Winter Embolism and Recovery in the Conifer Shrub Pinus mugo L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seasonal Course
2.3. Water Potential (Ψ)
2.4. Loss of Conductivity (LC) and Water Content (WC)
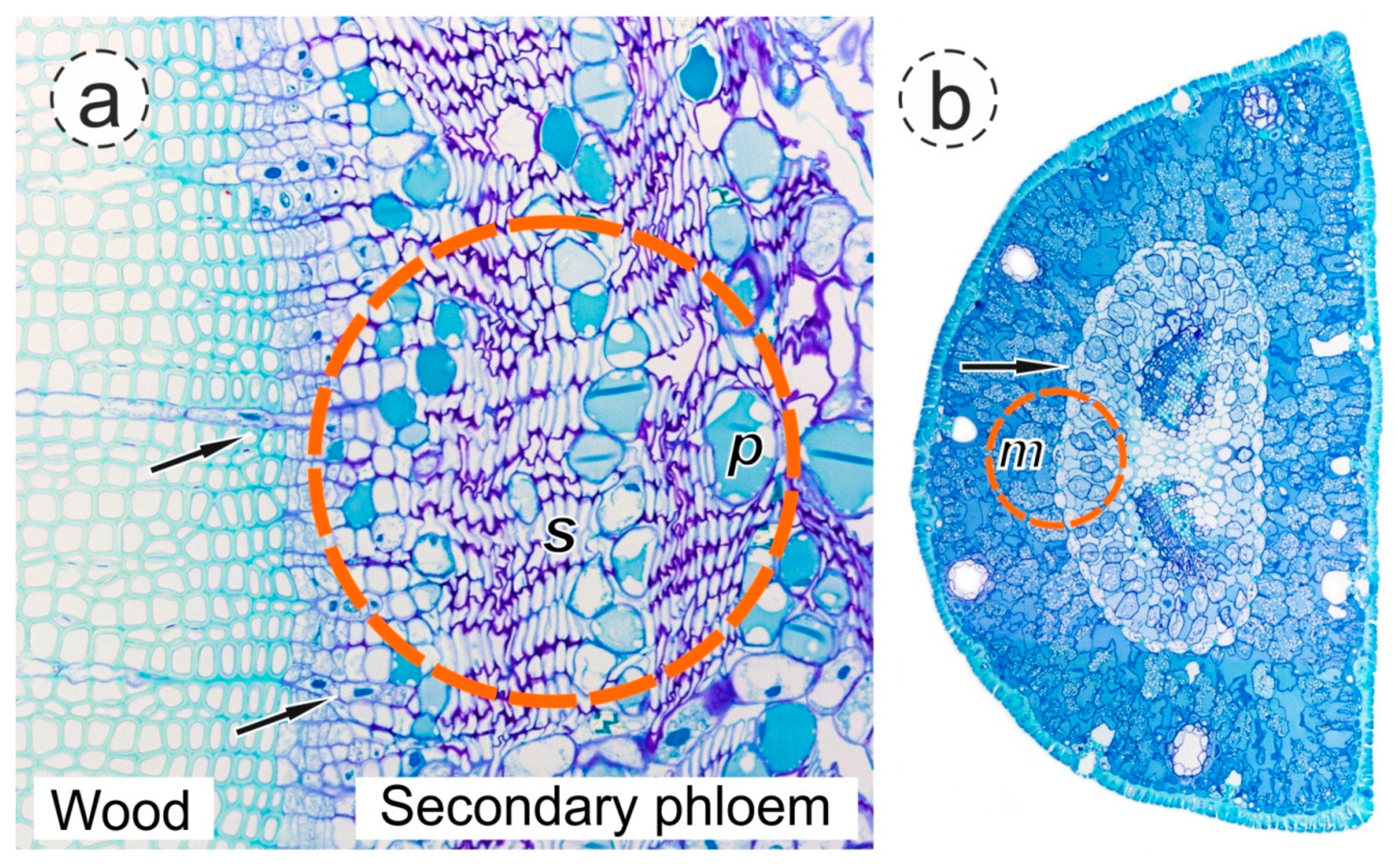
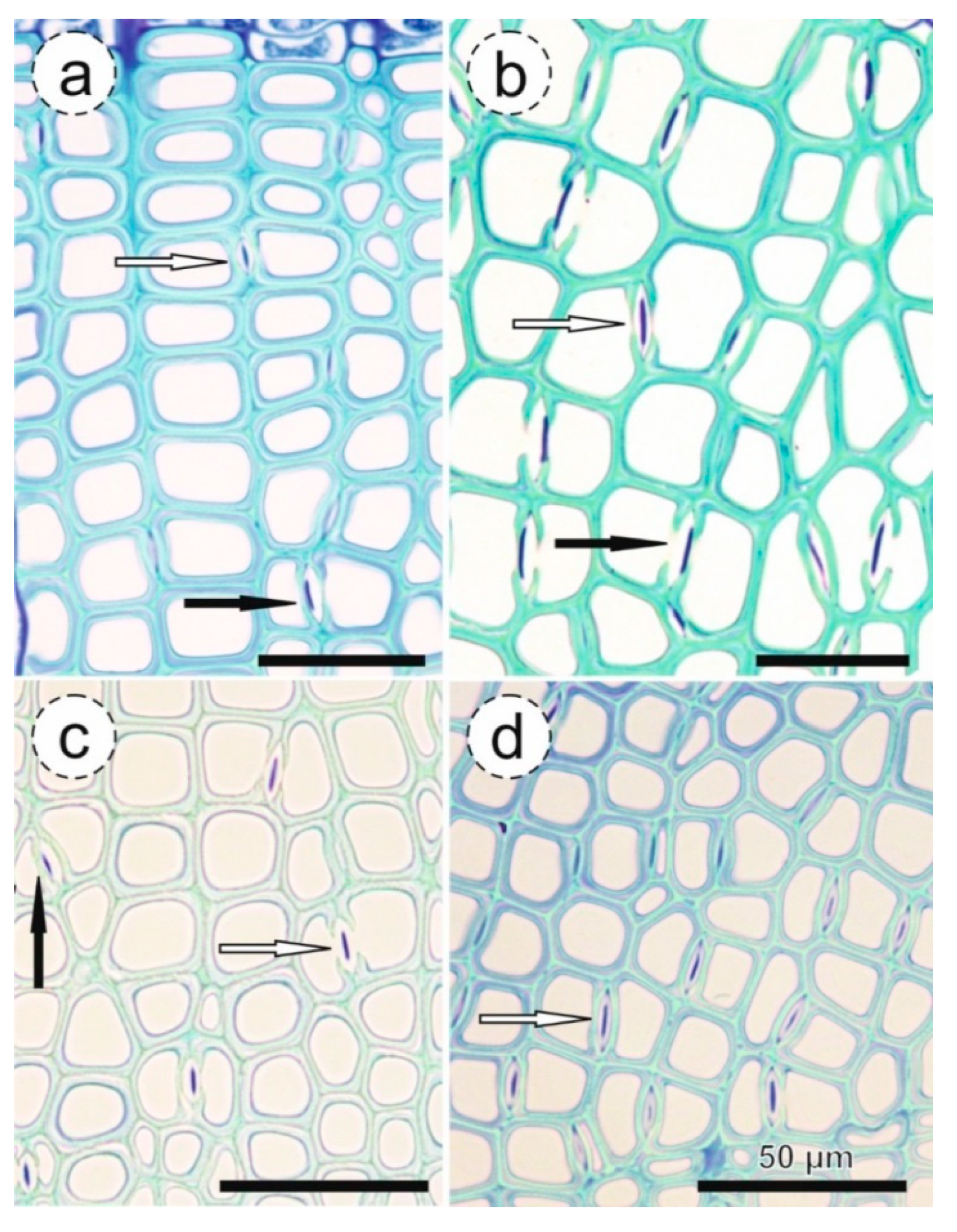
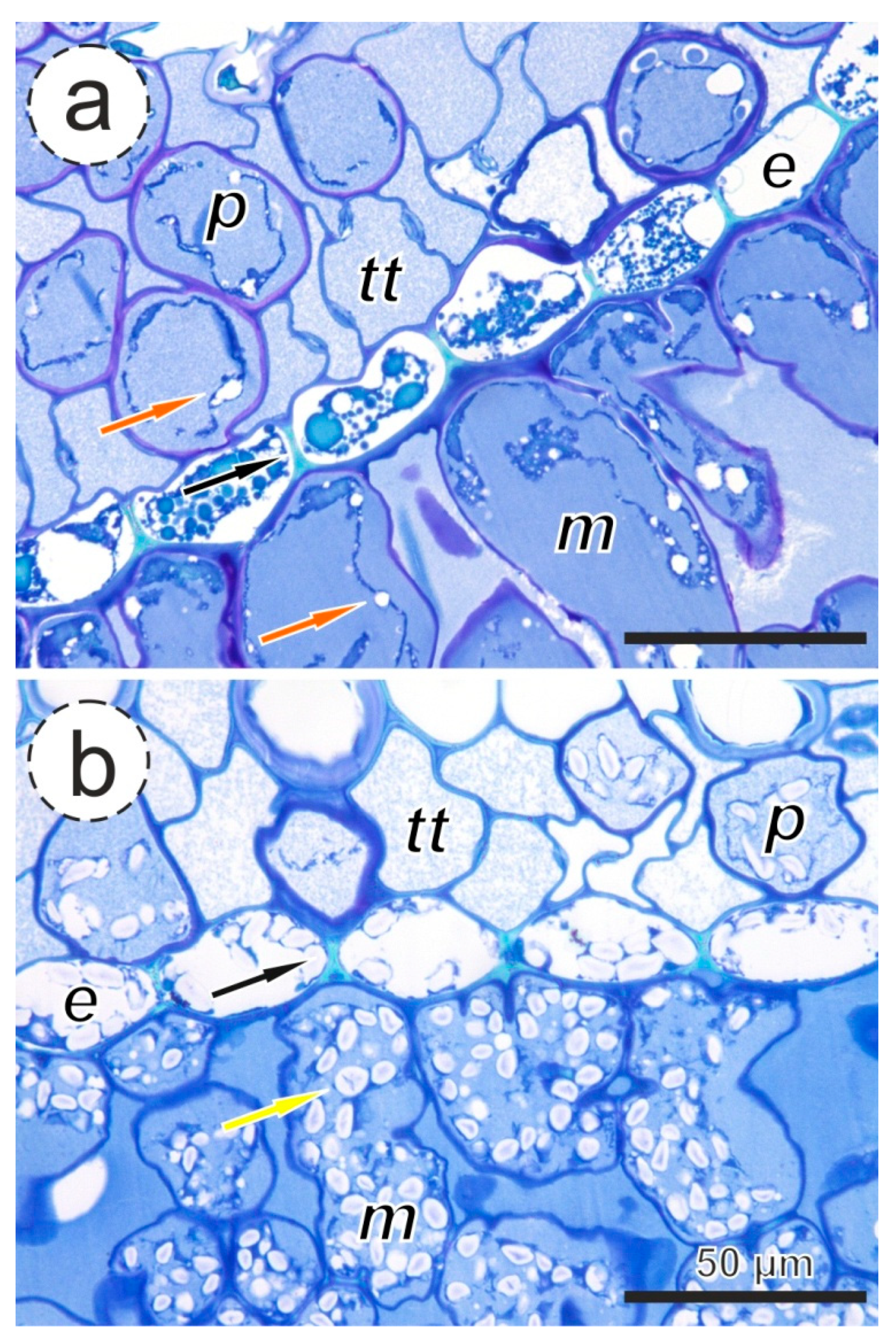
2.5. Anatomical Analysis
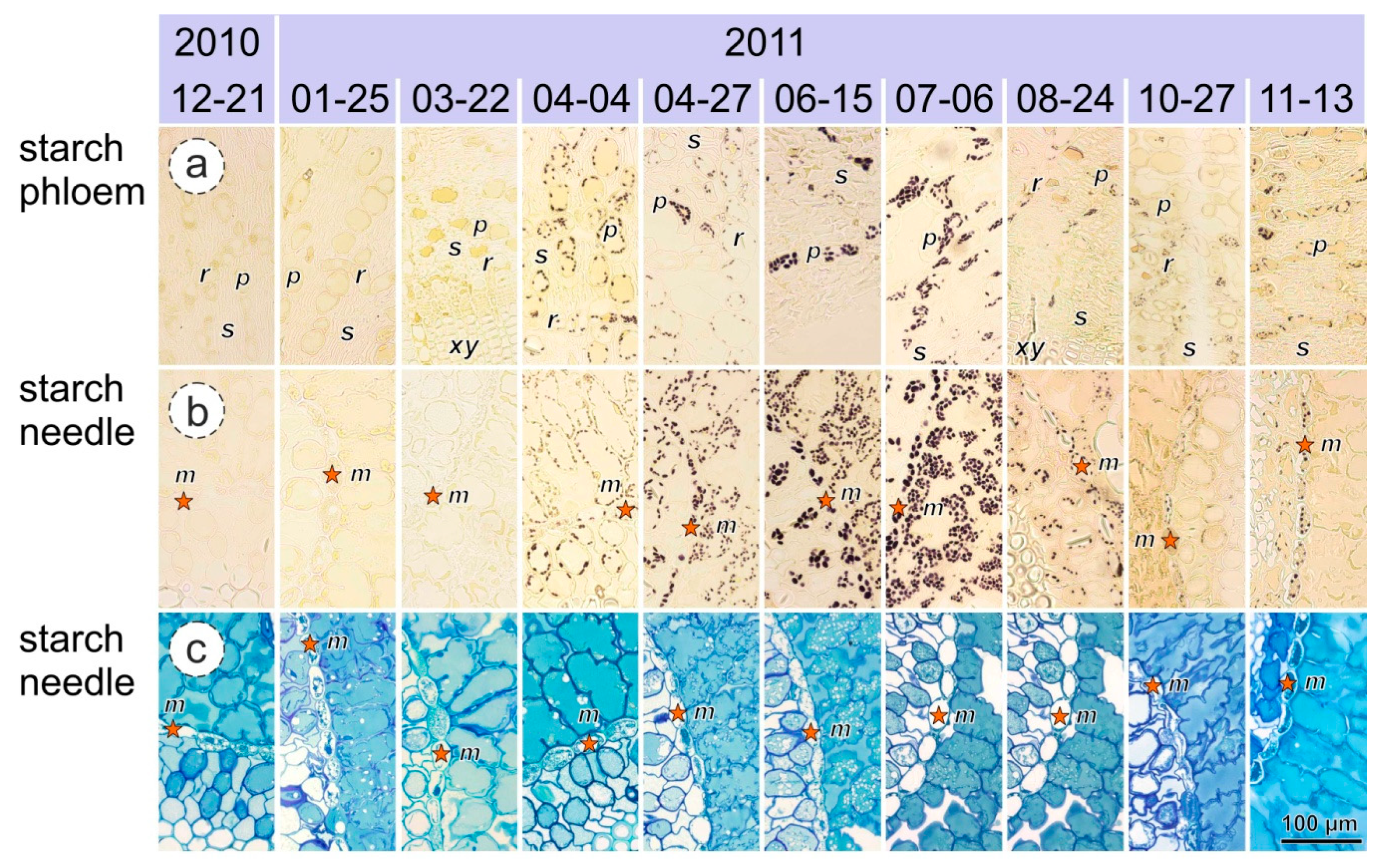
2.6. Glucose, Fructose, and Starch Content
2.7. Statistics
3. Results
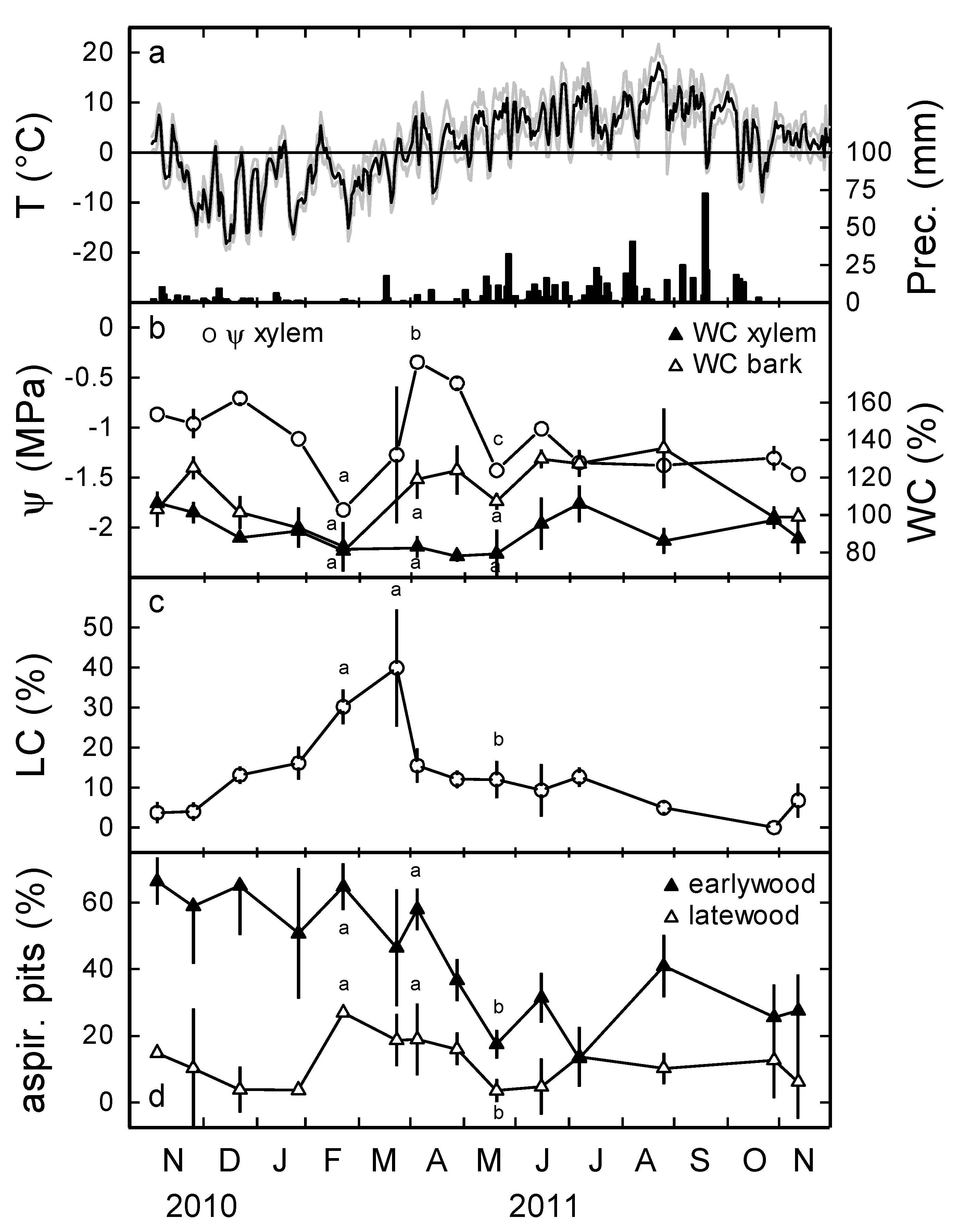
3.1. Water Potential and Embolism
3.2. Proportion of Aspirated Pits
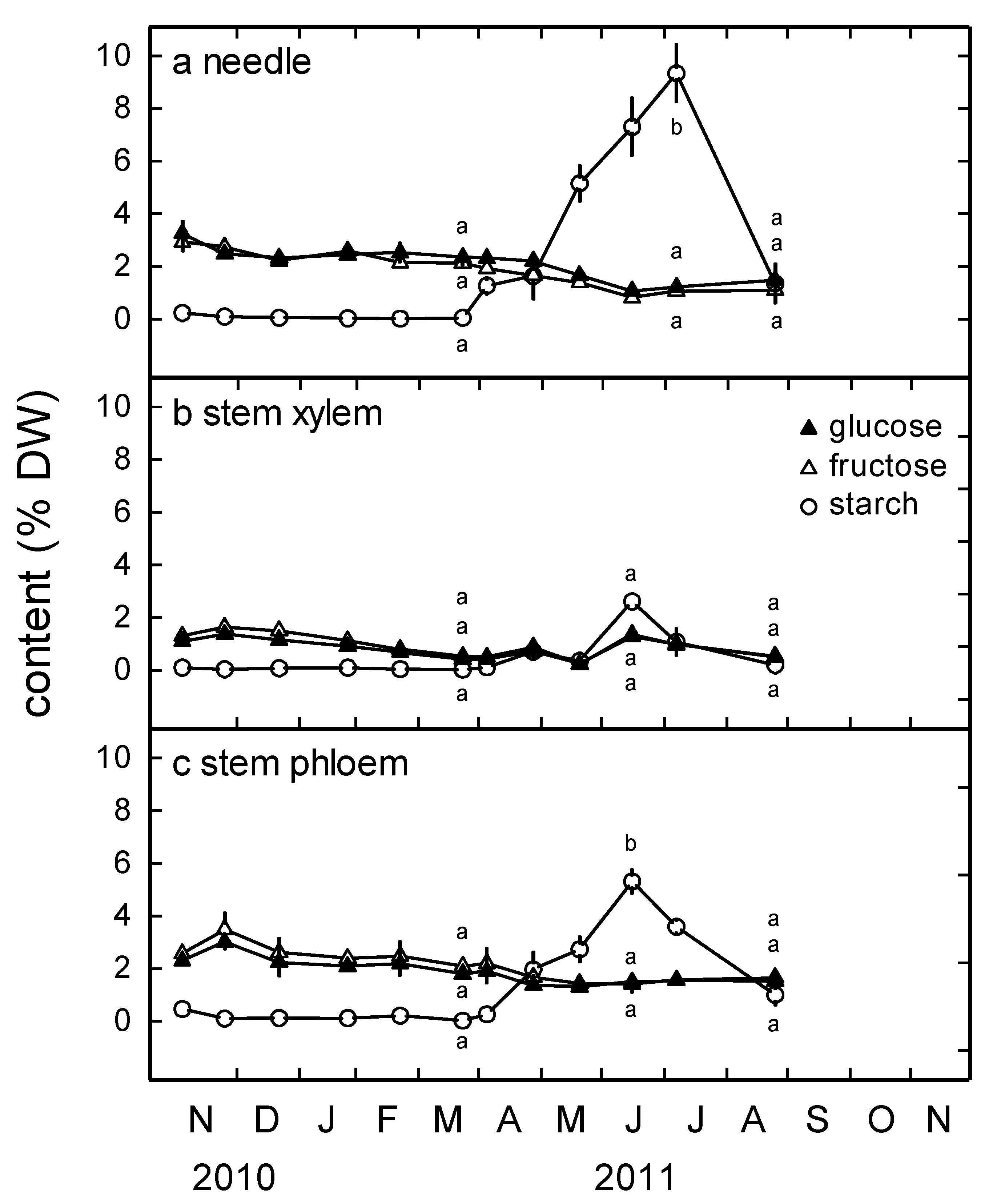
3.3. Glucose, Fructose and Starch Content
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayr, S.; Schmid, P.; Laur, J.; Rosner, S.; Charra-Vaskou, K.; Dämon, B.; Hacke, U.G. Uptake of water via branches helps timberline conifers refill embolized xylem in late winter. Plant Physiol. 2014, 164, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap, 2nd ed.; Springer: Berlin, Germany, 2002; p. 284. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S. Functional and ecological xylem anatomy. Perspect. Plant Ecol. Evol. Syst. 2001, 4, 97–115. [Google Scholar] [CrossRef]
- Sperry, J.S. Evolution of water transport and xylem structure. Int. J. Plant Sci. 2003, 164, S115–S127. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Field, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Pittermann, J.; Sperry, J.S. Analysis of freeze-thaw embolism in conifers. The interaction between cavitation pressure and tracheid size. Plant Physiol. 2006, 140, 374–382. [Google Scholar] [CrossRef]
- Mayr, S.; Schwienbacher, F.; Bauer, H. Winter at the alpine timberline. Why does embolism occur in Norway spruce but not in stone pine? Plant Physiol. 2003, 131, 780–792. [Google Scholar] [CrossRef]
- Mayr, S.; Wieser, G.; Bauer, H. Xylem temperatures during winter in conifers at the alpine timberline. Agric. For. Meteorol. 2006, 137, 81–88. [Google Scholar] [CrossRef]
- Sparks, J.P.; Black, R.A. Winter hydraulic conductivity end xylem cavitation in coniferous trees from upper and lower treeline. Arct. Antarct. Alp. Res. 2000, 32, 397–403. [Google Scholar] [CrossRef]
- Mayr, S.; Wolfschwenger, M.; Bauer, H. Winter-drought induced embolism in Norway spruce (Picea abies) at the Alpine timberline. Physiol. Plant. 2002, 115, 74–80. [Google Scholar] [CrossRef]
- Mayr, S.; Hacke, U.; Schmid, P.; Schwienbacher, F.; Gruber, A. Frost drought in conifers at the alpine timberline: Xylem dysfunction and adaptations. Ecology 2006, 87, 3175–3185. [Google Scholar] [CrossRef]
- Brodribb, T.; Cochard, H. Hydraulic failure defines the recovery and point of no return in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.M.; Yu, K.L.; Wilson, L.A.; Will, R.E.; Anderegg, W.R.; Adams, H.D. Dead or dying? Quantifying the point of no return from hydraulic failure in drought-induced tree mortality. New Phytol. 2019, 223, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Nichols, K.L.; Sullivan, J.E.M.; Eastlack, S.E. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 1994, 75, 1736–1752. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Johnson, D.M.; Meinzer, F.C.; Lachenbruch, B. An annual pattern of native embolism in upper branches of four tall conifer species. Am. J. Bot. 2011, 98, 1007–1015. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef]
- Gomes, D.; Agasse, A.; Thiébaud, P.; Delrot, S.; Gerós, H.; Chaumont, F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta 2009, 1788, 1213–1228. [Google Scholar] [CrossRef]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef]
- Holbrook, N.M.; Zwieniecki, M.A. Embolism repair and xylem tension: Do we need a miracle? Plant Physiol. 1999, 120, 7–10. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S. Limits to xylem refilling under negative pressure in Laurus nobilis and Acer negundo. Plant Cell Environ. 2003, 26, 303–311. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Holbrook, N.M. Confronting Maxwell’s demon: Biophysics of xylem embolism repair. Trends Plant Sci. 2009, 14, 530–534. [Google Scholar] [CrossRef]
- Nardini, A.; Lo Gullo, M.A.; Salleo, S. Refilling embolized xylem conduits: Is it a matter of phloem unloading? Plant Sci. 2011, 180, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Laur, J.; Hacke, U.G. Exploring Picea glauca aquaporins in the context of needle water uptake and xylem refilling. New Phytol. 2014, 203, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Katz, C.; Oren, R.; Schulze, E.-D.; Milburn, J.A. Uptake of water and solutes through twigs of Picea abies (L.) Karst. Trees 1989, 3, 33–37. [Google Scholar] [CrossRef]
- Sparks, J.P.; Campbell, G.S.; Black, R.A. Water content, hydraulic conductivity, and ice formation in winter stems of Pinus contorta: A TDR case study. Oecologia 2001, 127, 468–475. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Dawson, T.E. The contribution of fog to the water relations of Sequoia sempervirens (D. Don): Foliar uptake and prevention of dehydration. Plant Cell Environ. 2004, 27, 1023–1034. [Google Scholar] [CrossRef]
- Limm, E.B.; Simonin, K.A.; Bothman, A.G.; Dawson, T.E. Foliar water uptake: A common water acquisition strategy for plants of the redwood forest. Oecologia 2009, 161, 449–459. [Google Scholar] [CrossRef]
- Pittermann, J.; Sperry, J.S.; Hacke, U.G.; Wheeler, J.K.; Sikkema, E.H. Torus-margo pits help conifers compete with angiosperms. Science 2005, 310, 1924. [Google Scholar] [CrossRef]
- Hacke, U.G.; Jansen, S. Embolism resistance of three boreal conifer species varies with pit structure. New Phytol. 2009, 182, 675–686. [Google Scholar] [CrossRef]
- Plavcová, L.; Jansen, S.; Klepsch, M.; Hacke, U.G. Nobody’s perfect: Can irregularities in pit structure influence vulnerability to cavitation? Front. Plant Sci. 2013, 12, 453. [Google Scholar] [CrossRef]
- Mayr, S.; Charra-Vaskou, K. Winter at the alpine timberline causes complex within-tree patterns of water potential and embolism in Picea abies. Physiol. Plant. 2007, 131, 131–139. [Google Scholar] [CrossRef]
- Mayr, S.; Gruber, A.; Bauer, H. Repeated freeze-thaw cycles induce embolism in drought stressed conifers (Norway spruce, stone pine). Planta 2003, 217, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Mayr, S.; Gruber, A.; Schweinbacher, F.; Dämon, B. Winter-embolism in a “Krummholz”-Shrub (Pinus mugo) growing at the alpine timberline. Austrian J. For. Sci. 2003, 1, 29–38. [Google Scholar]
- Sperry, J.S.; Donnelly, J.R.; Tyree, M.T. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Liese, W.; Bauch, J. On the closure of bordered pits in conifers. Wood Sci. Technol. 1967, 1, 1–13. [Google Scholar] [CrossRef]
- Rosner, S.; Gierlinger, N.; Klepsch, M.; Karlsson, B.; Evans, R.; Lundqvist, S.-O.; Světlík, J.; Børja, I.; Dalsgaard, L.; Andreassen, K.; et al. Hydraulic and mechanical dysfunction of Norway spruce sapwood due to extreme summer drought in Scandinavia. For. Ecol. Manag. 2018, 409, 527–540. [Google Scholar] [CrossRef]
- Bauer, H.; Plattner, K.; Volgger, W. Photosynthesis in Norway spruce seedlings infected by the needle rust Chrysomyxa rhododendri. Tree Physiol. 2000, 20, 211–216. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Bernt, E. Sucrose. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; Volume 3, pp. 1176–1179. [Google Scholar]
- Hacke, U.G.; Sperry, J.S.; Pittermann, J. Analysis of circular bordered pit function–II. Gymnosperm tracheids with torus-margo pit membranes. Am. J. Bot. 2004, 91, 386–400. [Google Scholar] [CrossRef]
- Murmanis, L.; Evert, R.F. Parenchyma cells of secondary phloem in Pinus strobus. Planta 1967, 73, 301–318. [Google Scholar] [CrossRef]
- Ziegler, H. Storage, Mobilization and Distribution of Reserve Material in Trees. In The Formation of Wood in Forest Trees; Zimmermann, M.H., Ed.; Academic Press: New York, NY, USA, 1964; pp. 303–320. [Google Scholar]
- Höll, W. Storage and mobilization of carbohydrates and lipids. In Trees-Contributions to Modern Tree Physiology; Rennenberg, H., Eschrich, W., Ziegler, H., Eds.; Blackhuhys Publishers: Leiden, The Netherlands, 1997; pp. 197–211. [Google Scholar]
- Bucci, S.J.; Scholz, F.G.; Goldstein, G.; Meinzer, F.C.; Sternberg, L.D.L. Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: Factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ. 2003, 26, 1633–1645. [Google Scholar] [CrossRef]
- Secchi, F.; Zwieniecki, M.A. Analysis of xylem sap from functional (nonembolized) and nonfunctional (embolized) vessels of Populus nigra: Chemistry of refilling. Plant Physiol. 2012, 160, 955–964. [Google Scholar] [CrossRef]
- Barnard, D.M.; Lachenbruch, B.; McCulloh, K.A.; Kitin, P.; Meinzer, F.C. Do ray cells provide a pathway for radial water movement in the stems of conifer trees? Am. J. Bot. 2013, 100, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Böhm, R.; Auer, I.; Brunetti, M.; Maugeri, M.; Nanni, T.; Schöner, W. Regional Temperature variability in the European Alps: 1760-1998 from homogenized instrumental time. Int. J. Climatol. 2001, 1801, 1779–1801. [Google Scholar] [CrossRef]
- Rebetez, M.; Reinhard, M. Monthly air temperature trends in Switzerland 1901–2000 and 1975–2004. Theor. Appl. Climatol. 2008, 91, 27–34. [Google Scholar] [CrossRef]
- Ciccarelli, N.; von Hardenberg, J.; Provenzale, A.; Ronchi, C.; Vargiu, A.; Pelosini, R. Climate variability in north-western Italy during the second half of the 20th century. Glob. Planet. Chang. 2008, 63, 185–195. [Google Scholar] [CrossRef]
- Beniston, M. Mountain climates and climatic change: An overview of processes focusing on the European Alps. Pure Appl. Geophys. 2005, 162, 1587–1606. [Google Scholar] [CrossRef]
- Elkin, C.; Gutiérrez, A.G.; Leuzinger, S.; Manusch, C.; Temperli, C.; Rasche, L.; Bugmann, H. A 2 °C warmer world is not safe for ecosystem services in the European Alps. Glob. Chang. Biol. 2013, 19, 1827–1840. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayr, S.; Schmid, P.; Rosner, S. Winter Embolism and Recovery in the Conifer Shrub Pinus mugo L. Forests 2019, 10, 941. https://doi.org/10.3390/f10110941
Mayr S, Schmid P, Rosner S. Winter Embolism and Recovery in the Conifer Shrub Pinus mugo L. Forests. 2019; 10(11):941. https://doi.org/10.3390/f10110941
Chicago/Turabian StyleMayr, Stefan, Peter Schmid, and Sabine Rosner. 2019. "Winter Embolism and Recovery in the Conifer Shrub Pinus mugo L." Forests 10, no. 11: 941. https://doi.org/10.3390/f10110941
APA StyleMayr, S., Schmid, P., & Rosner, S. (2019). Winter Embolism and Recovery in the Conifer Shrub Pinus mugo L. Forests, 10(11), 941. https://doi.org/10.3390/f10110941




