Dendroclimatic Assessment of Ponderosa Pine Radial Growth along Elevational Transects in Western Montana, U.S.A.
Abstract
:1. Introduction
2. Materials and Methods
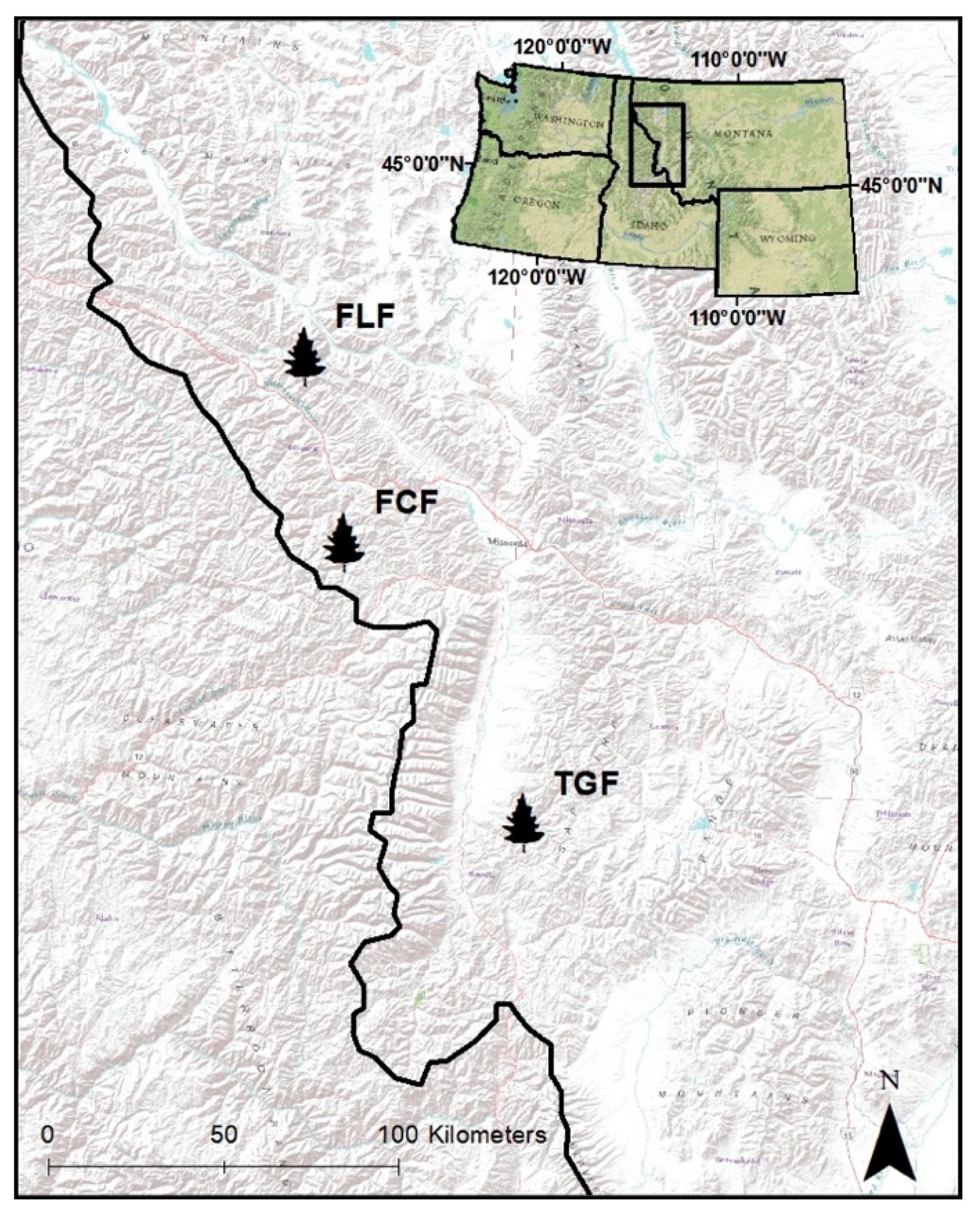
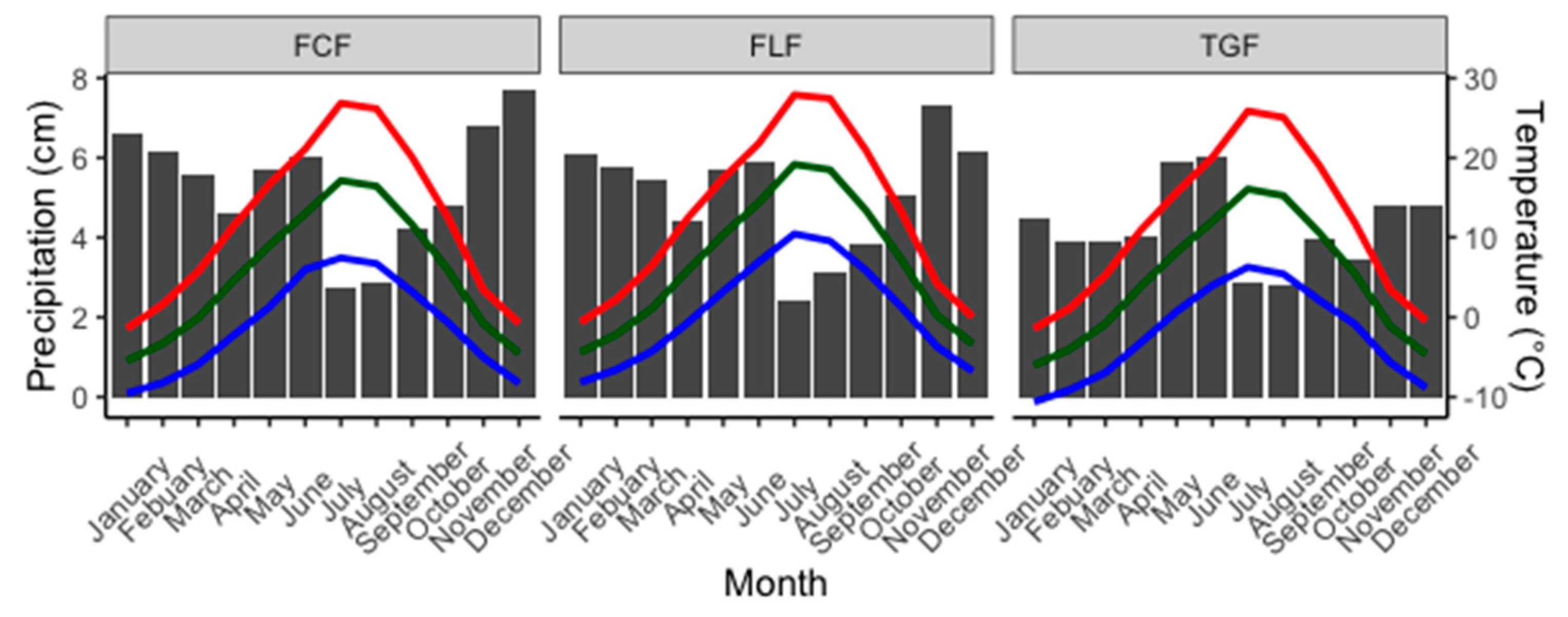
2.1. Study Area
2.2. Chronology Development
2.3. Statistical Analysis
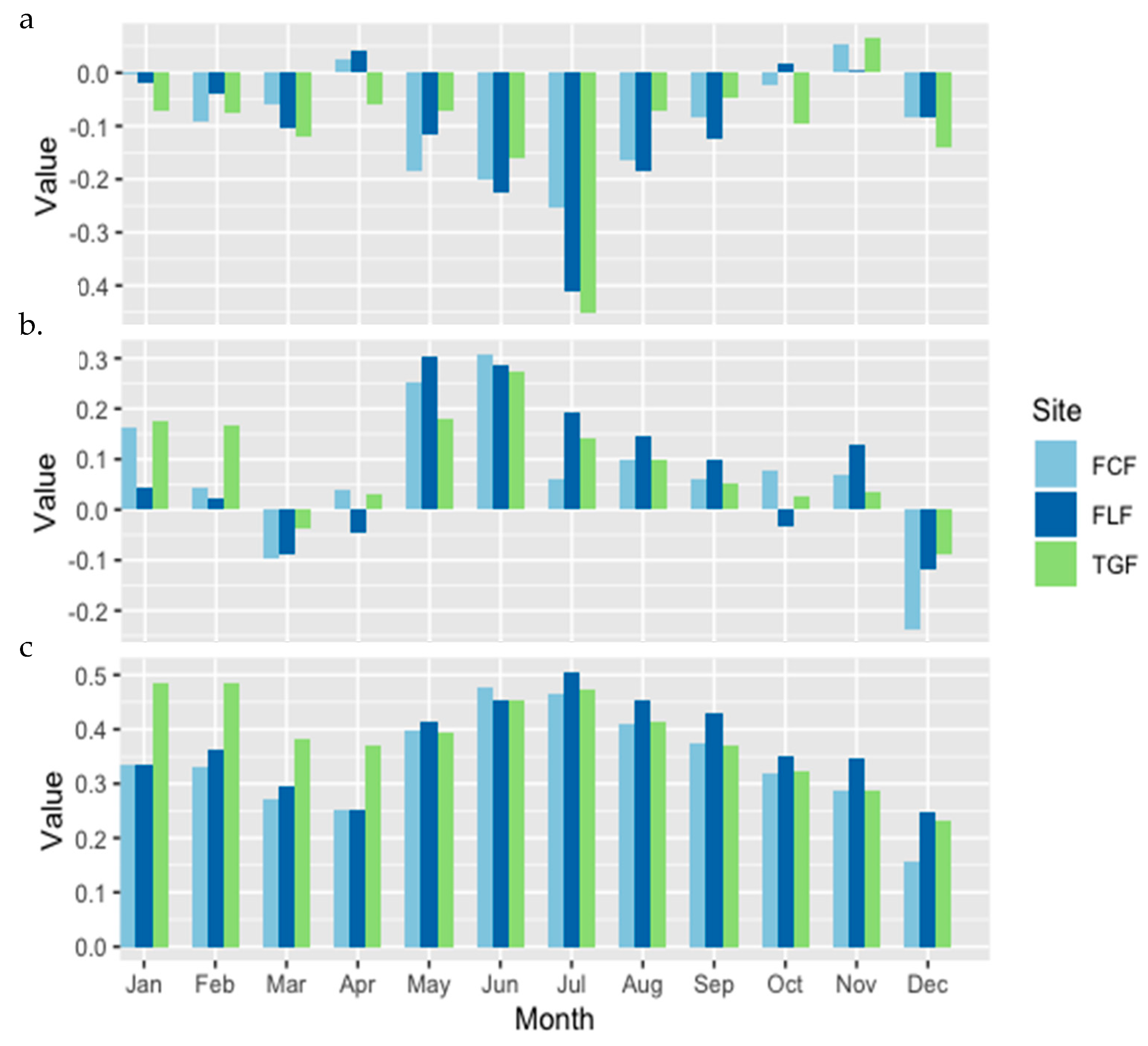
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinus ponderosa—The Gymonsperm Database. Available online: https://www.conifers.org/pi/Pinus_ponderosa.php (accessed on 2 September 2019).
- Plant Guide. Available online: https://plants.usda.gov/plantguide/pdf/pg_pipo.pdf (accessed on 7 September 2019).
- Joyce, L.A.; Talbert, M.; Sharp, D.; Stevenson, J. Historical and Projected Climate in the Northern Rockies Region. In Climate Change and Rocky Mountain Ecosystems. Advances in Global Change Research; Halofsky, J., Peterson, D., Eds.; Springer: New York, NY, USA, 2018; pp. 17–23. [Google Scholar]
- Littell, J.S.; McKenzie, D.; Kerns, B.K.; Cushman, S.; Shaw, C.G. Managing uncertainty in climate-driven ecological models to inform adaptation to climate change. Ecosphere 2011, 9, 1–19. [Google Scholar] [CrossRef]
- Riley, K.L.; Loehman, R.A. Mid-21st-century climate changes increase predicted fire occurrence and fire season length, Northern Rocky Mountains, United States. Ecosphere 2016, 7, 1–19. [Google Scholar] [CrossRef]
- Keane, R.E.; Mahalovich, M.F. Effects of Climate Change on Forest Vegetation in the Northern Rockies Region. Aspen Bibliogr. 2018, 374, 128–273. [Google Scholar]
- Whitlock, C.; Cross, W.; Maxwell, B.; Silverman, N.; Wade, A.A. Montana Climate Assessment; Montana Institute on Ecosystems, Montana State University and University of Montana, Bozeman and Missoula: Missoula, MT, USA, 2017. [Google Scholar]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase western US forest wildfire activity. Science 2006, 5789, 940–943. [Google Scholar] [CrossRef]
- Hamlet, A.F.; Lettenmaier, D.P. Effects of 20th century warming and climate variability on flood risk in the western US. Water Resour Res. 2007, 6, 1–17. [Google Scholar]
- Soulé, P.T.; Knapp, P.A. Radial Growth Rates of Two Co-occurring Coniferous Trees in the Northern Rockies during the Past Century. J. Arid Environ. 2013, 94, 87–95. [Google Scholar] [CrossRef]
- Soulé, P.T.; Knapp, P.A. Analyses of intrinsic water-use efficiency indicate performance differences of ponderosa pine and Douglas-fir in response to CO2 enrichment. J. Biogeogr. 2015, 42, 144–155. [Google Scholar] [CrossRef]
- Petrie, M.D.; Wildeman, A.M.; Bradford, J.B.; Hubbard, R.M.; Lauenroth, W.K. A review of precipitation and temperature control on seedling emergence and establishment for ponderosa and lodgepole pine forest regeneration. For. Ecol. Manag. 2016, 361, 328–338. [Google Scholar] [CrossRef]
- Marquardt, P.E. Investigation into Climatic Effects on the Growth and Genetic Structure of Sky Island Ponderosa Pine. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2018. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Marshall, L. Growth, carbon-isotope discrimination, and drought-associated mortality across a Pinus ponderosa elevational transect. Glob. Chang. Biol. 2010, 16, 399–415. [Google Scholar] [CrossRef]
- Ganey, J.L.; Vojta, S.C. Tree mortality in drought-stressed mixed-conifer and ponderosa pine forests, Arizona, USA. For. Ecol. Manag. 2011, 261, 162–168. [Google Scholar] [CrossRef]
- Tague, C.L.; McDowell, N.G.; Allen, C.D. An integrated model of environmental effects on growth, carbohydrate balance, and mortality of Pinus ponderosa forests in the southern Rocky Mountains. PLoS ONE 2013, 8, e80286. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Rehfeldt, G.E. Genetic differentiation among populations of Pinus ponderosa from the upper Colorado River Basin. Bot. Gaz. 1990, 1, 125–137. [Google Scholar] [CrossRef]
- Zhang, J.; Cregg, B.M. Growth and physiological responses to varied environments among populations of Pinus ponderosa. For. Ecol. Manag. 2005, 1, 1–12. [Google Scholar] [CrossRef]
- De Luis, M.; Čufar, K.; Di Filippo, A.; Novak, K.; Papadopoulos, A.; Piovesan, G.; Rathgeber, C.B.; Raventós, J.; Saz, M.A.; Smith, K.T. Plasticity in dendroclimatic response across the distribution range of Aleppo pine (Pinus halepensis). PLoS ONE 2013, 12, e83550. [Google Scholar] [CrossRef]
- Evenden, A.G.; Moeur, M.; Shelly, J.S.; Kimball, S.F.; Wellner, C.A. Research natural areas on National Forest System lands in Idaho, Montana, Nevada, Utah, and western Wyoming: A Guidebook for Scientists, Managers, and Educators; General Technical Report RMRS-GTR-69; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2001; p. 69. [Google Scholar]
- PRISM Climate Group, Oregon State University. Available online: http://prism.oregonstate.edu (accessed on 23 October 2019).
- Stokes, M.A. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Yamaguchi, D.K. A simple method for cross-dating increment cores from living trees. Can. J. For. Res. 1991, 3, 414–416. [Google Scholar] [CrossRef]
- Regent Instruments Canada Inc. WINDENDRO for Tree-Ring Analysis; Canada Inc.: Québec, QC, Canada, 2013. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Dannenberg, M.P.; Wise, E.K. Seasonal climate signals from multiple tree ring metrics: A case study of Pinus ponderosa in the upper Columbia River Basin. J Geophys. Res. Biogeosci. 2016, 121, 1178–1189. [Google Scholar] [CrossRef]
- Zhang, J.; Finley, K.A.; Johnson, N.G.; Ritchie, M.W. Lowering stand density enhances resiliency of ponderosa pine forests to disturbances and climate change. For. Sci. 2019, 65, 496–507. [Google Scholar] [CrossRef]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Palmer, W.C. Meteorological Drought; Paper 45, 58 pp; US Department of Commerce, Weather Bureau: Washington, DC, USA, 1965. [Google Scholar]
- Stout, D.L.; Sala, A. Xylem vulnerability to cavitation in Pseudotsuga menziesii and Pinus ponderosa from contrasting habitats. Tree Physiol. 2003, 23, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Affolter, P.; Büntgen, U.; Esper, J.; Rigling, A.; Weber, P.; Luterbacher, J.; Frank, D. Inner Alpine conifer response to 20th century drought swings. Eur. J. For. Res. 2010, 129, 289–298. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fulé, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread Increase of Tree Mortality Rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef]
- Knutson, K.C.; Pyke, D.A. Western juniper and ponderosa pine ecotonal climate–growth relationships across landscape gradients in southern Oregon. Can. J. For. Res. 2008, 38, 3021–3032. [Google Scholar] [CrossRef]
- Lascoux, M.; Palmé, A.E.; Cheddadi, R.; Latta, R.G. Impact of Ice Ages on the genetic structure of trees and shrubs. Philos. Trans. R. Soc. Lond. Ser. B 2004, 359, 197–207. [Google Scholar] [CrossRef]
- Latta, R.G.; Mitton, J.B. Historical separation and present gene flow through a zone of secondary contact in ponderosa pine. Evolution 1999, 53, 769–776. [Google Scholar] [CrossRef]
- McCullough, I.M.; Davis, F.W.; Williams, A.P. A range of possibilities: Assessing geographic variation in climate sensitivity of ponderosa pine using tree rings. For. Ecol. Manag. 2017, 402, 223–233. [Google Scholar] [CrossRef]
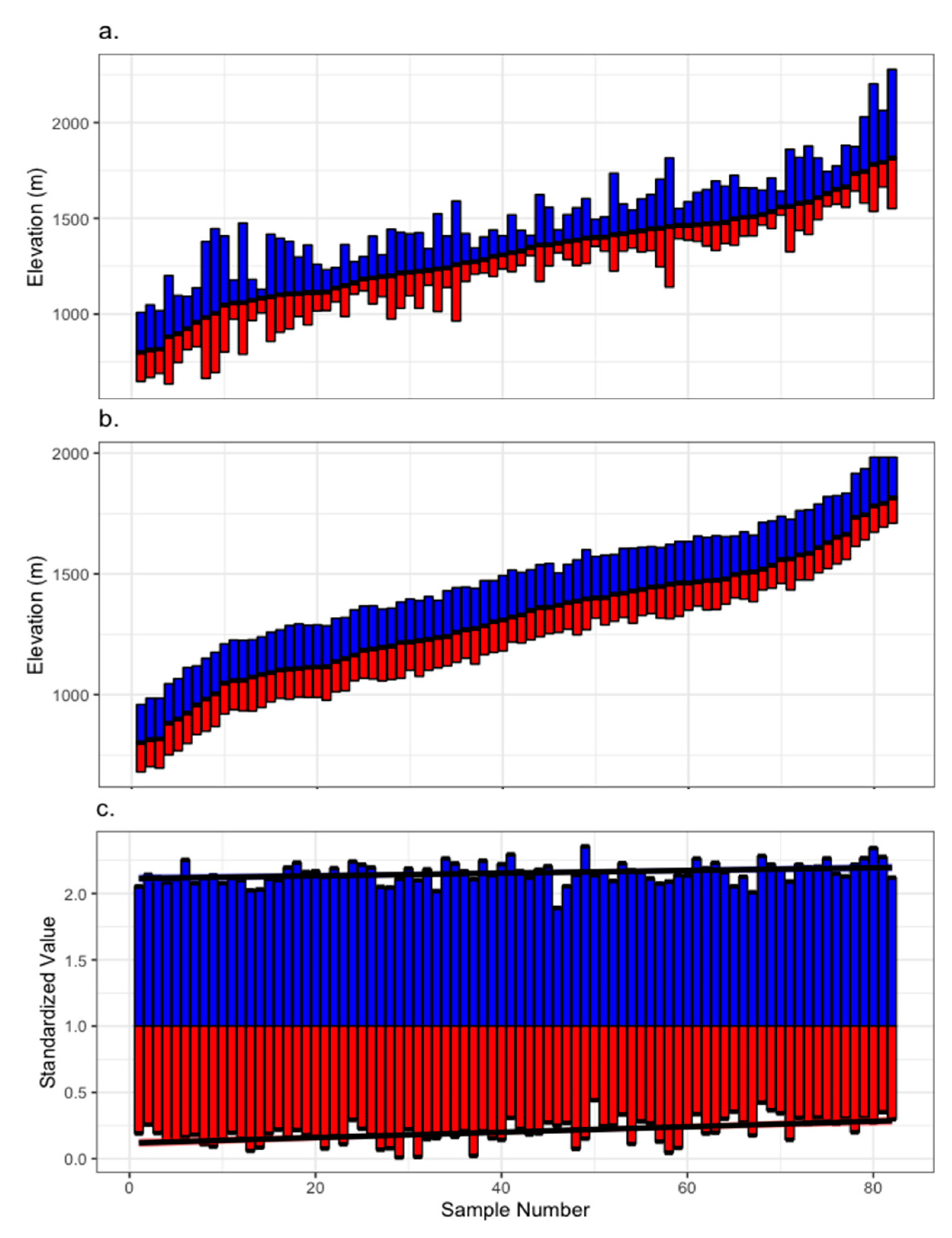
| Chronology | Mean DBH (cm) | Number of Samples | Mean Age (years) |
|---|---|---|---|
| FLF_ALL | 73.5 | 57 | 182 |
| FLF_TOP | 73.5 | 20 | 208 |
| FLF_BOTTOM | 72.9 | 19 | 145 |
| FCF_ALL | 64.7 | 47 | 233 |
| FCF_TOP | 63.0 | 17 | 241 |
| FCF_BOTTOM | 65.2 | 15 | 230 |
| TGF_ALL | 75.8 | 50 | 223 |
| TGF_TOP | 78.5 | 18 | 171 |
| TGF_BOTTOM | 78.5 | 18 | 275 |
| ALL | 70.6 | 154 | 213 |
| ALL_TOP | 74.1 | 53 | 211 |
| ALL_BOTTOM | 69.0 | 55 | 193 |
| July Temperature | June Precipitation | July PSDI | |
|---|---|---|---|
| FLF_Top | −0.410 | 0.258 | 0.542 |
| p-Value | 0.000 | 0.005 | 0.000 |
| FLF_Bottom | −0.326 | 0.299 | 0.442 |
| p-Value | 0.000 | 0.001 | 0.000 |
| FCF_Top | −0.297 | 0.286 | 0.493 |
| p-Value | 0.001 | 0.002 | 0.000 |
| FCF_Bottom | −0.193 | 0.287 | 0.421 |
| p-Value | 0.037 | 0.002 | 0.000 |
| TGF_Top | −0.410 | 0.328 | 0.461 |
| p-Value | 0.000 | 0.000 | 0.000 |
| TGF_Bottom | −0.448 | 0.240 | 0.452 |
| p-Value | 0.000 | 0.009 | 0.000 |
| July Temperature | June Precipitation | July PSDI | |
|---|---|---|---|
| ALL | −0.440 | 0.307 | 0.558 |
| p-Value | 0.000 | 0.001 | 0.000 |
| ALL_Top | −0.477 | 0.257 | 0.533 |
| p-Value | 0.000 | 0.005 | 0.000 |
| ALL_Bottom | −0.355 | 0.310 | 0.489 |
| p-Value | 0.000 | 0.001 | 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montpellier, E.E.; Soulé, P.T.; Knapp, P.A.; Maxwell, J.T. Dendroclimatic Assessment of Ponderosa Pine Radial Growth along Elevational Transects in Western Montana, U.S.A. Forests 2019, 10, 1094. https://doi.org/10.3390/f10121094
Montpellier EE, Soulé PT, Knapp PA, Maxwell JT. Dendroclimatic Assessment of Ponderosa Pine Radial Growth along Elevational Transects in Western Montana, U.S.A. Forests. 2019; 10(12):1094. https://doi.org/10.3390/f10121094
Chicago/Turabian StyleMontpellier, Evan E., Peter T. Soulé, Paul A. Knapp, and Justin T. Maxwell. 2019. "Dendroclimatic Assessment of Ponderosa Pine Radial Growth along Elevational Transects in Western Montana, U.S.A." Forests 10, no. 12: 1094. https://doi.org/10.3390/f10121094
APA StyleMontpellier, E. E., Soulé, P. T., Knapp, P. A., & Maxwell, J. T. (2019). Dendroclimatic Assessment of Ponderosa Pine Radial Growth along Elevational Transects in Western Montana, U.S.A. Forests, 10(12), 1094. https://doi.org/10.3390/f10121094





