Temperature, Wind, Cloud, and the Postglacial Tree Line History of Sub-Antarctic Campbell Island
Abstract
1. Introduction
2. Physical Geography and Vegetation Cover of Campbell Island
2.1. Geology, Topography, Soils, and Climate
2.2. Vegetation
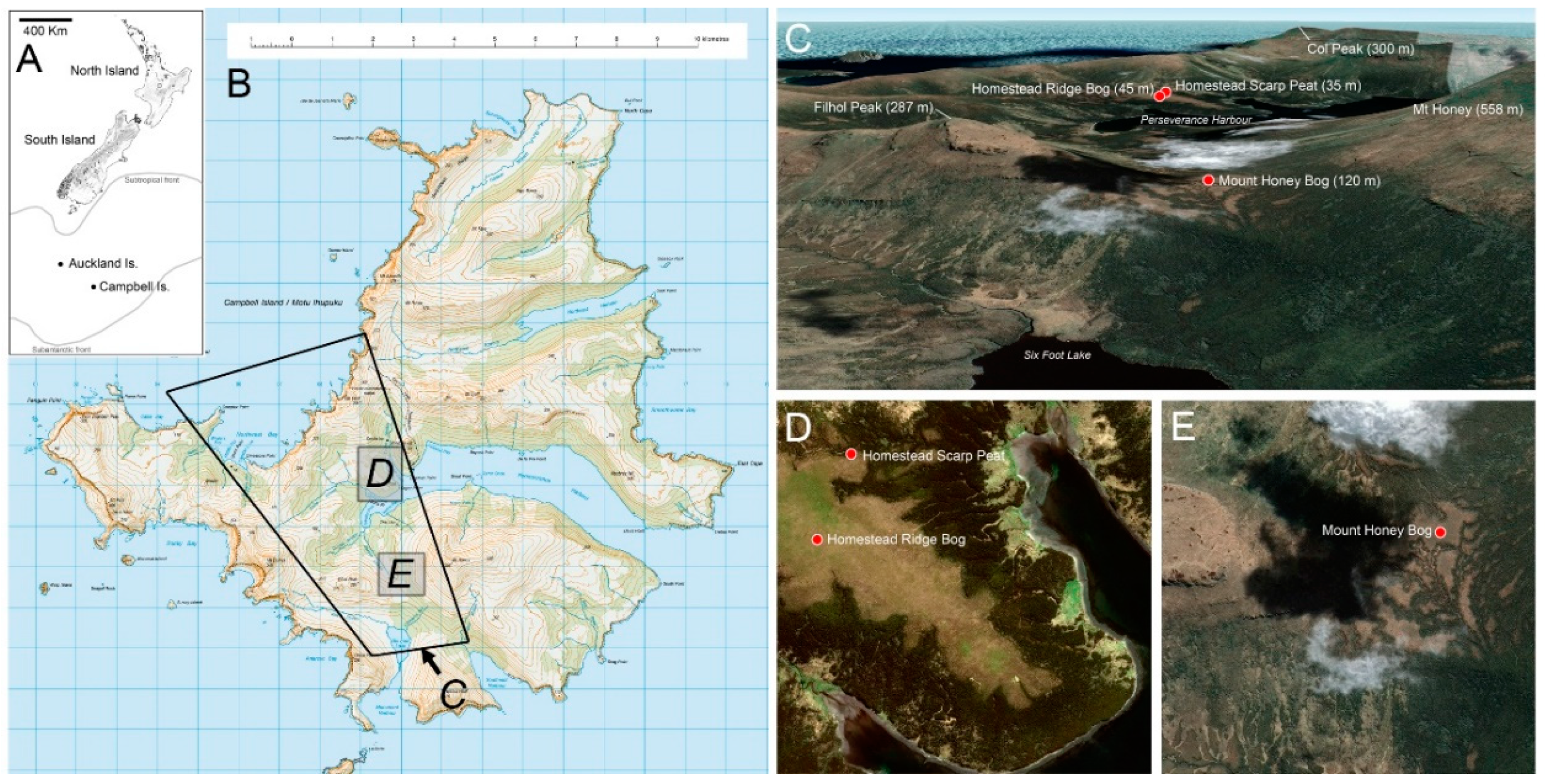
3. Study Sites (Figure 1)
3.1. Homestead Ridge Bog (HRB)
3.2. Homestead Scarp Peat (HSP)
3.3. Mount Honey Saddle Bog (MHB)
4. Methods
4.1. Water Table, Vegetation Cover, and Surface Wetness Index
4.2. Peat Sampling
4.3. Radiocarbon
4.4. Peat Humification
4.5. Pollen Analysis
5. Results
5.1. Chronostratigraphy
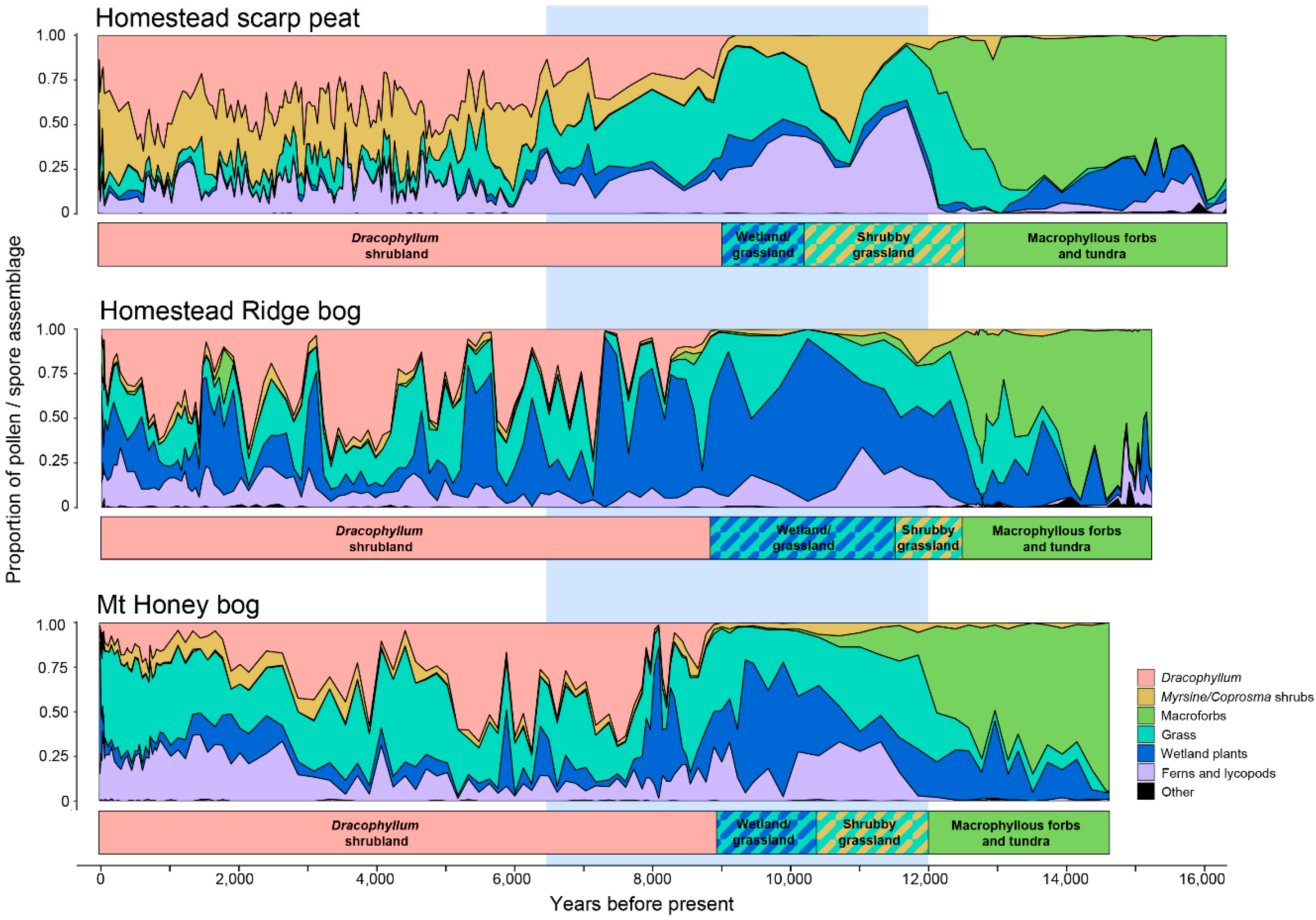
5.2. Vegetation Sequences Based on Pollen Stratigraphy
- Macrophyllous forb: The oldest sediments are dominated by macrophyllous forbs and include occurrences of the poorly dispersed Stilbocarpa polaris, and have relatively little wetland, low shrub, grassland, or fern representation. The small ground fern Grammitis is characteristic of the earliest sediments. While Grammitis spores cannot be identified to species, it is likely, given its association with tundra dominants, to be the dwarf alpine fern G. poeppigiana, which forms mats on rocks in the tundra zone [19]. A sparse macrophyllous forb tundra growing in a rock-covered and silt-covered landscape was, therefore, the first postglacial vegetation. The absence of abundant grass or wetland pollen types points to the tundra zone above about 300–400 m as the closest modern analogue. In contrast to the modern tundra, few fern spores are recorded, aside from the alpine Grammitis. Since fern spores are among the few well dispersed types on the island [24], tundra-like conditions extended right to the contemporary sea level about 100 m lower than the present day.
- Grassland-shrub-wetland: At all three sites, there is an abrupt to gradual transition toward less macrophyllous forbs and more grasses, which is followed by increased shrubs (Coprosma and Myrsine divaricata) and ferns. This sequence is best exemplified at well drained HSP where a Myrsine/Coprosma shrub-dominated zone (11,400-10,200 BP) not apparent at the other sites is recognised.
- Wetland-grassland: Wetland types begin to predominate in Astelia and Centrolepis. HSP, being the best drained of the three sites, has only a weak wetland response, but wetland types are more abundant at the other two sites than elsewhere in the profile. Hymenophyllum ferns, which are abundant in the preceding zone, are also common in this region.
- Dracophyllum-scrub: The island-wide simultaneous spread of Dracophyllum marks the onset of this zone, but grassland and ferns remain abundant, and wetland at the bog sites, for two to three thousand years longer. All sites have significant fluctuations in abundance of the major components, but most markedly in the raised bog, HRB, where Dracophyllum oscillates in response to wetter or drier conditions. This stage ended with the settlement of the island in the late 19th century and the changes it brought with fire and sheep grazing [34,35,36,37].
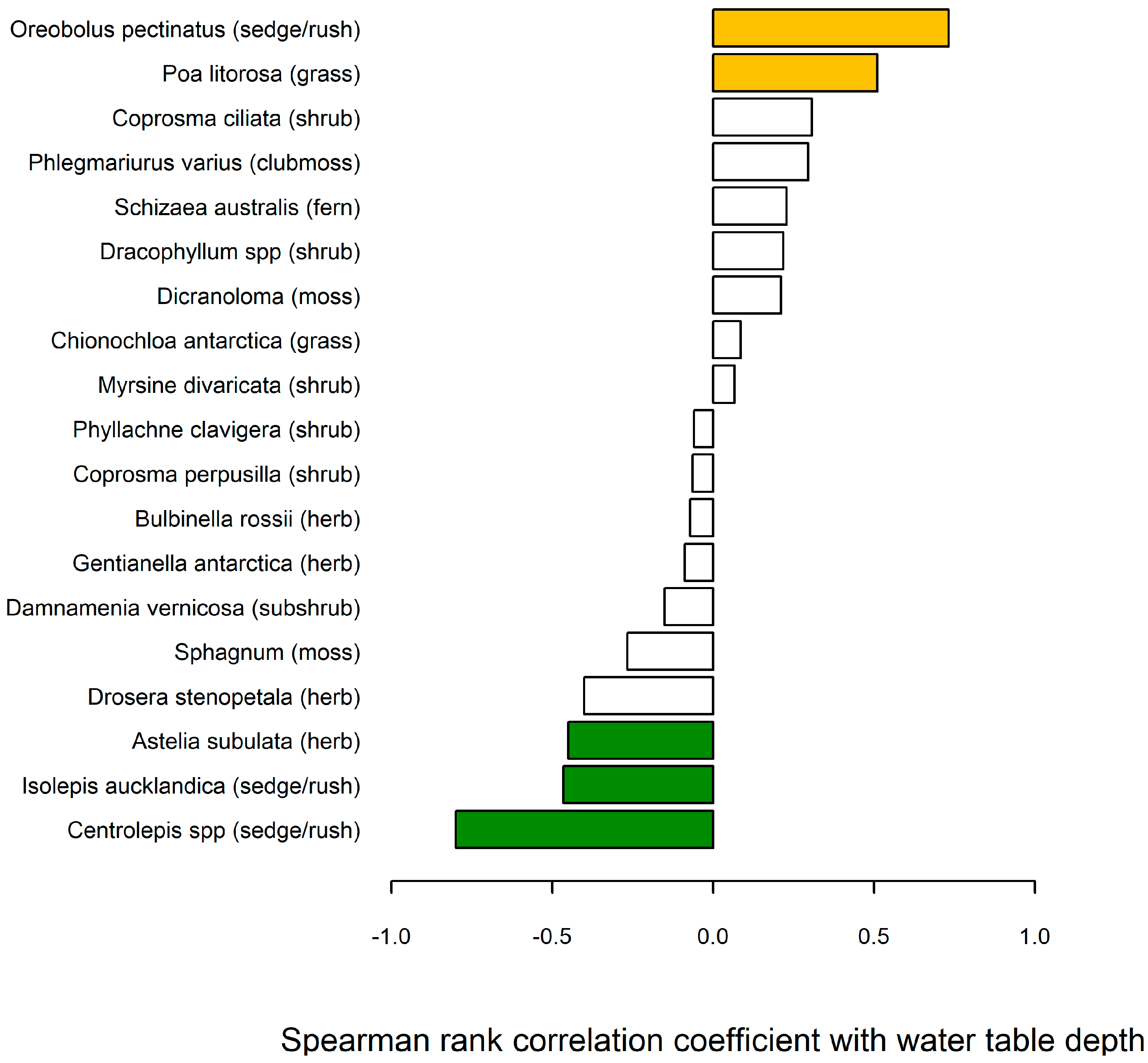
5.3. Water Table Relationship with Bog Vegetation
5.4. Peat Vertical Growth, Surface Wetness, and Humification
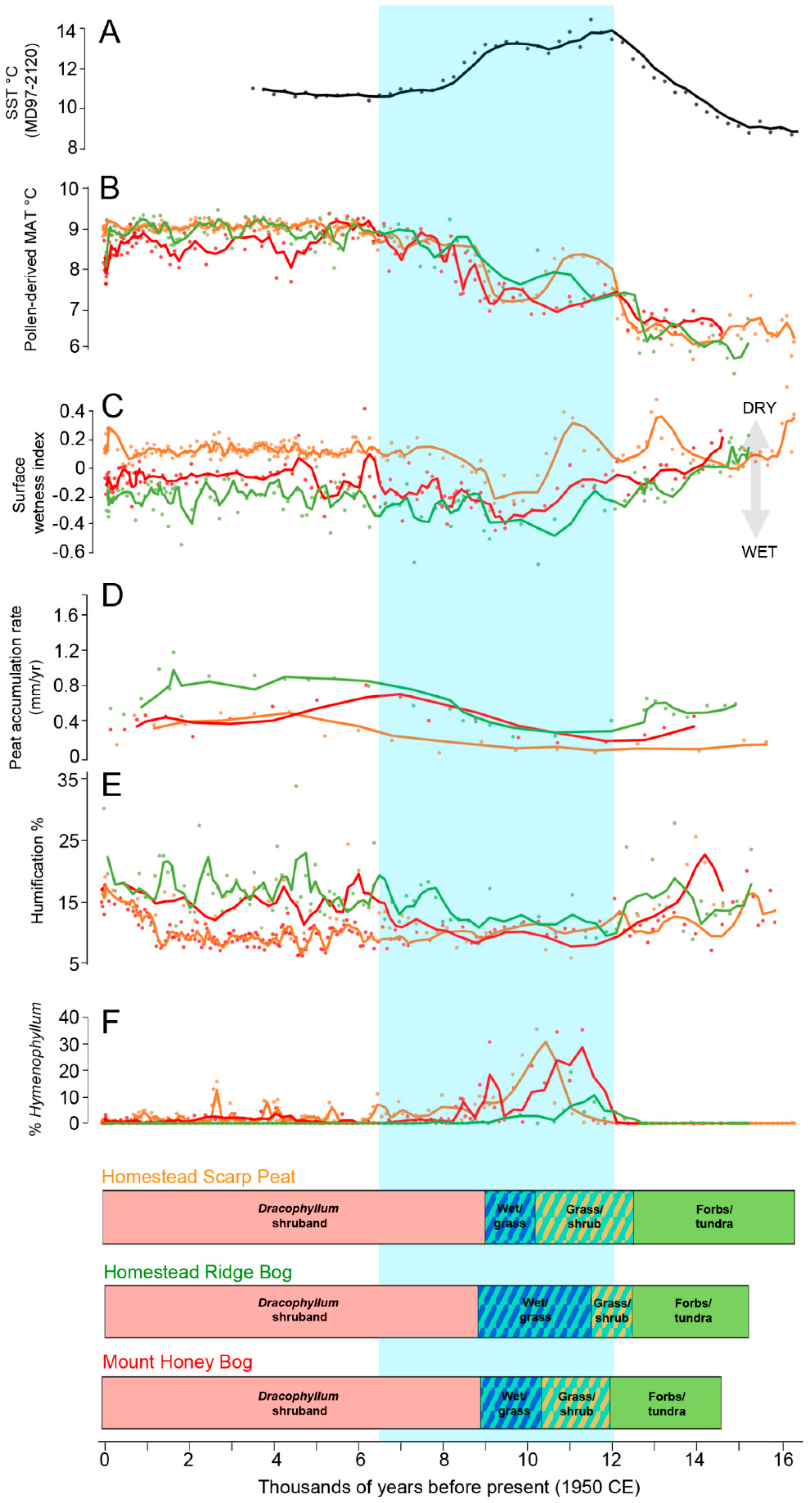
6. Discussion
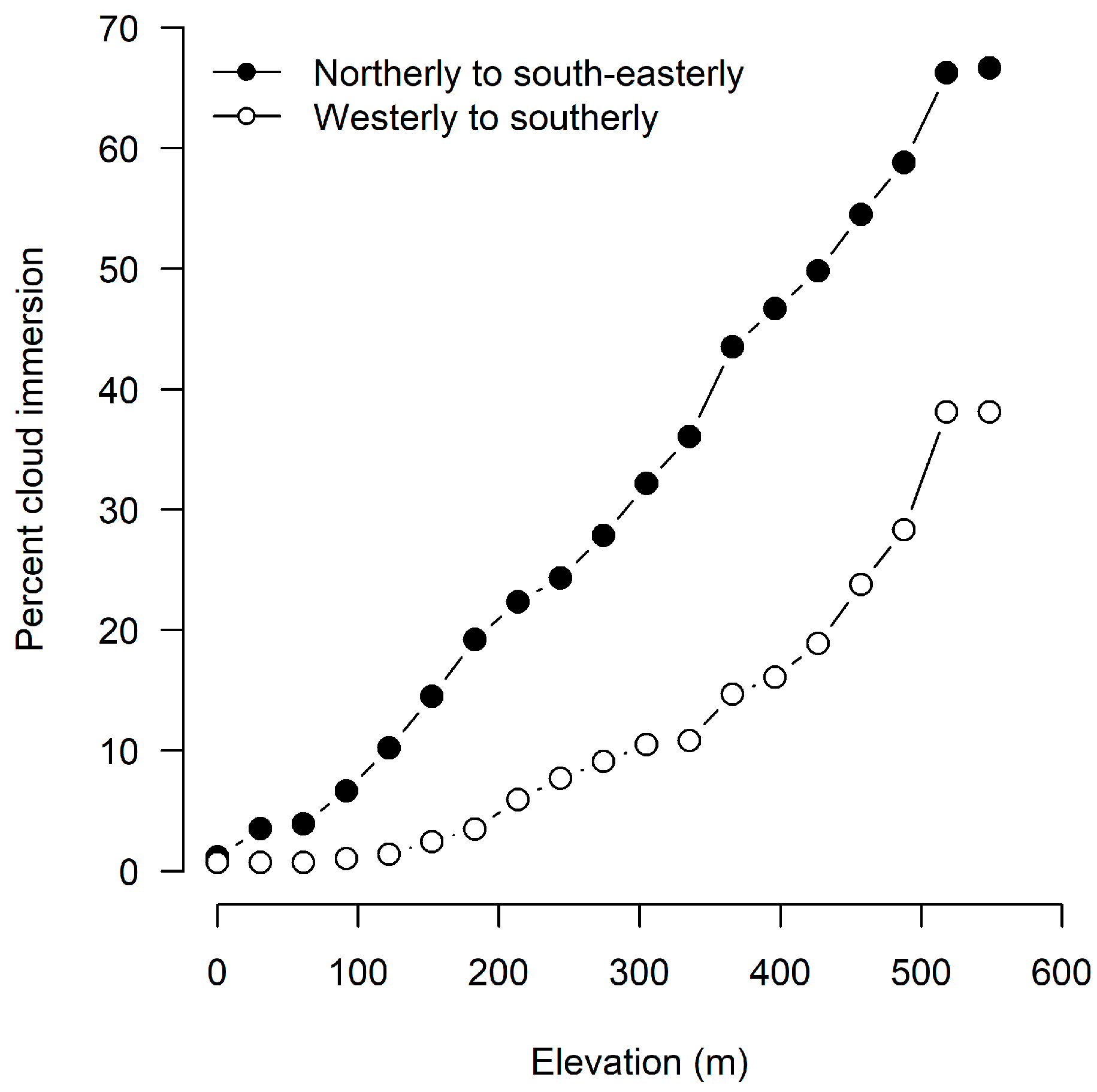
6.1. Climatic Interpretation
6.2. The Sea Surface Temperature versus Island Temperature Anomaly
6.3. Southern Ocean Context
7. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1132. [Google Scholar]
- Halloy, S.R.P.; Mark, A.F. Climate-change effects on alpine plant biodiversity: A New Zealand perspective on quantifying the threat. Arct. Antarct. Alp. Res. 2003, 35, 248–254. [Google Scholar] [CrossRef]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Cieraad, E.; McGlone, M.S.; Huntley, B. Southern hemisphere temperate tree lines are not climatically depressed. J. Biogeogr. 2014, 41, 1456–1466. [Google Scholar] [CrossRef]
- McGlone, M.S. The late Quaternary peat, vegetation and climate history of the southern oceanic islands of New Zealand. Quat. Sci. Rev. 2002, 21, 683–707. [Google Scholar] [CrossRef]
- Korner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Paulsen, J.; Körner, C. A climate-based model to predict potential treeline position around the globe. Alp. Bot. 2014, 124, 1–12. [Google Scholar] [CrossRef]
- McGlone, M.S.; Turney, C.S.M.; Wilmshurst, J.M.; Renwick, J.; Pahnke, K. Divergent trends in land and ocean temperature in the southern ocean over the past 18,000 years. Nat. Geosci. 2010, 3, 622–626. [Google Scholar] [CrossRef]
- Prebble, J.G.; Bostock, H.C.; Cortese, G.; Lorrey, A.M.; Hayward, B.W.; Calvo, E.; Northcote, L.C.; Scott, G.H.; Neil, H.L. Evidence for a Holocene climatic optimum in the southwest pacific: A multiproxy study. Paleoceanography 2017, 32, 763–779. [Google Scholar] [CrossRef]
- McGlone, M.S.; Meurk, C.D.; Crompton, M.B. Climate and Water Table Depth Data from Campbell Island, New Zealand Subantarctic; Manaaki Whenua-Landcare Research Contract Report; Landcare Research NZ Ltd.: Lincoln, New Zealand, 2019. [Google Scholar] [CrossRef]
- Campbell, I.B. Soil pattern of Campbell Island. N. Z. J. Sci. 1981, 24, 111–135. [Google Scholar]
- McGlone, M.S.; Moar, N.T.; Wardle, P.; Meurk, C.D. Late-glacial and Holocene vegetation and environment of Campbell Island, far southern new zealand. Holocene 1997, 7, 1–12. [Google Scholar] [CrossRef]
- De Lisle, J.F. Weather and climate of Campbell Island. Pac. Insect Monogr. 1964, 7, 34–44. [Google Scholar]
- Ross, D.J.; Campbell, I.B.; Bridger, B.A. Biochemical activities of organic soils from sub-antarctic tussock grasslands on Campbell Island. 1. Oxygen uptakes and nitrogen mineralization. N. Z. J. Sci. 1979, 22, 161–171. [Google Scholar]
- Foggo, M.N.; Meurk, C.D. A bioassay of some Campbell Island soils. N. Z. J. Ecol. 1983, 6, 121–124. [Google Scholar]
- Meurk, C.D.; Foggo, M.N.; Thomson, B.M.; Bathurst, E.T.J.; Crompton, M.B. Ion-rich precipitation and vegetation pattern on sub-antarctic Campbell Island. Arct. Alp. Res. 1994, 26, 281–289. [Google Scholar] [CrossRef]
- Meurk, C.D.; Blaschke, P.M. How Representative Can Restored Islands Really Be?: An Analysis of Climo-Edaphic Environments in New Zealand; Department of Conservation: Wellington, New Zealand, 1990.
- Meurk, C.D.; Given, D.R. Vegetation Map of Campbell Island. Land Resources; Department of Scientific and Industrial Research: Christchurch, New Zealand, 1990. [Google Scholar]
- Meurk, C.D.; Foggo, M.N.; Wilson, J.B. The vegetation of sub-antarctic Campbell Island. N. Z. J. Ecol. 1994, 18, 123–168. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P. Vegan: Community Ecology Package. R Package v. 2.5-4. 2018. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 3 October 2019).
- Warton, D.I.; Foster, S.D.; De’ath, G.; Stoklosa, J.; Dunstan, P.K. Model-based thinking for community ecology. Plant Ecol. 2015, 216, 669–682. [Google Scholar] [CrossRef]
- Wang, Y.; Naumann, U.; Wright, S.T.; Warton, D.I. Mvabund—An R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- McGlone, M.S.; Meurk, C.D. Modern pollen rain, subantarctic Campbell Island, New Zealand. N. Z. J. Ecol. 2000, 24, 181–194. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bruce, M.; Grace, J.; Urban, D. Analysis of Ecological Communities; MJM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Bengtsson, L.; Enell, M.; Berglund, B. Handbook of Holocene palaeoecology and palaeohydrology. Chem. Anal. 1986, 423–451. [Google Scholar]
- Stuiver, M.; Reimer, P.J.; Bard, E.; Beck, J.W.; Burr, G.S.; Hughen, K.A.; Kromer, B.; McCormac, G.; Van Der Plicht, J.; Spurk, M. Intcal98 radiocarbon age calibration, 24,000–0 cal bp. Radiocarbon 1998, 40, 1041–1083. [Google Scholar] [CrossRef]
- Blackford, J.; Chambers, F. Determining the degree of peat decomposition for peat-based palaeoclimatic studies. Int. Peat J. 1993, 5, 7–24. [Google Scholar]
- Moore, P.; Webb, J.; Collinson, M. Pollen Analysis; Blackwell Scientific: Oxford, UK, 1991. [Google Scholar]
- Moar, N.T.; Wilmshurst, J.M.; McGlone, M.S. Standardizing names applied to pollen and spores in New Zealand Quaternary palynology. N. Z. J. Bot. 2011, 49, 201–229. [Google Scholar] [CrossRef]
- Heenan, P.B.; Smissen, R.D. Revised circumscription of Nothofagus and recognition of the segregate genera Fuscospora, Lophozonia, and Trisyngyne (Nothofagaceae). Phytotaxa 2013, 142, 1–31. [Google Scholar] [CrossRef]
- Grimm, E.C. TILIA Software Version 1.7.16; Illinois State Museum, Research and Collection Center: Springfield, IL, USA, 2011. Available online: http://intra.museum.state.ilus/pub/grim?m/tilia/ (accessed on 3 October 2019).
- McGlone, M.; Wilmshurst, J.; Meurk, C. Climate, fire, farming and the recent vegetation history of subantarctic Campbell Island. Earth Environ. Sci. Trans. R. Soc. Edinb. 2007, 98, 71–84. [Google Scholar] [CrossRef]
- Bestic, K.L.; Duncan, R.P.; McGlone, M.S.; Wilmshurst, J.M.; Meurk, C.D. Population age structure and recent dracophyllum spread on subantarctic Campbell Island. N. Z. J. Ecol. 2005, 29, 291–297. [Google Scholar]
- Wilmshurst, J.M.; Bestic, K.L.; Meurk, C.D.; McGlone, M.S. Recent spread of Dracophyllum scrub on subantarctic Campbell Island, New Zealand: Climatic or anthropogenic origins? J. Biogeogr. 2004, 31, 401–413. [Google Scholar] [CrossRef]
- Bestic, K.L. Dracophyllum Scrub Expansion on Subantarctic Campbell Island, New Zealand; Lincoln University: Lincoln, New Zealand, 2002. [Google Scholar]
- Pahnke, K.; Sachs, J.P. Sea surface temperatures of southern midlatitudes 0–160 kyr bp. Paleoceanography 2006, 21. [Google Scholar] [CrossRef]
- Pedro, J.B.; Bostock, H.C.; Bitz, C.M.; He, F.; Vandergoes, M.J.; Steig, E.J.; Chase, B.M.; Krause, C.E.; Rasmussen, S.O.; Markle, B.R. The spatial extent and dynamics of the antarctic cold reversal. Nat. Geosci. 2016, 9, 51. [Google Scholar] [CrossRef]
- Charman, D. Peatlands and Environmental Change; John Wiley & Sons: London, UK, 2002. [Google Scholar]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Ise, T.; Dunn, A.L.; Wofsy, S.C.; Moorcroft, P.R. High sensitivity of peat decomposition to climate change through water-table feedback. Nat. Geosci. 2008, 1, 763. [Google Scholar] [CrossRef]
- Reinhardt, K.; Smith, W.K.; Carter, G.A. Clouds and cloud immersion alter photosynthetic light quality in a temperate mountain cloud forest. Botany-Botanique 2010, 88, 462–470. [Google Scholar] [CrossRef]
- Crawford, R.M.M.; Jeffree, C.E.; Rees, W.G. Paludification and forest retreat in northern oceanic environments. Ann. Bot. 2003, 91, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Harsch, M.A.; McGlone, M.S.; Wilmshurst, J.M. Winter climate limits subantarctic low forest growth and establishment. PLoS ONE 2014, 9, e93241. [Google Scholar] [CrossRef] [PubMed]
- Cieraad, E.; McGlone, M.S. Thermal environment of New Zealand’s gradual and abrupt treeline ecotones. N. Z. J. Ecol. 2014, 38, 12–25. [Google Scholar]
- Bostock, H.C.; Prebble, J.G.; Cortese, G.; Hayward, B.; Calvo, E.; Quiros-Collazos, L.; Kienast, M.; Kim, K. Paleoproductivity in the sw pacific ocean during the early Holocene climatic optimum. Paleoceanogr. Paleoclimatol. 2019, 34, 580–599. [Google Scholar] [CrossRef]
- Turney, C.S.; M c Glone, M.; Palmer, J.; Fogwill, C.; Hogg, A.; Thomas, Z.A.; Lipson, M.; Wilmshurst, J.M.; Fenwick, P.; Jones, R.T. Intensification of southern hemisphere westerly winds 2000–1000 years ago: Evidence from the subantarctic Campbell and Auckland Islands (52–50° s). J. Quat. Sci. 2016, 31, 12–19. [Google Scholar] [CrossRef]
- McGlone, M.S.; Moar, N.T. Pollen-vegetation relationships on the subantarctic Auckland Islands, New Zealand. Rev. Palaeobot. Palynol. 1997, 96, 317–338. [Google Scholar] [CrossRef]
- Browne, I.M.; Moy, C.M.; Riesselman, C.R.; Neil, H.L.; Curtin, L.G.; Gorman, A.R.; Wilson, G.S. Late Holocene intensification of the westerly winds at the subantarctic Auckland Islands (51 degrees S), New Zealand. Clim. Past 2017, 13, 1301–1322. [Google Scholar] [CrossRef]
- Schaefer, J.M.; Denton, G.H.; Kaplan, M.; Putnam, A.; Finkel, R.C.; Barrell, D.J.A.; Andersen, B.G.; Schwartz, R.; Mackintosh, A.; Chinn, T.; et al. High-frequency holocene glacier fluctuations in New Zealand differ from the northern signature. Science 2009, 324, 622–625. [Google Scholar] [CrossRef]
- McGlone, M.S.; Wilmshurst, J.M.; Wiser, S.K. Lateglacial and Holocene vegetation and climatic change on Auckland Island, subantarctic New Zealand. Holocene 2000, 10, 719–728. [Google Scholar] [CrossRef]
- Fleming, C.A.; Mildenhall, D.C.; Moar, N.T. Quaternary sediments and plant microfossils from Enderby Island, Auckland Islands. J. R. Soc. N. Z. 1976, 6, 433. [Google Scholar] [CrossRef]
- Turney, C.; Wilmshurst, J.; Jones, R.; Wood, J.; Palmer, J.; Hogg, A.; Fenwick, P.; Crowley, S.; Privat, K.; Thomas, Z. Reconstructing atmospheric circulation over southern new zealand: Establishment of modern westerly airflow 5500 years ago and implications for southern hemisphere Holocene climate change. Quat. Sci. Rev. 2017, 159, 77–87. [Google Scholar] [CrossRef]
- Anderson, H.J.; Moy, C.M.; Vandergoes, M.J.; Nichols, J.E.; Riesselman, C.R.; Van Hale, R. Southern hemisphere westerly wind influence on southern New Zealand hydrology during the lateglacial and Holocene. J. Quat. Sci. 2018, 33, 689–701. [Google Scholar] [CrossRef]
- McGlone, M.S.; Basher, L. Holocene vegetation change at treeline, Cropp Valley, Southern Alps, New Zealand. In Peopled Landscapes: Archaeological and Biogeographic Approaches to Landscape; Haberle, S., David, B., Eds.; ANU E Press: Canberra, Australia, 2012; Volume 34, pp. 343–358. [Google Scholar]
- Jara, I.A.; Newnham, R.M.; Vandergoes, M.J.; Foster, C.R.; Lowe, D.J.; Wilmshurst, J.M.; Moreno, P.I.; Renwick, J.A.; Homes, A.M. Pollen-climate reconstruction from northern South Island, New Zealand (41 degrees S), reveals varying high- and low-latitude teleconnections over the last 16,000 years. J. Quat. Sci. 2015, 30, 817–829. [Google Scholar] [CrossRef]
- Heusser, C. Late Quaternary vegetation and climate of southern Tierra del Fuego. Quatern. Res. 1989, 31, 396–406. [Google Scholar] [CrossRef]
- Heusser, C.J. Three late Quaternary pollen diagrams from southern Patagonia and their paleoecological implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1995, 118, 1–24. [Google Scholar] [CrossRef]
- McCulloch, R.D.; Davies, S.J. Late-glacial and Holocene palaeoenvironmental change in the central Strait of Magellan, southern Patagonia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 173, 143–173. [Google Scholar] [CrossRef]
- Fesq-Martin, M.; Friedmann, A.; Peters, M.; Behrmann, J.; Kilian, R. Late-glacial and Holocene vegetation history of the Magellanic rain forest in southwestern Patagonia, Chile. Veg. Hist. Archaeobot. 2004, 13, 249–255. [Google Scholar] [CrossRef]
- Borromei, A.M.; Coronato, A.; Quattrocchio, M.; Rabassa, J.; Grill, S.; Roig, C. Late Pleistocene-Holocene environments in Valle Carbajal, Tierra del Fuego, Argentina. J. S. Am. Earth Sci. 2007, 23, 321–335. [Google Scholar] [CrossRef]
- Borromei, A.M.; Coronato, A.; Franzen, L.G.; Ponce, J.F.; Saez, J.A.L.; Maidana, N.; Rabassa, J.; Candel, M.S. Multiproxy record of Holocene paleoenvironmental change, Tierra del Fuego, Argentina. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 286, 1–16. [Google Scholar] [CrossRef]
- Markgraf, V. Paleoenvironments and paleoclimates in Tierra del Fuego and southernmost Patagonia, South America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1993, 102, 53–68. [Google Scholar] [CrossRef]
- Pendall, E.; Markgraf, V.; White, J.W.C.; Dreier, M.; Kenny, R. Multiproxy record of late Pleistocene-Holocene climate and vegetation changes from a peat bog in Patagonia. Quatern. Res. 2001, 55, 168–178. [Google Scholar] [CrossRef]
- Barrow, C.J. Postglacial pollen diagrams from South Georgia (sub-antarctic) and West Falkland Island (South Atlantic). J. Biogeogr. 1978, 5, 251–274. [Google Scholar] [CrossRef]
- Smith, R.I.L.; Prince, P.A. The natural history of Beauchêne Island. Biol. J. Linn. Soc. 1985, 24, 233–283. [Google Scholar] [CrossRef]
- Nanavati, W.P.; Whitlock, C.; Iglesias, V.; de Porras, M.E. Postglacial vegetation, fire, and climate history along the eastern Andes, Argentina and Chile (lat. 41–55 degrees s). Quat. Sci. Rev. 2019, 207, 145–160. [Google Scholar] [CrossRef]
- Morrison, K.W.; Battley, P.F.; Sagar, P.M.; Thompson, D.R. Population dynamics of eastern rockhopper penguins on Campbell Island in relation to sea surface temperature 1942–2012: Current warming hiatus pauses a long-term decline. Polar Biol. 2015, 38, 163–177. [Google Scholar] [CrossRef]
- Cullen, L.E.; Stewart, G.H.; Duncan, R.P.; Palmer, J.G. Disturbance and climate warming influences on New Zealand Nothofagus tree-line population dynamics. J. Ecol. 2001, 89, 1061–1071. [Google Scholar] [CrossRef]
- Wardle, P.; Coleman, M.; Buxton, R.; Wilmshurst, J. Climatic warming and the upper forest limit. Canterb. Bot. Soc. J. 2005, 39, 90–98. [Google Scholar]


| Vegetation Zones | Homestead Ridge Bog (HRB: X9901) | Homestead Scarp Peat (HSP: X9911) | Mt Honey Bog (MHB: X9903) |
|---|---|---|---|
| Macrophyllous forbs-tundra | 15,300–12,500 | 16,800–12,500 | 14,700–12,000 |
| Grassland-shrub | 12,500–11,500 | 12,500–10,200 | 12,000–10,400 |
| Wetland-grassland | 11,500–8800 | 10,200–9000 | 10,400–8900 |
| Dracophyllum | 8900–55 | 9000–55 | 8900–55 |
| Site Vegetation Cover (Elevation) | Annual Mean Water Table Depth (cm) | Standard Deviation |
|---|---|---|
| Cushion vegetation, Homestead Ridge (45 m) | 5.8 | 7.1 |
| Sedge fen, Met. Station (10 m) | 8.1 | 9.9 |
| Macrophyllous forbs/cushion, Homestead Ridge, raised bog (45 m) | 17.0 | 12.0 |
| Tussock grassland, Met. Station (10 m) | 34.2 | 19.0 |
| Dracophyllum low forest slope marginal to Homestead Ridge, raised bog (35 m) | 58.2 | 20.3 |
| Dracophyllum/grassland, Met. Station (10 m) | 78.1 | 13.2 |
| Dracophyllum/Coprosma forest, Met. Station (20 m) | 109.5 | 7.6 |
| Wind Directions | Observations with all Peaks Clear (%) | Mean Daily Temperature (°C) | % Observations |
|---|---|---|---|
| All | 39.5 | 6.1 | 100.0 |
| North to east-southeast | 21.0 | 8.1 | 27.4 |
| North-northwest to west northwest | 20.8 | 5.1 | 22.7 |
| West to southeast | 58.7 | 5.0 | 45.3 |
| Calm | 53.9 | 9.3 | 4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGlone, M.S.; Wilmshurst, J.M.; Richardson, S.J.; Turney, C.S.M.; Wood, J.R. Temperature, Wind, Cloud, and the Postglacial Tree Line History of Sub-Antarctic Campbell Island. Forests 2019, 10, 998. https://doi.org/10.3390/f10110998
McGlone MS, Wilmshurst JM, Richardson SJ, Turney CSM, Wood JR. Temperature, Wind, Cloud, and the Postglacial Tree Line History of Sub-Antarctic Campbell Island. Forests. 2019; 10(11):998. https://doi.org/10.3390/f10110998
Chicago/Turabian StyleMcGlone, Matt S., Janet M. Wilmshurst, Sarah J. Richardson, Chris S.M. Turney, and Jamie R. Wood. 2019. "Temperature, Wind, Cloud, and the Postglacial Tree Line History of Sub-Antarctic Campbell Island" Forests 10, no. 11: 998. https://doi.org/10.3390/f10110998
APA StyleMcGlone, M. S., Wilmshurst, J. M., Richardson, S. J., Turney, C. S. M., & Wood, J. R. (2019). Temperature, Wind, Cloud, and the Postglacial Tree Line History of Sub-Antarctic Campbell Island. Forests, 10(11), 998. https://doi.org/10.3390/f10110998





