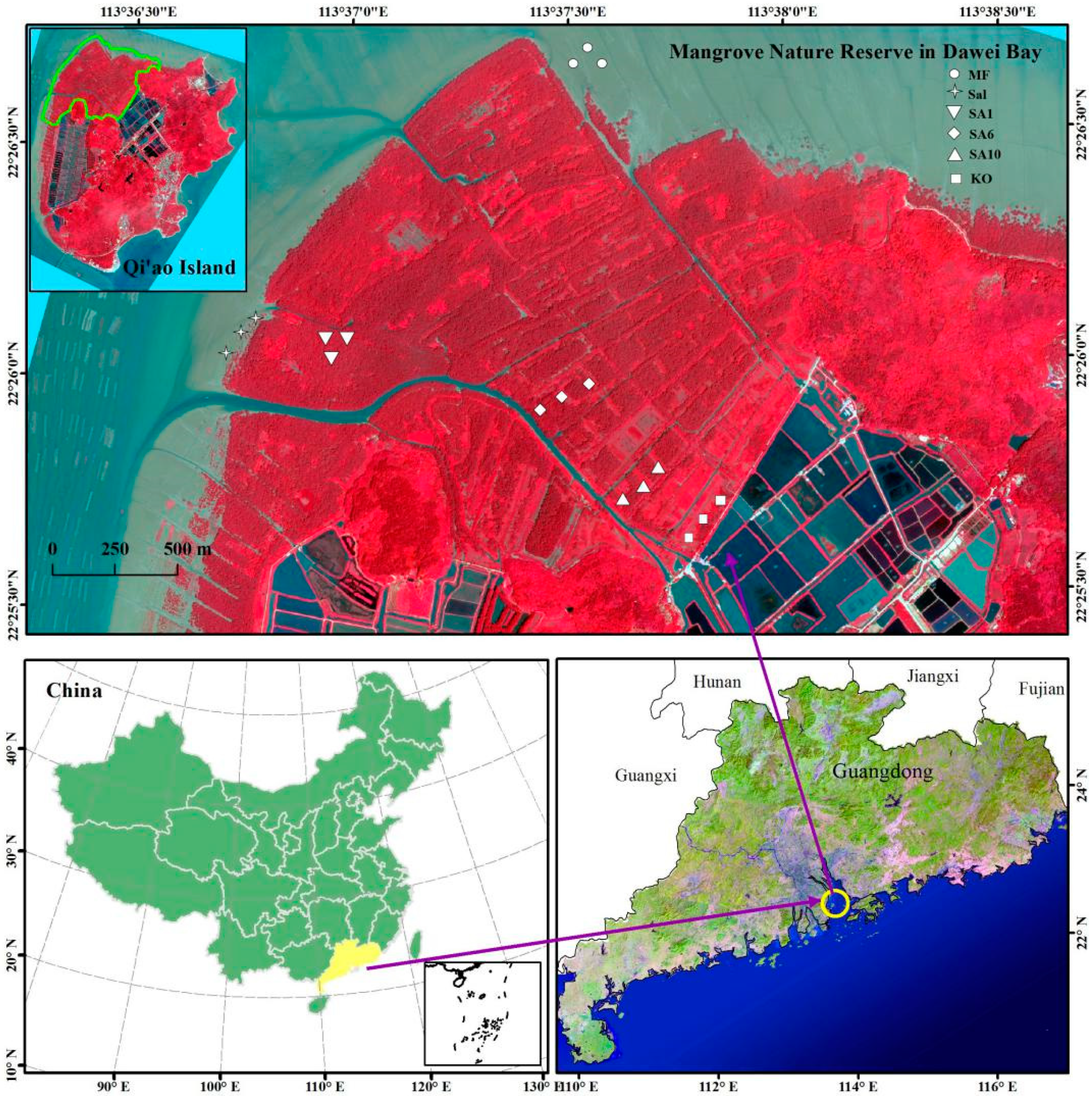
2.1. Study Area
2.2. Sample Collection and Measurement
2.3. Statistical Analysis
3. Results
3.1. Effects of Invasion and Restoration on Soil Physicochemical Parameters
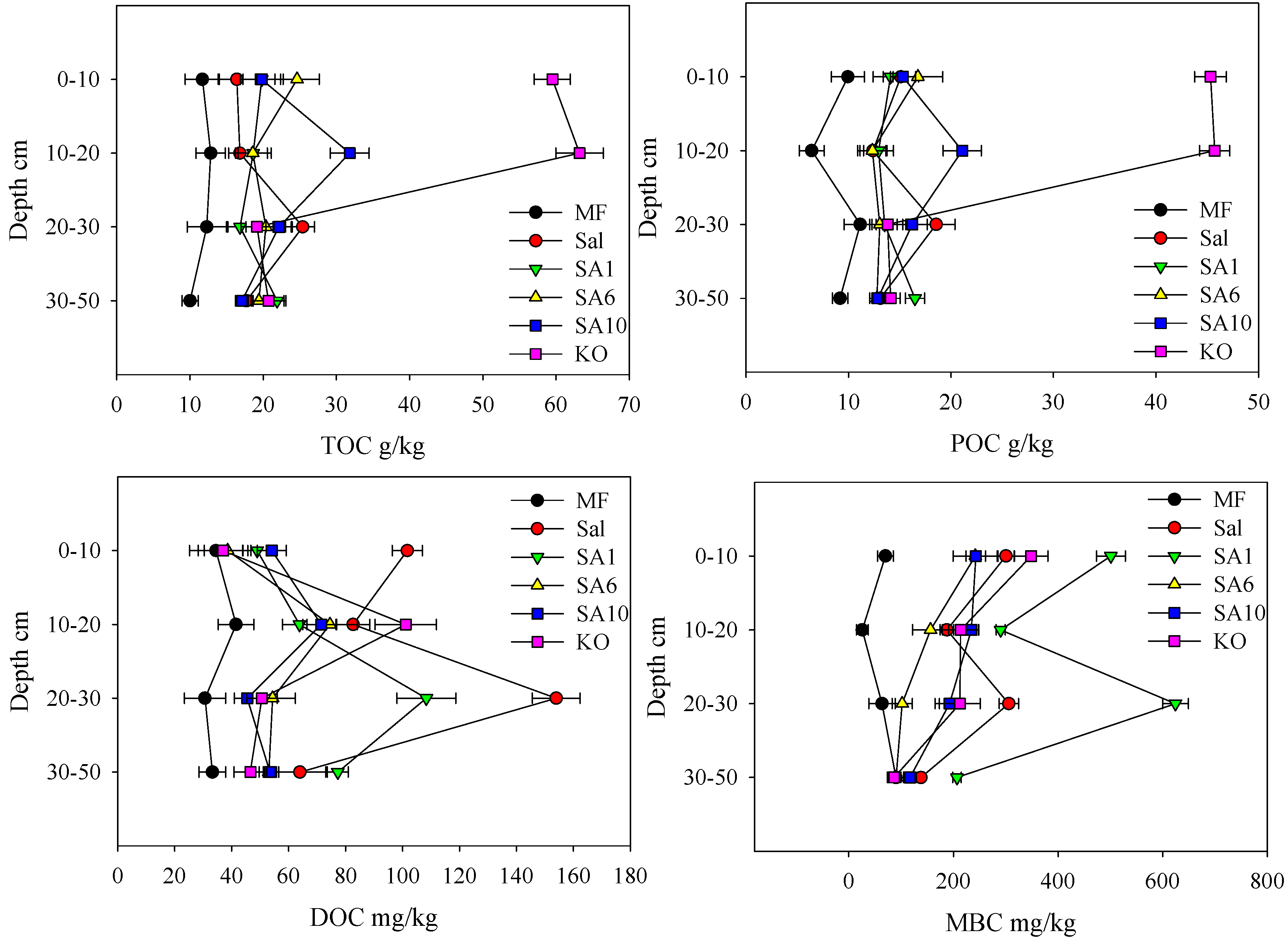
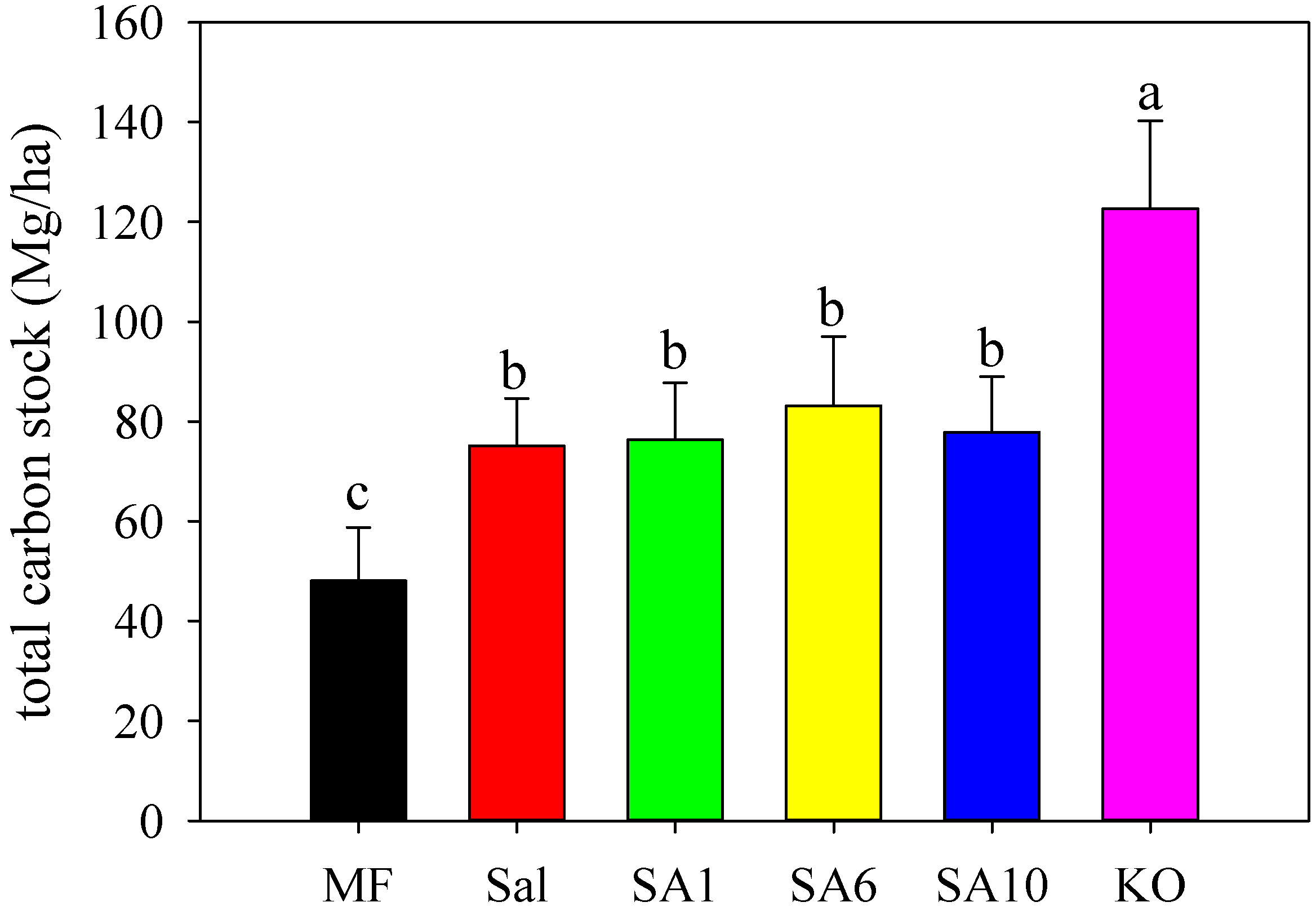
3.2. Effects of Invasion and Restoration on Soil Carbon Fractions and Carbon Stock
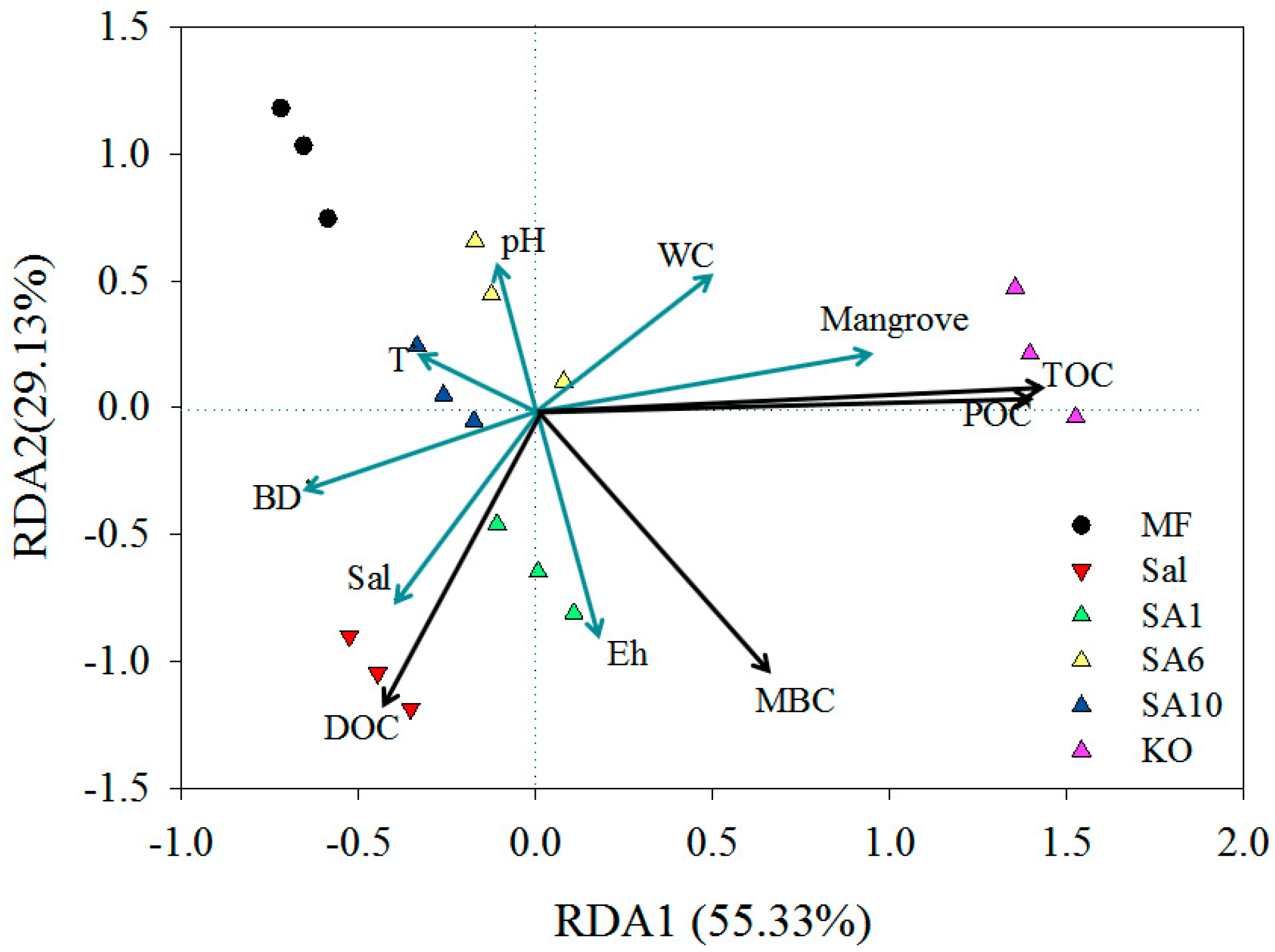
3.3. Relationship between Carbon Contents and Soil Physicochemical and Plant Community Age
4. Discussion
4.1. Effects of S. alterniflora and Restored Mangroves on Soil Organic Carbon Contents
4.2. Effects of Invasive S. alterniflora and Restored Mangroves on Soil Organic Carbon Fractions
4.3. Effects of S. alterniflora and Restored Mangroves on Soil Organic Carbon Stock
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Xiao, J.; Liu, F.; Zhang, Y.; Liu, C.A.; Lin, G. Contrasting ecosystem CO2 fluxes of inland and coastal wetlands: A meta-analysis of eddy covariance data. Glob. Chang. Biol. 2017, 23, 1180–1198. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ding, W.; Liu, D.; Kang, H.; Freeman, C.; Xiang, J.; Lin, Y. Exotic Spartina alterniflora invasion alters ecosystem-atmosphere exchange of CH4 and N2O and carbon sequestration in a coastal salt marsh in China. Glob. Chang. Biol. 2015, 21, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chen, G.C.; Ye, Y. Coastal vegetation invasion increases greenhouse gas emission from wetland soils but also increases soil carbon accumulation. Sci. Total. Environ. 2015, 526, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Lunstrum, A.; Chen, L.Z. Soil carbon stocks and accumulation in young mangrove forests. Soil Biol. Biochem. 2014, 75, 223–232. [Google Scholar] [CrossRef]
- Xiong, Y.; Liao, B.; Wang, F. Mangrove vegetation enhances soil carbon storage primarily through in situ inputs rather than increasing allochthonous sediments. Mar. Pollut. Bull. 2018, 131, 378–385. [Google Scholar] [CrossRef]
- Cheng, X.; Luo, Y.; Chen, J.; Lin, G.; Li, B. Short-term C4 plant Spartina alterniflora invasions change the soil carbon in C3 plant-dominated tidal wetlands on a growing estuarine Island. Soil Biol. Biochem. 2006, 38, 3380–3386. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, J.; Wang, L.; Cui, X.; Ning, C.; Wu, H.; Zhu, X.; Lin, G. Effects of short-term invasion of Spartina alterniflora and the subsequent restoration of native mangroves on the soil organic carbon, nitrogen and phosphorus stock. Chemosphere 2017, 184, 774–783. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Jiang, L.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Invasion of Spartina alterniflora enhanced ecosystem carbon and nitrogen stocks in the Yangtze Estuary, China. Ecosystems 2007, 10, 1351–1361. [Google Scholar] [CrossRef]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2007, 177, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; An, S.; Zhao, H.; Xu, L.; Qiao, Y.; Cheng, X. Impacts of Spartina alterniflora invasion on soil organic carbon and nitrogen pools sizes, stability, and turnover in a coastal salt marsh of eastern China. Ecol. Eng. 2016, 86, 174–182. [Google Scholar] [CrossRef]
- Liu, W.; Maung-Douglass, K.; Strong, D.R.; Pennings, S.C.; Zhang, Y. Geographical variation in vegetative growth and sexual reproduction of the invasive Spartina alterniflora in China. J. Ecol. 2015, 84, 129–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.; Wang, W.; Chen, L.; Lin, G. Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 2012, 93, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, J.; Luo, Y.; Henderson, R.; An, S.; Zhang, Q.; Chen, J.; Li, B. Assessing the effects of short-term Spartina alterniflora invasion on labile and recalcitrant C and N pools by means of soil fractionation and stable C and N isotopes. Geoderma 2008, 145, 177–184. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, H.; Chen, X.; Yin, S.; Cheng, X.; An, S. Consequences of short-term C4 plant Spartina alterniflora invasions for soil organic carbon dynamics in a coastal wetland of Eastern China. Ecol. Eng. 2013, 61, 50–57. [Google Scholar] [CrossRef]
- Davidson, I.C.; Cott, G.M.; Devaney, J.L.; Simkanin, C. Differential effects of biological invasions on coastal blue carbon: A global review and meta-analysis. Glob. Chang. Biol. 2018, 24, 5218–5230. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Wang, M.; Nie, M.; Qiu, S.; Wang, Q.; Quan, Z.; Xiao, M.; Li, B. Responses of soil nitrogen fixation to Spartina alterniflora invasion and nitrogen addition in a Chinese salt marsh. Sci. Rep. 2016, 6, 20384. [Google Scholar] [CrossRef]
- Feng, J.; Guo, J.; Huang, Q.; Jiang, J.; Huang, G.; Yang, Z.; Lin, G. Changes in the community structure and diet of benthic macrofauna in invasive Spartina alterniflora wetlands following restoration with native mangroves. Wetlands 2014, 34, 673–683. [Google Scholar] [CrossRef]
- Feng, J.; Huang, Q.; Chen, H.; Guo, J.; Lin, G. Restoration of native mangrove wetlands can reverse diet shifts of benthic macrofauna caused by invasive cordgrass. J. Appl. Ecol. 2017, 55, 905–916. [Google Scholar] [CrossRef]
- Gratton, C.; Denno, R.F. Restoration of arthropod assemblages in a Spartina salt marsh following removal of the invasive plant Phragmites australis. Restor. Ecol. 2005, 13, 358–372. [Google Scholar] [CrossRef]
- Li, B.; Liao, C.Z.; Zhang, X.D.; Chen, H.L.; Wang, Q.; Chen, Z.Y.; Gan, X.J.; Wu, J.H.; Zhao, B.; Ma, Z.J.; et al. Spartina alterniflora invasions in the Yangtze River estuary, China: An overview of current status and ecosystem effects. Ecol. Eng. 2009, 35, 511–520. [Google Scholar] [CrossRef]
- Chen, H.; Liao, B.W.; Liu, B.E.; Peng, C.H.; Zhang, Y.; Guan, W.; Zhu, Q.A.; Yang, G. Eradicating invasive Spartina alterniflora with alien Sonneratia apetala and its implications for invasion controls. Ecol. Eng. 2014, 73, 367–372. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, S.; Feng, Z.; Liu, G.; Gan, Q.; Peng, S. Use of exotic plants to control Spartina alterniflora invasion and promote mangrove restoration. Sci. Rep. 2015, 5, 12980. [Google Scholar] [CrossRef] [PubMed]
- Walcker, R.; Gandois, L.; Proisy, C.; Corenblit, D.; Mougin, E.; Laplanche, C.; Ray, R.; Fromard, F. Control of “blue carbon” storage by mangrove ageing: Evidence from a 66-year chronosequence in French Guiana. Glob. Chang. Biol. 2018, 24, 2325–2338. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Z.; Yang, S.C.; Chen, L.Z.; Wang, W.Q.; Du, X.N.; Wang, C.M.; Ma, Y.; Lin, G.X.; Lin, G.H. Changes in carbon pool and stand structure of a native subtropical mangrove forest after inter-planting with exotic species Sonneratia apetala. PLoS ONE 2014, 9, e91238. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Chen, H.; Li, Z.A.; Han, W. Biomass accumulation and carbon storage of four different aged Sonneratia apetala plantations in Southern China. Plant Soil 2010, 327, 279–291. [Google Scholar] [CrossRef]
- Lian, Z.; Jiang, Z.; Huang, X.; Liu, S.; Zhang, J.; Wu, Y. Labile and recalcitrant sediment organic carbon pools in the Pearl River Estuary, southern China. Sci. Total Environ. 2018, 640, 1302–1311. [Google Scholar] [CrossRef]
- Gunina, A.; Ryzhova, I.; Dorodnikov, M.; Kuzyakov, Y. Effect of plant communities on aggregate composition and organic matter stabilisation in young soils. Plant Soil 2015, 387, 265–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, W.; Luo, J.; Donnison, A. Changes in soil organic carbon dynamics in an Eastern Chinese coastal wetland following invasion by a C4 plant Spartina alterniflora. Soil Biol. Biochem. 2010, 42, 1712–1720. [Google Scholar] [CrossRef]
- Chen, G.; Gao, D.; Chen, G.; Zeng, C.; Wang, W. Effects of Spartina alterniflora invasion on soil carbon fractions in mangrove wetland of China. J. Water Soil Conserv. 2017, 31, 249–256, (In Chinese with English abstract). [Google Scholar]
- Chen, L.Z.; Zeng, X.Q.; Tam, N.F.Y.; Lu, W.Z.; Luo, Z.K.; Du, X.N.; Wang, J. Comparing carbon sequestration and stand structure of monoculture and mixed mangrove plantations of Sonneratia caseolaris and S. apetala in Southern China. For. Ecol. Manag. 2012, 284, 222–229. [Google Scholar] [CrossRef]
- Chen, L.Z.; Wang, W.Q.; Zhang, Y.H.; Lin, G.H. Recent progresses in mangrove conservation, restoration and research in China. J. Plant Ecol. 2009, 2, 45–54. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate Structure and Carbon, Nitrogen, and Phosphorus in Native and Cultivated Soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Vance, E.D.; Brooks, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-3. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 16 February 2019).
- Liu, J.E.; Zhou, H.; Qin, P.; Zhou, J. Effects of Spartina alterniflora salt marshes on organic carbon acquisition in intertidal zones of Jiangsu Province, China. Ecol. Eng. 2007, 30, 240–249. [Google Scholar] [CrossRef]
- Liu, J.-E.; Han, R.-M.; Su, H.-R.; Wu, Y.-P.; Zhang, L.-M.; Richardson, C.J.; Wang, G.-X. Effects of exotic Spartina alterniflora on vertical soil organic carbon distribution and storage amount in coastal salt marshes in Jiangsu, China. Ecol. Eng. 2017, 106, 132–139. [Google Scholar] [CrossRef]
- Yu, X.Q.; Yang, J.; Liu, L.M.; Tian, Y.; Yu, Z. Effects of Spartina alterniflora invasion on biogenic elements in a subtropical coastal mangrove wetland. Environ. Sci. Pollut. Res. 2015, 22, 3107–3115. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Power, M.E.; Comin, F.A.; Yockteng, R. Structural and functional Loss in restored wetland ecosystems. PLoS Biol. 2012, 10, e1001247. [Google Scholar] [CrossRef]
- Liao, J.D.; Boutton, T.W.; Jastrow, J.D. Storage and dynamics of carbon and nitrogen in soil physical fractions following woody plant invasion of grassland. Soil Biol. Biochem. 2006, 38, 3184–3196. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Xiong, Y.; Liao, B.; Proffitt, E.; Guan, W.; Sun, Y.; Wang, F.; Liu, X. Soil carbon storage in mangroves is primarily controlled by soil properties: A study at Dongzhai Bay, China. Sci. Total Environ. 2018, 619, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, T.; Patricia, J.F.; Pando-Bahuon, A.; Girardin, C.; Patrick, L. Soil organic matter dynamics along a rice chronosequence in north-eastern Argentina: Evidence from natural 13C abundance and particle size fractionation. Soil Biol. Biochem. 2006, 38, 2753–2761. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Xu, X.J.; Li, Y.; Huang, L.D.; Xie, X.J.; Dong, J.M.; Yang, S.Q. Effects of Spartina alterniflora invasion and exogenous nitrogen on soil nitrogen mineralization in the coastal salt marshes. Ecol. Eng. 2016, 87, 281–287. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Francioso, O.; Concheri, G.; Muscolo, A.; Nardi, S. Land use affects the soil C sequestration in alpine environment, NE Italy. Forests 2017, 8, 197. [Google Scholar] [CrossRef]
- He, N.; Wu, L.; Wang, Y.; Han, X. Changes in carbon and nitrogen in soil particle-size fractions along a grassland restoration chronosequence in northern China. Geoderma 2009, 150, 302–308. [Google Scholar] [CrossRef]
- Wang, J.; Song, C.; Wang, X.; Song, Y. Changes in labile soil organic carbon fractions in wetland ecosystems along a latitudinal gradient in Northeast China. Catena 2012, 96, 83–89. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Allison, M.A.; Zhao, J.; Li, X.; Comeaux, R.S.; Feagin, R.A.; Kulawardhana, R.W. Historical reconstruction of mangrove expansion in the Gulf of Mexico: Linking climate change with carbon sequestration in coastal wetlands. Estuar. Coast. Shelf Sci. 2013, 119, 7–16. [Google Scholar] [CrossRef]
- Kelleway, J.J.; Saintilan, N.; Macreadie, P.I.; Skilbeck, C.G.; Zawadzki, A.; Ralph, P.J. Seventy years of continuous encroachment substantially increases “blue carbon” capacity as mangroves replace intertidal salt marshes. Glob. Chang. Biol. 2016, 22, 1097–1109. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Ewers Lewis, C.J.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Liu, H.; Ren, H.; Hui, D.; Wang, W.; Liao, B.; Cao, Q. Carbon stocks and potential carbon storage in the mangrove forests of China. J. Environ. Manag. 2014, 133, 86–93. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Peng, Y.; Guan, D.; Hu, Z.; Chen, Y.; Lee, S.Y. Appearance can be deceptive: Shrubby native mangrove species contributes more to soil carbon sequestration than fast-growing exotic species. Plant Soil 2018, 432, 425–436. [Google Scholar] [CrossRef]
- Cheng, X.; Luo, Y.; Xu, Q.; Lin, G.; Zhang, Q.; Chen, J.; Bo, L. Seasonal variation in CH4 emission and its 13C-isotopic signature from Spartina alterniflora and Scirpus mariqueter soils in an estuarine wetland. Plant Soil 2010, 327, 85–94. [Google Scholar] [CrossRef]
- Gao, G.F.; Li, P.F.; Shen, Z.J.; Qin, Y.Y.; Zhang, X.M.; Ghoto, K.; Zhu, X.Y.; Zheng, H.L. Exotic Spartina alterniflora invasion increases CH4 while reduces CO2 emissions from mangrove wetland soils in southeastern China. Sci. Rep. 2018, 8, 9243. [Google Scholar] [CrossRef] [PubMed]
| Item | Depth (cm) | MF | Sal | SA1 | SA6 | SA10 | KO |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 0–10 | 31.3 ± 1.05a | 28.97 ± 0.67ab | 29.67 ± 0.67ab | 30.30 ± 1.18ab | 27.77 ± 0.61b | 28.27 ± 1.12b |
| 10–20 | 29.53 ± 0.45a | 27.8 ± 0.89ab | 27.33 ± 0.47b | 28.17 ± 0.95ab | 26.87 ± 0.65b | 26.63 ± 0.57b | |
| 20–30 | 28.00 ± 0.36a | 27.27 ± 0.55ab | 27.60 ± 0.50ab | 28.33 ± 0.85a | 25.93 ± 0.57c | 26.13 ± 0.46c | |
| 30–50 | 27.65 ± 0.27a | 27.23 ± 0.23a | 27.72 ± 0.34a | 28.18 ± 0.32a | 25.68 ± 0.24b | 25.92 ± 0.29b | |
| Water content (%) | 0–10 | 0.54 ± 0.03ab | 0.46 ± 0.02c | 0.44 ± 0.02 | 0.48 ± 0.03bc | 0.53 ± 0.02ab | 0.57 ± 0.02a |
| 10–20 | 0.49 ± 0.01bc | 0.46 ± 0.02c | 0.43 ± 0.02d | 0.44 ± 0.02d | 0.51 ± 0.01b | 0.59 ± 0.02a | |
| 20–30 | 0.41 ± 0.01e | 0.48 ± 0.01bc | 0.47 ± 0.01bc | 0.46 ± 0.02cd | 0.52 ± 0.01a | 0.42 ± 0.01de | |
| 30–50 | 0.45 ± 0.01ab | 0.45 ± 0.01ab | 0.48 ± 0.01a | 0.46 ± 0.01ab | 0.48 ± 0.01a | 0.42 ± 0.02b | |
| Bulk density (g/cm3) | 0–10 | 0.78 ± 0.03a | 0.80 ± 0.02a | 0.79 ± 0.03a | 0.79 ± 0.04a | 0.65 ± 0.02b | 0.60 ± 0.04b |
| 10–20 | 0.70 ± 0.05b | 0.82 ± 0.04ab | 0.8 ± 0.04ab | 0.84 ± 0.05a | 0.71 ± 0.03b | 0.52 ± 0.04c | |
| 20–30 | 0.92 ± 0.05a | 0.65 ± 0.03c | 0.71 ± 0.03bc | 0.80 ± 0.02b | 0.74 ± 0.04bc | 0.95 ± 0.05a | |
| 30–50 | 0.91 ± 0.02a | 0.89 ± 0.02a | 0.77 ± 0.01bc | 0.81 ± 0.02abc | 0.75 ± 0.02c | 0.88 ± 0.05ab | |
| pH | 0–10 | 7.43 ± 0.25a | 6.84 ± 0.22b | 6.85 ± 0.19b | 7.01 ± 0.18ab | 7.12 ± 0.14ab | 7.05 ± 0.17ab |
| 10–20 | 6.99 ± 0.19a | 6.91 ± 0.14a | 6.85 ± 0.16a | 7.24 ± 0.25a | 6.94 ± 0.17a | 7.33 ± 0.17a | |
| 20–30 | 7.64 ± 0.18a | 6.87 ± 0.28c | 7.06 ± 0.19bc | 7.14 ± 0.21abc | 7.17 ± 0.19abc | 6.98 ± 0.12bc | |
| 30–50 | 7.63 ± 0.07a | 6.98 ± 0.09b | 7.07 ± 0.07b | 7.03 ± 0.09b | 7.25 ± 0.08b | 7.05 ± 0.08b | |
| Eh | 0–10 | −29.00 ± 2.00d | 9.00 ± 1.00a | 9.00 ± 2.00a | −3.33 ± 1.53b | −9.67 ± 0.58c | −5.67 ± 1.53b |
| 10–20 | −17.33 ± 1.16de | 6.00 ± 1.00a | 6.00 ± 1.00a | −13.67 ± 2.08c | −9.67 ± 1.53b | −18.00 ± 1.00e | |
| 20–30 | −39.67 ± 1.53e | 6.67 ± 1.16a | −2.00 ± 0.00b | −8.00 ± 2.00c | −9.67 ± 1.53c | 4.33 ± 1.53a | |
| 30–50 | −37.00 ± 1.18c | −2.83 ± 1.01a | −3.50 ± 0.76a | −2.67 ± 0.84a | −12.50 ± 0.67b | −2.83 ± 2.77a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wang, S.; Wang, S.; Ying, R.; Yin, F.; Jiang, L.; Li, Z. Effects of Invasive Spartina alterniflora Loisel. and Subsequent Ecological Replacement by Sonneratia apetala Buch.-Ham. on Soil Organic Carbon Fractions and Stock. Forests 2019, 10, 171. https://doi.org/10.3390/f10020171
Feng J, Wang S, Wang S, Ying R, Yin F, Jiang L, Li Z. Effects of Invasive Spartina alterniflora Loisel. and Subsequent Ecological Replacement by Sonneratia apetala Buch.-Ham. on Soil Organic Carbon Fractions and Stock. Forests. 2019; 10(2):171. https://doi.org/10.3390/f10020171
Chicago/Turabian StyleFeng, Jianxiang, Shugong Wang, Shujuan Wang, Rui Ying, Fangmin Yin, Li Jiang, and Zufu Li. 2019. "Effects of Invasive Spartina alterniflora Loisel. and Subsequent Ecological Replacement by Sonneratia apetala Buch.-Ham. on Soil Organic Carbon Fractions and Stock" Forests 10, no. 2: 171. https://doi.org/10.3390/f10020171
APA StyleFeng, J., Wang, S., Wang, S., Ying, R., Yin, F., Jiang, L., & Li, Z. (2019). Effects of Invasive Spartina alterniflora Loisel. and Subsequent Ecological Replacement by Sonneratia apetala Buch.-Ham. on Soil Organic Carbon Fractions and Stock. Forests, 10(2), 171. https://doi.org/10.3390/f10020171




