Functional Diversity of the Soil Culturable Microbial Community in Eucalyptus Plantations of Different Ages in Guangxi, South China
Abstract
1. Introduction
2. Materials and Methods
2.1. Profile of the Study Site
2.2. Plot Selection and Soil Collection
2.3. Chemical Analysis and Biolog EcoPlate Technique
2.3.1. Soil Chemical Analysis
2.3.2. Soil Microbial Metabolic Activity Analysis
2.4. Statistical Analyses
3. Results
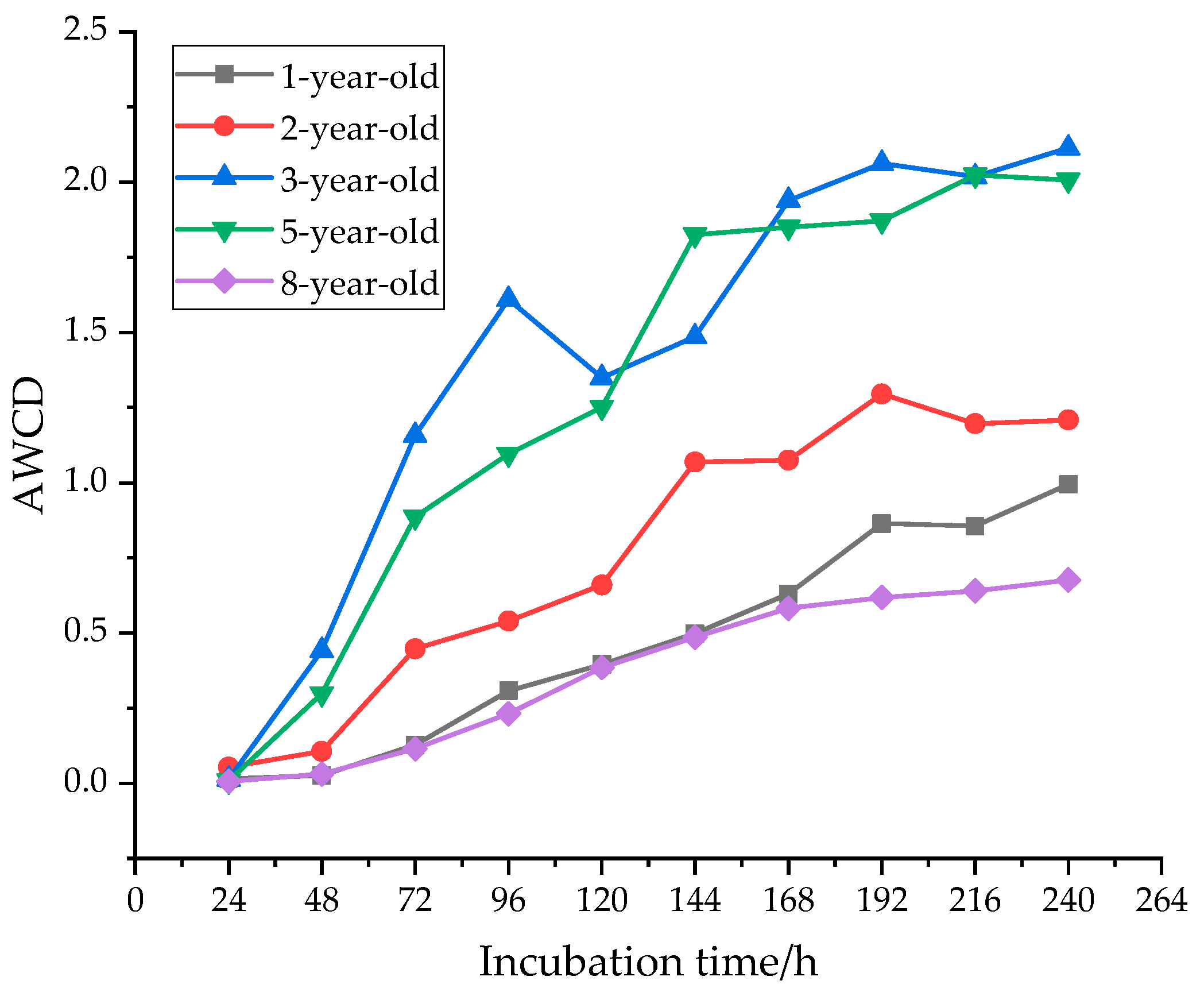
3.1. Changes in Soil Microbial AWCD at Different Forest Age with Time
3.2. Changes in Soil Microbial Diversity Index at Different Forest Age
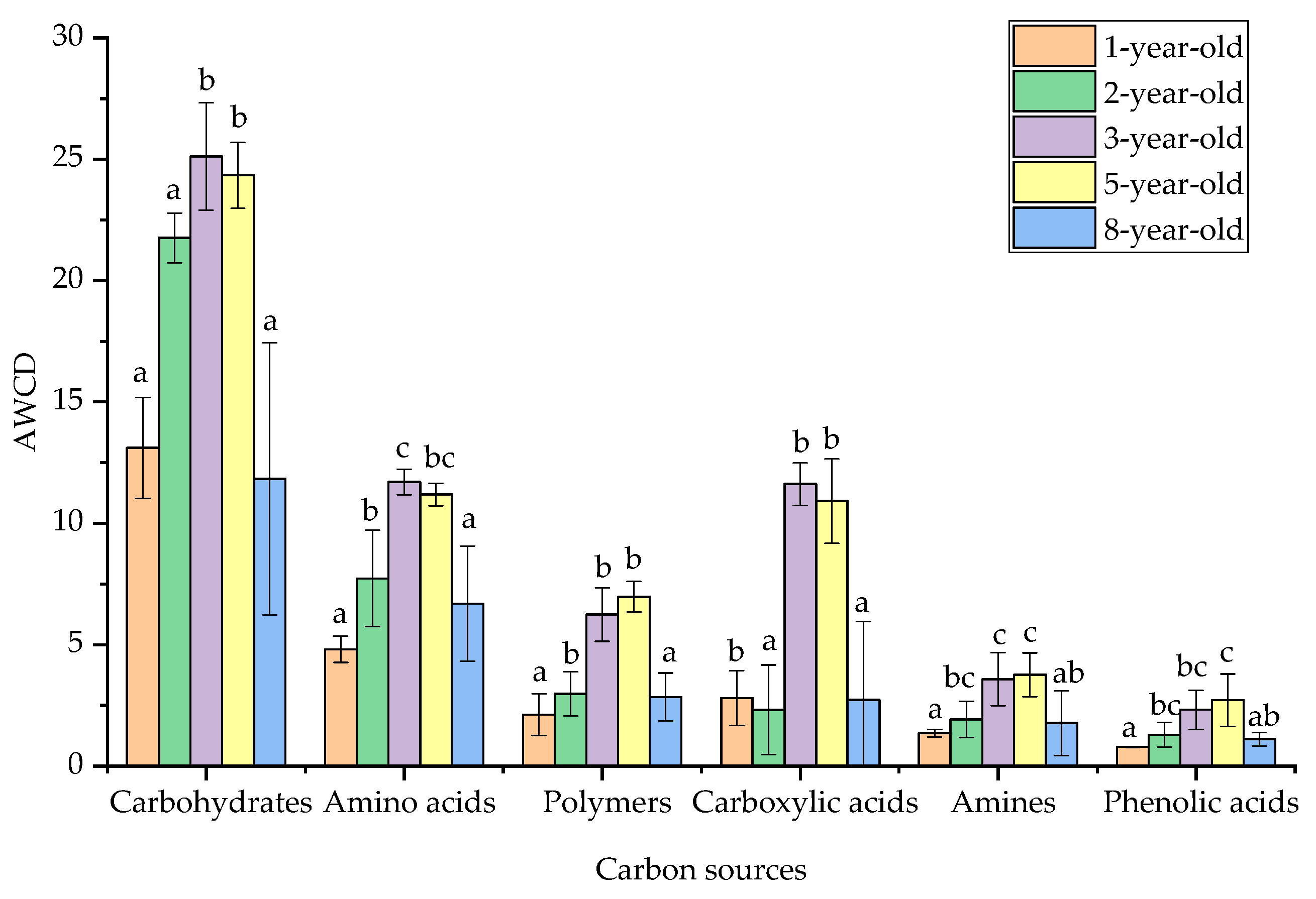
3.3. Carbon Source Utilization Level by Soil Microbiota
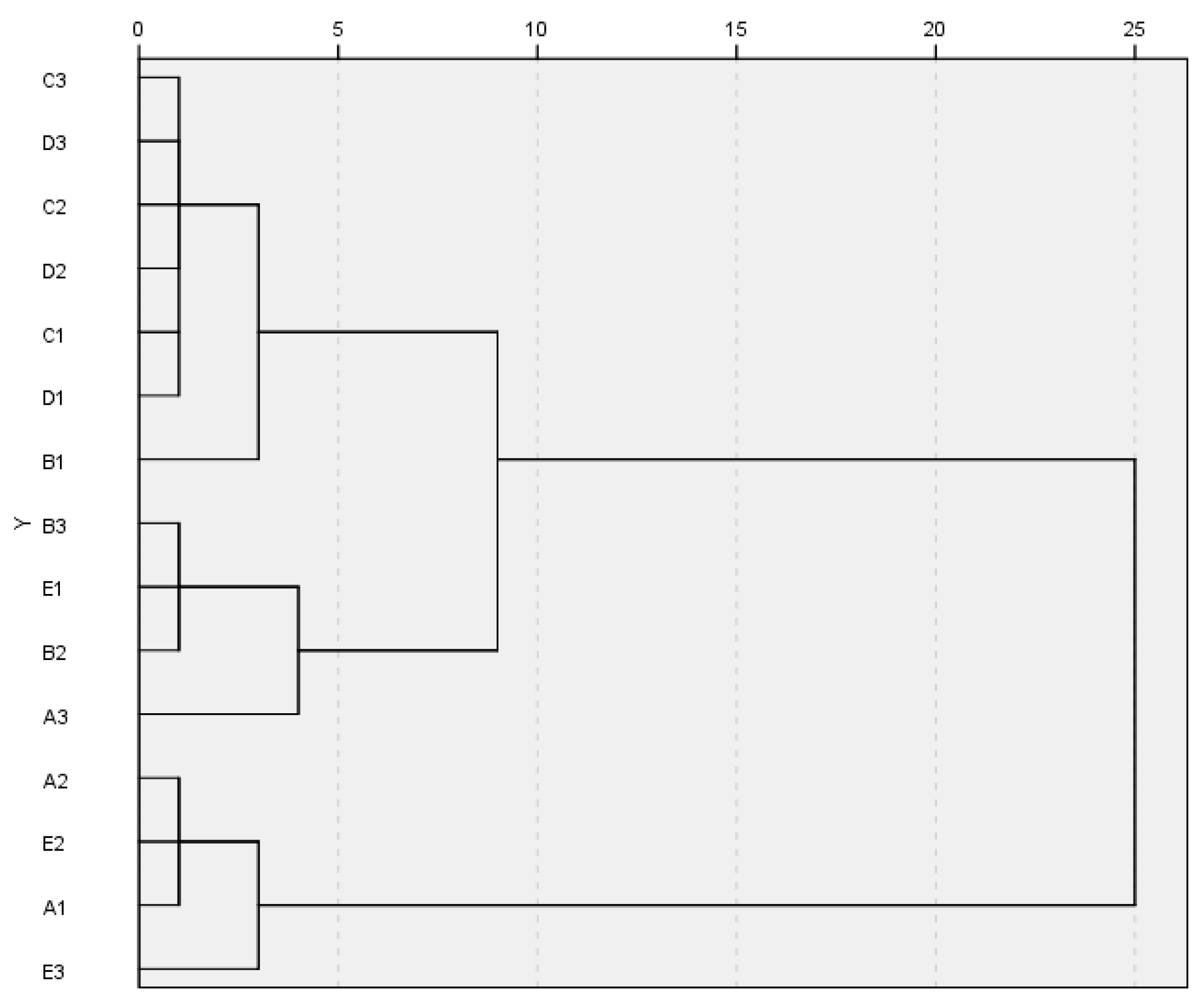
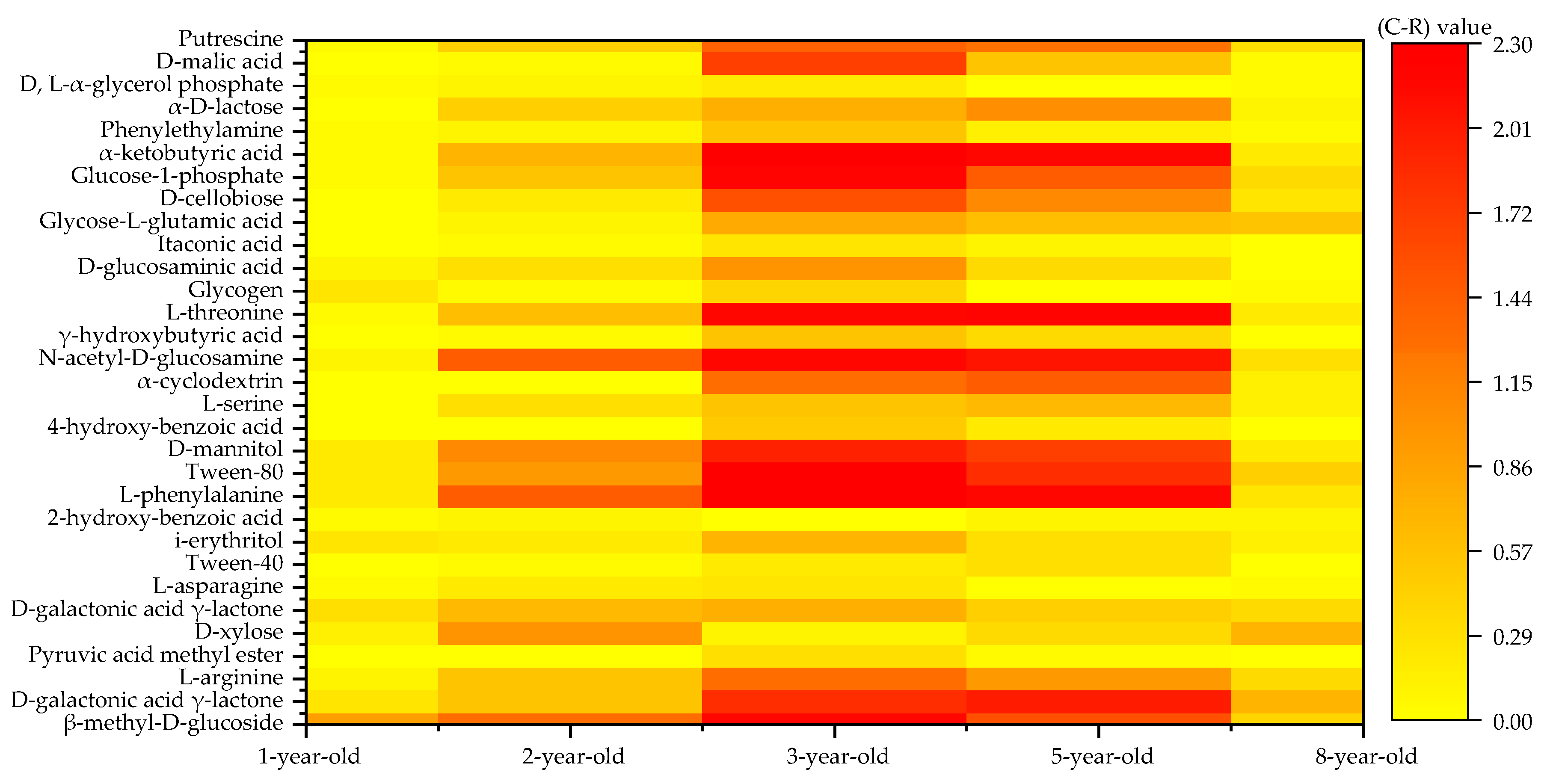
3.4. Carbon Source Species Utilized by the Soil Microbial Community in Eucalyptus Plantations at Various Forest Ages
3.5. Principal Component Analysis (PCA) of Carbon Source Utilization by Soil Microbiota
3.6. Relationship between Soil Microbial Community Functional Diversity with Soil Chemical Factors
4. Discussion
4.1. Variations of Soil Microbial Carbon Source Utilization Activity in Eucalyptus Forests at Various Forest Ages
4.2. Soil Microbial Functional Diversity of Eucalyptus Plantations at Different Ages
4.3. PCA and CCA of Soil Microbial Utilization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harris, J.; Birch, P. Soil microbial activity in opencast coal mine restorations. Soil Use Manag. 1989, 5, 155–160. [Google Scholar] [CrossRef]
- Guan, X.; Wang, J.; Zhao, H.; Wang, J.; Luo, X.; Liu, F.; Zhao, F. Soil bacterial communities shaped by geochemical factors and land use in a less-explored area, Tibetan Plateau. BMC Genom. 2013, 14, 820. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Grogan, P.; Sun, H.; Xiong, J.; Yang, Y.; Zhou, J.; Chu, H. Multi-Scale variability analysis reveals the importance of spatial distance in shaping Arctic soil microbial functional communities. Soil Biol. Biochem. 2015, 86, 126–134. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.-S.; Zepp, H. Effect of vegetation restoration on soil and water erosion and nutrient losses of a severely eroded clayey Plinthudult in southeastern China. Catena 2004, 57, 77–90. [Google Scholar] [CrossRef]
- Gałązka, A.; Grządziel, J. Fungal Genetics and Functional Diversity of Microbial Communities in the Soil under Long-Term Monoculture of Maize Using Different Cultivation Techniques. Front. Microbiol. 2018, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Tan, X.P.; He, W.X.; Geng, Z.C.; Liu, S.; She, D.; Hou, L. The metabolism characteristics of microbial community in different forest soil in Qinling Mountains Area. Acta Ecol. Sin. 2016, 36, 2978–2986. [Google Scholar] [CrossRef]
- Zhong, W.H.; Cai, Z.C. Diversity of carbon source metabolism of microbial community in farmland soils in an arsenic mining area. Chin. J. Appl. Ecol. 2013, 24, 473–480. [Google Scholar]
- Zheng, H.; Ouyang, Z.; Wang, X.; Fang, Z.; Zhao, T.; Miao, H. Effects of regenerating forest cover on soil microbial communities: A case study in hilly red soil region, Southern China. For. Ecol. Manag. 2005, 217, 244–254. [Google Scholar] [CrossRef]
- Smalla, K.; Wachtendorf, U.; Heuer, H.; Liu, W.-T.; Forney, L. Analysis of BIOLOG GN Substrate Utilization Patterns by Microbial Communities. Appl. Environ. Microbiol. 1998, 64, 1220–1225. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and characterisation of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar]
- Zak, J.; Willig, M.; Moorhead, D.; Wildman, H. Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
- Preston-Matham, J.; Boddy, L.; Randerson, P.F. Analysis of microbial community functional diversity using sole-carbon-source utilization profiles—acritique. FEMS Microbiol. Ecol. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, Z.; Zheng, H.; Wang, X.; Chen, F.; Zeng, J. Carbon metabolism of soil microbial communities of restored forests in Southern China. J. Soils Sediments 2011, 11, 789–799. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S.; Wang, H.; Hu, Z.; Li, Z.; You, Y. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, D.; Chen, X.; Wang, J.; Diao, J.J.; Zhang, J.Y.; Guan, Q.W. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar] [CrossRef]
- Grządziel, J.; Gałązka, A. Microplot long-term experiment reveals strong soil type influence on bacteria composition and its functional diversity. Appl. Soil Ecol. 2018, 124, 117–123. [Google Scholar] [CrossRef]
- Grządziel, J.; Furtak, K.; Gałązka, A. Community-Level Physiological Profiles of Microorganisms from Different Types of Soil That Are Characteristic to Poland—A Long-Term Microplot Experiment. Sustainability 2019, 11, 56. [Google Scholar] [CrossRef]
- Deng, J.J.; Zhu, W.X.; Zhou, Y.B.; Yin, Y.; Bai, X.J.; Zhang, H.Z.; Zhang, Y.M.; Qin, S.J. Effects of different land use patterns on the soil microbial community diversity in montane region of eastern Liaoning Province, China. J. Appl. Ecol. 2018, 29, 2269–2276. [Google Scholar] [CrossRef]
- Yin, R.; Deng, H.; Wang, H.-L.; Zhang, B. Vegetation type affects soil enzyme activities and microbial functional diversity following re-vegetation of a severely eroded red soil in sub-tropical China. Catena 2014, 115, 96–103. [Google Scholar] [CrossRef]
- Hu, X.; Yin, P.; Zeng, S.; Tong, C. Responses of Soil Macrofaunal Community and Diversity under Different Types of Vegetation on Soil Nutrient Pools in winter in Emei Mountain, China. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 022125. [Google Scholar] [CrossRef]
- Han, D.-X.; Wang, N.; Wang, N.-N.; Sun, X.; Feng, F.-J. Soil microbial functional diversity of different altitude Pinus koraiensis forests. J. Appl. Ecol. 2015, 26, 3649–3656. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Drenovsky, R.; Vo, D.; Graham, K.; Scow, K. Soil Water Content and Organic Carbon Availability Are Major Determinants of Soil Microbial Community Composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Grayston, S.J.; Prescott, C.E. Microbial communities in forest floors under four tree species in coastal British Columbia. Soil Biol. Biochem. 2005, 37, 1157–1167. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Xiao, Z.H.; Peng, W.X.; Du, H.; Song, M.; Liu, Y.X. Dynamics of plant diversity and community structure of Eucalyptus plantation at different ages class in Guangxi. J. Cent. South Univ. For. Technol. 2018, 38, 38–44. [Google Scholar] [CrossRef]
- Huang, G.Q.; Zhao, Q.G. The history, status quo, ecological problems and countermeasures of Eucalyptus plantations in Guangxi. Acta Ecol. Sin. 2014, 34, 5142–5152. [Google Scholar] [CrossRef]
- Behera, N.; Sahani, U. Soil microbial biomass and activity in response to Eucalyptus plantation and natural regeneration on tropical soil. For. Ecol. Manag. 2003, 174, 1–11. [Google Scholar] [CrossRef]
- Liu, H.Y. The Relationship of Soil Enzyme Activities between Soil Nutrients in A Long-Term Continuous Planting Eucalyptus Plantation; Guangxi University: Nanning, China, 2013. [Google Scholar]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Miltiadou, M.; Campbell, N.D.; Aracil, S.G.; Brown, T.; Grant, M.G. Detection of dead standing Eucalyptus camaldulensis without tree delineation for managing biodiversity in native Australian forest. Int. J. Appl. Earth Obs. Geoinf. 2018, 67, 135–147. [Google Scholar] [CrossRef]
- Leroy, G.; Bradley, L.; Rachel, B. Experimental evaluation of the initial effects of large-scale thinning on structure and biodiversity of river red gum (Eucalyptus camaldulensis) forests. Wildl. Res. 2018, 45, 397. [Google Scholar] [CrossRef]
- Li, F.Y.; Liang, Y.Y.; Shi, M.X. Ecological Benefit Analysis and Evaluation of Eucalyptus robusta Plantations in Guangxi. J. Anhui Agric. Sci. 2017, 45, 161–165. [Google Scholar] [CrossRef]
- Yang, X.; Li, D.; Mcgrouther, K.; Long, W.X.; Li, Y.L.; Chen, Y.K.; Lv, X.B.; Niazi, N.K.; Song, Z.L.; Wang, H.L. Effect of Eucalyptus forests on understory vegetation and soil quality. J. Soils Sediments 2017, 17, 2383–2389. [Google Scholar] [CrossRef]
- Hubbard, R.M.; Ferraz, S.; Stape, J.L.; Lemos, C.; Hakamada, R. Biomass production and potential water stress increase with planting density in four highly productive clonal Eucalyptus genotypes. South. For. A J. For. Sci. 2017, 79, 251–257. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Zeng, Z.; Du, H.; Zeng, F. Biomass and Carbon Sequestration by Juglans regia Plantations in the Karst Regions of Southwest China. Forests 2017, 8, 103. [Google Scholar] [CrossRef]
- Song, X.C.; Xiang, D.Y.; Yang, Z.N.; Zhang, Z.Y.; Cao, J.Z. Microbial functional diversity of rhizosphere soil of Eucalyptus plantation in Guangxi. J. Cent. South Univ. For. Technol. 2017, 37, 58–61. [Google Scholar] [CrossRef]
- Fu, W.B.; Peng, W.X.; Song, T.Q.; Zeng, F.P.; Du, H.; Wan, Y.G.; Xu, H.F. Biomass and its allocation characteristics of Eucalyptus urophylla × E. grandis plantations at different stand ages. Acta Ecol. Sin. 2014, 34, 5234–5241. [Google Scholar] [CrossRef]
- Du, H.; Song, T.Q.; Zeng, F.P.; Wang, K.L.; Fu, W.B.; Peng, W.X.; Li, S.S. Carbon storage and its controlling factors under different vegetation types in depressions between karst hills, Southwest China. Acta Ecol. Sin. 2015, 35, 4658–4667. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Zhang, W.; Chen, H.S.; Nie, Y.P.; Ye, Y.Y.; Wang, K.L. Temporal and spatial variation in surface soil moisture content of karst slopes in the dry season. Acta Ecol. Sin. 2015, 35, 6326–6334. [Google Scholar] [CrossRef]
- State Forestry Administration of the People’ s Republic of China. Methodology for Field Longterm Observation of Forest System (GB/T 33027-2016); China Standards Press: Beijing, China, 2017.
- Du, H.; Zeng, F.; Peng, W.; Song, T.; Hu, F. Plant community characteristics and their relationships with soil properties in a karst region of southwest China. Contemp. Probl. Ecol. 2017, 10, 707–716. [Google Scholar] [CrossRef]
- State Forestry Administration of the People’ s Republic of China. Determination of Available Phosphorus in Forest Soil (LY/T 1232-2015); China Standards Press: Beijing, China, 2016.
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 145–196. ISBN 9787109066441. [Google Scholar]
- Li, S.S.; Ma, D.L.; Zang, S.Y.; Wang, L.L.; Sun, H.Z. Structural and functional characteristics of soil microbial community in the Songjiang wetland under different interferences. Acta Ecol. Sin. 2018, 38, 7979–7989. [Google Scholar]
- Boyle, S.I.; Haskins, K.E.; Overby, S.T.; Hart, S.C.; Classen, A.T. Community-level physiological profiles of bacteria and fungi: plate type and incubation temperature influences on contrasting soils. FEMS Microbiol. Ecol. 2003, 44, 319–328. [Google Scholar] [CrossRef]
- Ratcliff, A.W.; Busse, M.D.; Shestak, C.J. Changes in microbial community structure following herbicide (glyphosate) additions to forest soils. Appl. Soil Ecol. 2006, 34, 114–124. [Google Scholar] [CrossRef]
- Kong, W.-D.; Zhu, Y.-G.; Fu, B.-J.; Marschner, P.; He, J.-Z.; Kong, W. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community. Environ. Pollut. 2006, 143, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Degens, B. Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 2000, 32, 189–196. [Google Scholar] [CrossRef]
- Choi, K.-H.; Dobbs, F.C. Comparison of two kinds of Biolog microplates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J. Microbiol. Methods 1999, 36, 203–213. [Google Scholar] [CrossRef]
- Tian, Y.N.; Wang, H.Q. Application of Biolog to study of environmental microbial function diversity. Environ. Sci. Technol. 2011, 34, 50–57. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Dong, Y.; Wei, Y.; Jiao, R. Unravelling the Functional Diversity of the Soil Microbial Community of Chinese Fir Plantations of Different Densities. Forests 2018, 9, 532. [Google Scholar] [CrossRef]
- Qu, Y.D.; Su, Z.Y.; Peng, G.X.; Liu, G. Soil microbial function aldiversity inamontaneevergreenbroadleaved forest of Chebaling following the huge ice storm in south China. Acta Ecol. Sin. 2009, 29, 6156–6164. [Google Scholar]
- Deng, J.; Zhou, Y.; Bai, X.; Luo, J.; Yin, Y.; Zhu, W. Soil Microbial Functional Diversity Responses to Different Revegetation Types in Baishilazi Nature Reserve. Pol. J. Environ. Stud. 2019, 28, 3675–3686. [Google Scholar] [CrossRef]
- Bending, G.D.; Turner, M.K.; E Jones, J. Interactions between crop residue and soil organic matter quality and the functional diversity of soil microbial communities. Soil Biol. Biochem. 2002, 34, 1073–1082. [Google Scholar] [CrossRef]
- Springob, G.; Kirchmann, H. Bulk soil C to N ratio as a simple measure of net N mineralization from stabilized soil organic matter in sandy arable soils. Soil Biol. Biochem. 2003, 35, 629–632. [Google Scholar] [CrossRef]
- Xie, L.X.; Chen, Q.P.; Wang, Z.H.; Liu, X.X. A Review of Effects of Soil Environmental Changes on Soil Microbe. Chin. J. Trop. Agric. 2004, 24, 39–47. [Google Scholar] [CrossRef]
- Xu, W.X.; Zhao, C.J.; Zhu, M.J.; Wu, Z.Y.; Han, Q.; Zheng, Q.Y. Soil microbial functional diversity of Eucalyptus plantation in Hainan Island. Guangdong Agric. Sci. 2016, 43, 65–72. [Google Scholar] [CrossRef]
- Gu, L.H.; Zhou, Y. Vegetation Structure and Species Diversity in Eucalypt Understory at Different Stand Age. Guangdong For. Technol. 2012, 28, 46–52. [Google Scholar] [CrossRef]
- Wang, C.Q.; Jiao, R.Z.; Dong, Y.H.; Hou, L.Y.; Zhao, J.J.; Zhao, S.R. Differences in Metabolic Functions of Soil Microbial Communities of Chinese Fir Plantations of Different Ages. Sci. Silvae Sin. 2019, 55, 36–45. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, A.; Yan, S.; Rafay, L.; Du, K.; Wang, D.; Ge, Y.; Li, J. Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot valley. J. Soils Sediments 2018, 19, 511–521. [Google Scholar] [CrossRef]
- O’Donnell, A.G.; Seasman, M.; Macrae, A.; Waite, I.; Davies, J.T. Plants and fertilisers as drivers of change in microbial community structure and function in soils. Plant Soil 2001, 232, 135–145. [Google Scholar] [CrossRef]
- Kang, M.M.; Chen, H.Y.; Chen, C.G.; Da, L.Q.; Wu, Q.T.; He, S.T. Microbial diversity in root zone soils of Acacia confusa and Eucalyptus urophylla polluted by heavy metals. J. East China Norm. Univ. (Nat. Sci.) 2009, 2, 50–60. [Google Scholar] [CrossRef]
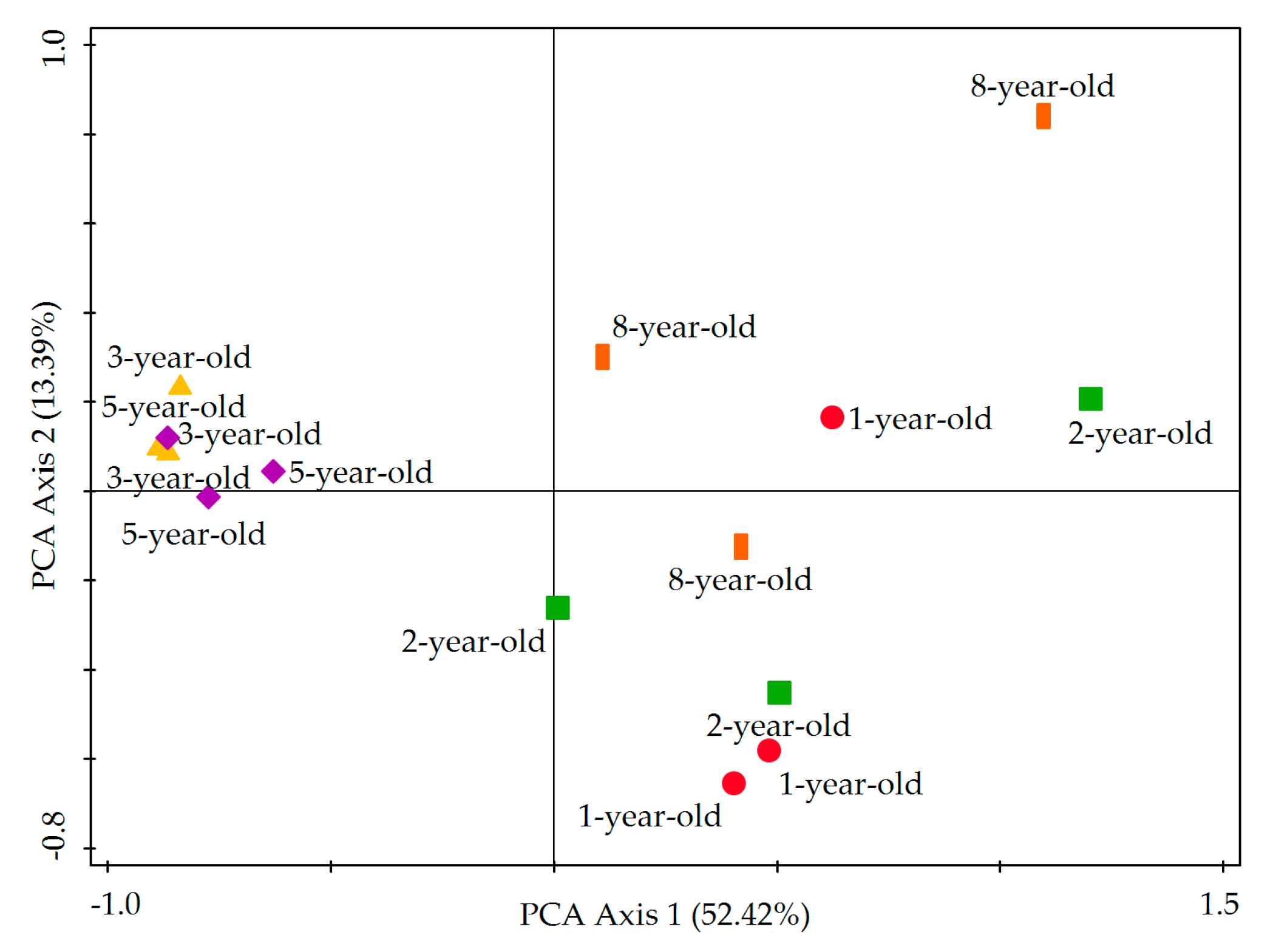
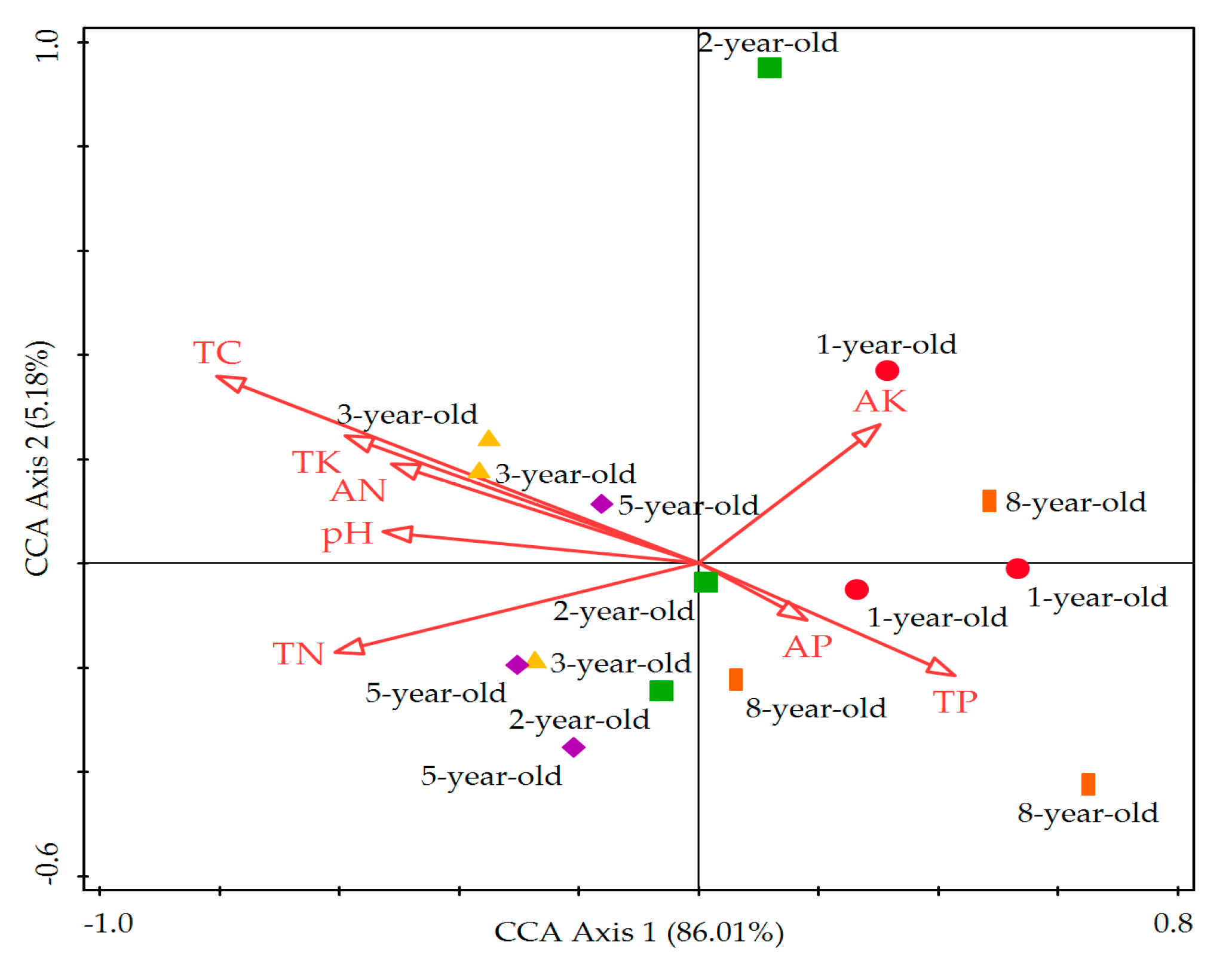
| Stand Ages | TC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | pH |
|---|---|---|---|---|---|---|---|---|
| 1-year-old | 17.32 ± 5.54b | 0.53 ± 0.23c | 0.23 ± 0.08c | 2.54 ± 1.52d | 68.79 ± 16.63a | 13.32 ± 5.64a | 92.28 ± 19.13b | 4.16 ± 0.20a |
| 2-year-old | 27.55 ± 4.93a | 1.57 ± 0.49a | 0.38 ± 0.06a | 15.58 ± 0.99a | 131.51 ± 25.30a | 5.76 ± 0.51a | 152.14 ± 15.27a | 4.14 ± 0.10a |
| 3-year-old | 30.59 ± 6.49a | 1.19 ± 0.22ab | 0.22 ± 0.04c | 11.03 ± 1.76b | 134.78 ± 8.03a | 8.41 ± 4.16a | 32.70 ± 6.63c | 4.46 ± 0.47a |
| 5-year-old | 30.68 ± 1.31a | 1.51 ± 0.09a | 0.25 ± 0.05c | 7.37 ± 2.51c | 204.94 ± 34.44a | 6.72 ± 1.05a | 37.49 ± 9.95c | 4.46 ± 0.31a |
| 8-year-old | 15.46 ± 2.84b | 0.87 ± 0.04bc | 0.50 ± 0.05a | 2.75 ± 0.88d | 85.99 ± 10.19a | 9.99 ± 6.70a | 44.26 ± 4.43c | 4.03 ± 0.15a |
| Type | Carbon Source | PC1 (52.42%) | PC2 (13.39%) |
|---|---|---|---|
| Carbohydrates | D-glucosaminic acid | 0.877 | −0.125 |
| Glucose-1-phosphate | 0.800 | 0.373 | |
| D-cellobiose | 0.787 | −0.005 | |
| α-D-lactose | 0.777 | 0.537 | |
| D-galacturonic acid | 0.698 | 0.290 | |
| D-galactonic acid γ-lactone | 0.695 | −0.211 | |
| D-mannitol | 0.681 | 0.529 | |
| D, L-α-glycerol phosphate | 0.649 | 0.200 | |
| N-acetyl-D-glucosamine | 0.599 | 0.536 | |
| i-erythritol | 0.515 | −0.033 | |
| β-methyl-D-glucoside | 0.105 | 0.442 | |
| D-xylose | -0.036 | 0.731 | |
| Amino acids | L-serine | 0.884 | 0.036 |
| Glycose-L-glutamic acid | 0.863 | 0.003 | |
| L-arginine | 0.824 | −0.169 | |
| L-phenylalanine | 0.789 | 0.235 | |
| L-threonine | 0.756 | −0.152 | |
| L-asparagine | 0.549 | 0.212 | |
| Carboxylic acids | γ-hydroxybutyric acid | 0.913 | −0.165 |
| D-malic acid | 0.885 | −0.323 | |
| Itaconic acid | 0.883 | −0.236 | |
| Pyruvic acid methyl ester | 0.781 | −0.199 | |
| α-Ketobutyric acid | 0.781 | −0.114 | |
| Polymers | α-cyclodextrin | 0.935 | −0.278 |
| Tween-80 | 0.873 | 0.185 | |
| Tween-40 | 0.818 | −0.189 | |
| Glycogen | 0.222 | −0.437 | |
| Amines | Phenylethylamine | 0.931 | 0.011 |
| Putrescine | 0.853 | −0.218 | |
| Phenolic acids | 4-hydroxy-benzoic acid | 0.886 | −0.260 |
| 2-hydroxy-benzoic acid | 0.588 | 0.151 |
| Stand Ages | H | J | Ds | U | S |
|---|---|---|---|---|---|
| 1-year-old | 3.19 ± 0.11b | 0.90 ± 0.01b | 0.93 ± 0.04a | 5.64 ± 0.76c | 23.00 ± 1.73c |
| 2-year-old | 3.20 ± 0.06ab | 0.93 ± 0.19ab | 0.95 ± 0.01a | 8.44 ± 0.65b | 25.00 ± 3.61ba |
| 3-year-old | 3.36 ± 0.05a | 0.98 ± 0.014a | 0.96 ± 0.01a | 11.80 ± 0.29a | 30.33 ± 1.15a |
| 5-year-old | 3.33 ± 0.03ab | 0.97 ± 0.01a | 0.96 ± 0.00a | 11.57 ± 0.53a | 29.67 ± 1.15a |
| 8-year-old | 3.10 ± 0.16b | 0.90 ± 0.05b | 0.95 ± 0.01a | 6.01 ± 2.41c | 24.00 ± 4.00cb |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, X.; Du, H.; Peng, W.; Liu, Y.; Fang, Z.; Song, T. Functional Diversity of the Soil Culturable Microbial Community in Eucalyptus Plantations of Different Ages in Guangxi, South China. Forests 2019, 10, 1083. https://doi.org/10.3390/f10121083
Lan X, Du H, Peng W, Liu Y, Fang Z, Song T. Functional Diversity of the Soil Culturable Microbial Community in Eucalyptus Plantations of Different Ages in Guangxi, South China. Forests. 2019; 10(12):1083. https://doi.org/10.3390/f10121083
Chicago/Turabian StyleLan, Xiu, Hu Du, Wanxia Peng, Yongxian Liu, Zhilian Fang, and Tongqing Song. 2019. "Functional Diversity of the Soil Culturable Microbial Community in Eucalyptus Plantations of Different Ages in Guangxi, South China" Forests 10, no. 12: 1083. https://doi.org/10.3390/f10121083
APA StyleLan, X., Du, H., Peng, W., Liu, Y., Fang, Z., & Song, T. (2019). Functional Diversity of the Soil Culturable Microbial Community in Eucalyptus Plantations of Different Ages in Guangxi, South China. Forests, 10(12), 1083. https://doi.org/10.3390/f10121083






