Decomposition of Forest Litter and Feces of Armadillidium vulgare (Isopoda: Oniscidea) Produced from the Same Litter Affected by Temperature and Litter Quality
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Microcosms and Incubation
2.3. Substrate Analyses
2.4. Data Analyses
3. Results
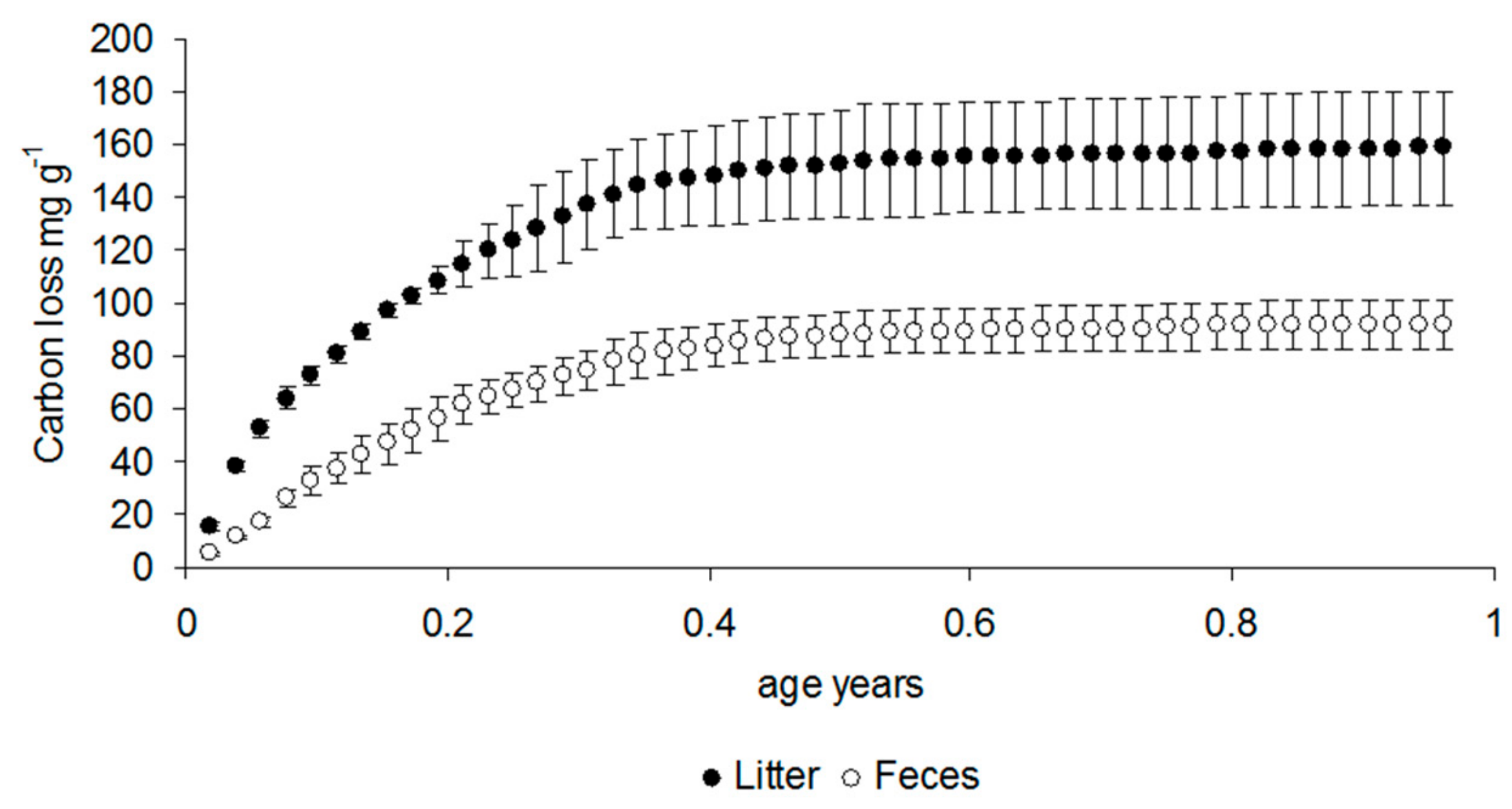
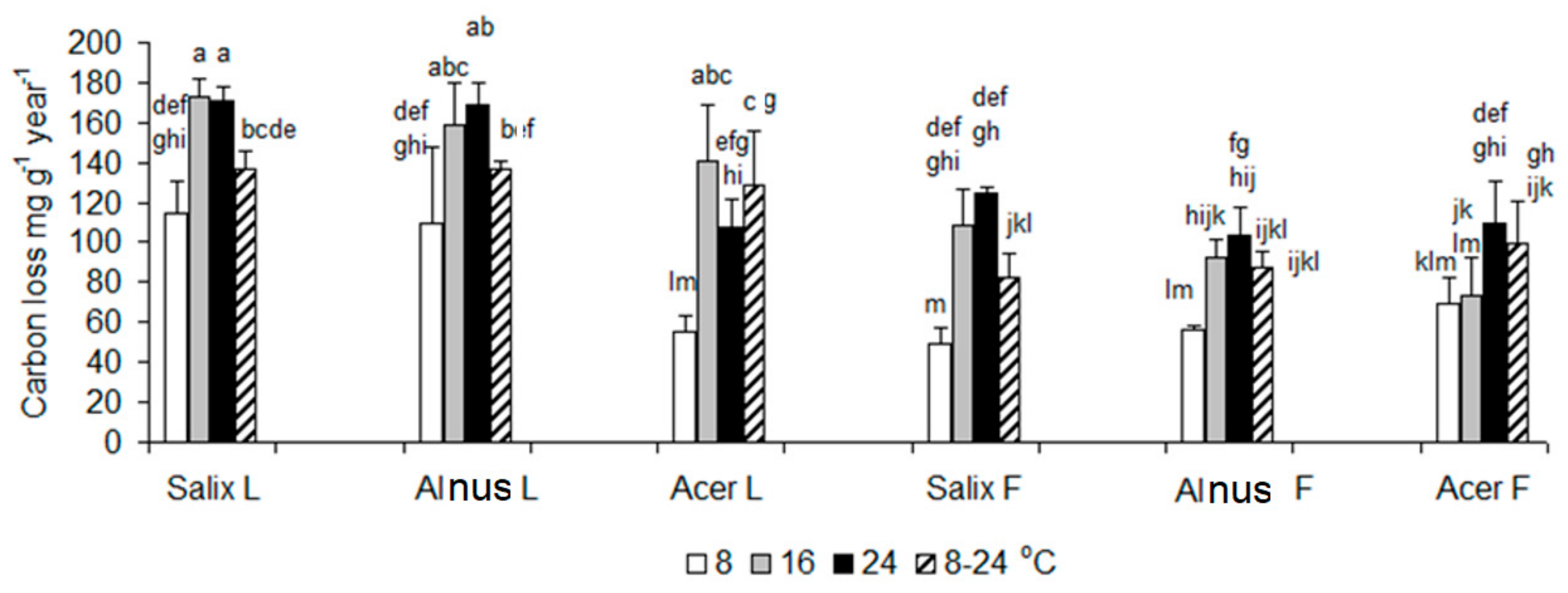
3.1. Respiration Data
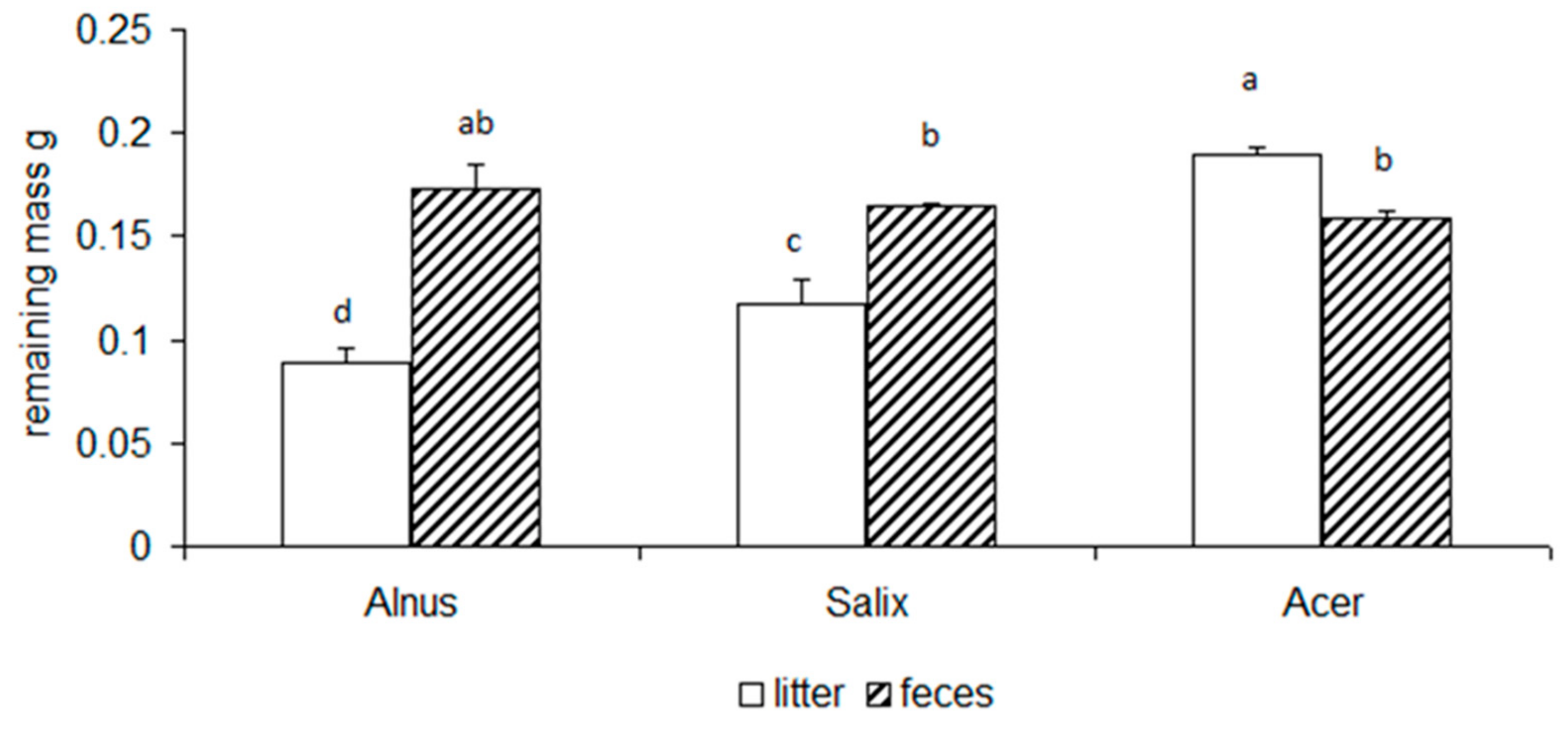
3.2. Chemical Changes of Litter and Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mellillo, J.M.; Aber, J.B.; Muratore, J.F. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 1982, 63, 621–626. [Google Scholar] [CrossRef]
- Wolters, V. Invertebrate control of soil organic matter stability. Biol. Fertil. Soils 2000, 31, 1–19. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.H.; Bradford, M.A.; St. John, M.G.; Trofymow, J.A.; Behan-Pelletier, V.; Bignell, D.E. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Chang. Biol. 2008, 14, 2661–2677. [Google Scholar] [CrossRef]
- David, J.F.; Gillon, D. Combined effects of elevated temperatures and reduced leaf litter quality on the life-history parameters of a saprophagous macroarthropod. Glob. Chang. Biol. 2009, 15, 156–165. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savege, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 2004. 85, 1771–1789. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C: N: P stoichiometry in soils: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Zimmer, M.; Topp, W. Species-specific utilization of food sources by sympatric woodlice (Isopoda: Oniscidea). J. Anim. Ecol. 2002, 69, 1071–1082. [Google Scholar] [CrossRef]
- Xuluc-Tolosa, F.J.; Vester, H.F.M.; Ramírez-Marcial, N.; Castellanos-Albores, J.; Lawrence, D. Leaf litter decomposition of tree species in three successional phases of tropical dry secondary forest in Campeche, Mexico. For. Ecol. Manag. 2003, 174, 401–412. [Google Scholar] [CrossRef]
- Salgado, P.R.; Favarin, J.L.; Leandro, R.A.; de Lima Filho, O.F. Total phenol concentrations in coffee tree leaves during fruit development. Sci. Agric. 2008, 65, 354–359. [Google Scholar] [CrossRef]
- Zimmer, M.; Danko, J.P.; Pennings, S.C.; Danford, A.R.; Carefoot, T.H.; Ziegler, A.; Uglow, R.F. Cellulose digestion and phenol oxidation in coastal isopods (Crustacea: Isopoda). Mar. Biol. 2002, 140, 1207–1213. [Google Scholar]
- Graça, M.A.S.; Bärlocher, F. Radial diffusion assay for tannins. In Methods to Study Litter Decomposition: A Practical Guide; Graça, M.A.S., Bärlocher, F., Gessner, M.O., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 101–105. [Google Scholar]
- Frouz, J.; Li, X.; Brune, A.; Pizl, V.; Abakumov, E.V. Effect of soil invertebrates on the formation of humic substances under laboratory conditions. Eurasian Soil Sci. 2011, 44, 893–896. [Google Scholar] [CrossRef]
- Frouz, J.; Špaldoňová, A.; Lhotáková, Z.; Cajthaml, T. Major mechanisms contributing to the macrofauna-mediated slow down of litter decomposition. Soil Biol. Biochem. 2015, 91, 23–31. [Google Scholar] [CrossRef]
- Frouz, J.; Lin, Q.; Li, X.; Šustr, V. Utilization of dietary protein in the litter-dwelling larva of Bibio marci (Diptera: Bibionidae). Euroasian Soil Sci. 2019. accepted. [Google Scholar]
- Zimmer, M.; Topp, W. Do woodlice (Isopoda: Oniscidea) produce endogenous cellulases? Biol. Fertil. Soil 1998, 26, 155–156. [Google Scholar] [CrossRef]
- Zimmer, M. The fate and effects of ingested hydrolysable tannins in Porcellio scaber. J. Chem. Ecol. 1999, 25, 611–628. [Google Scholar] [CrossRef]
- Hartenstein, R. Soil macroinvertebrates, aldehyde oxidase, catalase, cellulase and peroxidase. Soil Biol. Biochem. 1982, 14, 387–391. [Google Scholar] [CrossRef]
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Lavelle, P.; Bignell, D.; Lepage, M.; Wolters, W.; Roger, P.; Ineson, P.; Heal, O.W.; Dhillion, S. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 1997, 33, 159–193. [Google Scholar]
- David, J.F.; Handa, I.T. The ecology of saprophagous macroarthropods (millipedes, woodlice) in the context of global change. Biol. Rev. 2010, 85, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, P.; Martin, A. Small-scale and large-scale effects of endogeic earthworms on soil organic matter dynamics in soils of the humid tropics. Soil Biol. Biochem. 1992, 24, 1491–1498. [Google Scholar] [CrossRef]
- Hassall, M.; Turner, J.G.; Rands, M.R.W. Effects of terrestrial isopods on the decomposition of woodland leaf litter. Oecologia 1987, 72, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Frouz, J.; Roubíčková, A.; Heděnec, P.; Tajovský, K. Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies. Eur. J. Soil Biol. 2015, 68, 18–24. [Google Scholar] [CrossRef]
- Frouz, J.; Ali, A.; Frouzová, J.; Lobinske, R.J. Horizontal and vertical distribution of soil macroarthropods on spatio-temporal moisture gradient in subtropical central Florida. Environ. Entomol. 2004, 33, 1282–1295. [Google Scholar] [CrossRef]
- Rouifed, S.; Handa, I.T.; David, J.F.; Hattenschwiler, S. The importance of biotic factors in predicting global change effects on decomposition of temperature forest leaf litter. Oecologia 2010, 163, 247–256. [Google Scholar] [CrossRef]
- Špaldoňová, A.; Frouz, J. The role of Armadillidium vulgare (Isopoda: Oniscidea) in litter decomposition and soil organic matter stabilization. Appl. Soil Ecol. 2014, 83, 186–192. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis. Part. 2. Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Singleton, V.L.; Rossi, V.A. Colorimetry of total phenolics and phosphomolybdicphosphotungistic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Frouz, J.; Šimek, M. Short term and long term effects of bibionid (Diptera: Bibionidae) larvae feeding on microbial respiration and alder litter decomposition. Eur. J. Soil Biol. 2009, 45, 192–197. [Google Scholar] [CrossRef]
- Kaneda, S.; Frouz, J.; Baldrian, P.; Cajthaml, T.; Kristufek, V. Does the addition of leaf litter affect soil respiration in the same way as addition of macrofauna excrements (of Bibio marci Diptera larvae) produced from the same litter? Appl. Soil Ecol. 2013, 72, 7–13. [Google Scholar] [CrossRef]
- Suzuki, Y.; Grayston, S.J.; Prescott, C.E. Effects of leaf litter consumption by millipedes (Harpaphe haydeniana) on subsequent decomposition depend on litter type. Soil Biol. Biochem. 2013, 57, 116–123. [Google Scholar] [CrossRef]
- Frouz, J.; Elhotová, D.; Pižl, V.; Tajovský, K.; Šourková, M.; Picek, T.; Malý, S. The effect of litter quality and soil faunal composition on organic matter dynamics in post-mining soil: A laboratory study. Appl. Soil Ecol. 2007, 37, 72–80. [Google Scholar] [CrossRef]
- Taylor, B.R.; Parkinson, D.; Parsons, W.F.J. Nitrogen and lignin content as predictors of litter decay rates: A microcosm test. Ecology 1989, 70, 97–104. [Google Scholar] [CrossRef]
- Cepáková, Š.; Frouz, J. Changes in chemical composition of litter during decomposition: A review of published 13C NMR spectra. J. Soil Sci. Plant. Nutr. 2015, 15, 805–815. [Google Scholar] [CrossRef]
- Singh, P.P.; Ambika; Chauhan, S.M.S. Activity guided isolation of antioxidants from the leaves of Ricinus communis L. Food Chem. 2009, 114, 1069–1072. [Google Scholar] [CrossRef]
- Wood, C.T.; Schlindwein, C.C.D.; Soares, G.L.G.; Araujo, P.B. Feeding rates of Balloniscus sellowii (Crustacea, Isopoda, Oniscidea): The effect of leaf litter decomposition and its relation to the phenolic and flavonoid content. Zoo Keys 2012, 176, 231–245. [Google Scholar] [CrossRef]
- Kefeli, V.I.; Kalewitch, M.V.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol. 2003, 2, 13–18. [Google Scholar]

| df | F | p | Post Hoc | |
|---|---|---|---|---|
| 3–way ANOVA all data | ||||
| 1–temperature | 3 | 26.5 | <0.0001 | 8a 8–24b 16bc 24c |
| 2–litter type | 2 | 7.5 | 0.0015 | |
| 3–litter vs. feces | 1 | 90.6 | <0.0001 | |
| interactions 1 × 2 | 6 | 1.5 | 0.1926 | |
| interactions 1 × 3 | 3 | 2.2 | 0.0968 | |
| interactions 2 × 3 | 2 | 7.0 | 0.0021 | |
| 2–way ANOVA litter only | ||||
| 1–temperature | 3 | 13.6 | <0.0001 | 8a 8–24b 24bc 16c |
| 2–litter type | 2 | 10.9 | <0.0001 | maple a, alder b, willow b |
| interactions 1 × 2 | 6 | 1.3 | 0.2782 | |
| 2–way ANOVA feces only | ||||
| 1–temperature | 3 | 1.7 | 0.1913 | |
| 2–litter type | 2 | 1.2 | 0.3186 | |
| interactions 1 × 2 | 6 | 1.7 | 0.1704 | |
| df | F | p | |
|---|---|---|---|
| 1–litter type | 1 | 42.5 | >0.0001 |
| 2–fauna vs. feces | 2 | 25.2 | 0.0001 |
| interaction 1 × 2 | 2 | 40.8 | >0.0001 |
| . | df | F | p |
|---|---|---|---|
| 1–before after decomposition | 5 | 5.7 | 0.0002 |
| 2–litter type | 2 | 28.1 | >0.0001 |
| 3–litter vs. feces | 1 | 18.5 | 0.0001 |
| interaction 1 × 2 | 10 | 1.0 | 0.4153 |
| interaction 1 × 3 | 5 | 2.6 | 0.0341 |
| interaction 2 × 3 | 2 | 6.4 | 0.0029 |
| Alder | Willow | Maple | Mean | |
|---|---|---|---|---|
| Litter before | 188 | 221 | 189 | 199.2 ± 15.4a |
| Feces before | 19 | 12 | 11 | 14.1 ± 3.6b |
| Litter after 8 °C | 10 | 7 | 13 | 9.8 ± 2.7b |
| Litter after 16 °C | 8 | 4 | 4 | 5.5 ± 1.9b |
| Litter after 24 °C | 7 | 5 | 2 | 4.4 ± 2.2b |
| Litter after 8–24 °C | 7 | 7 | 10 | 7.8 ± 1.4b |
| Feces after 8 °C | 12 | 8 | 5 | 8.1 ± 3.1b |
| Feces after 16 °C | 6 | 4 | 4 | 4.7 ± 1.0b |
| Feces after 24 °C | 5 | 4 | 6 | 4.7 ± 0.8b |
| Feces after 8–24 °C | 6 | 4 | 3 | 4.5 ± 1.0b |
| df | F | p | ||
| Litter type effect | 2 | 0.5 | 0.6405 | |
| Feeding and decomposition | 9 | 250.7 | >0.0001 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Špaldoňová, A.; Frouz, J. Decomposition of Forest Litter and Feces of Armadillidium vulgare (Isopoda: Oniscidea) Produced from the Same Litter Affected by Temperature and Litter Quality. Forests 2019, 10, 939. https://doi.org/10.3390/f10110939
Špaldoňová A, Frouz J. Decomposition of Forest Litter and Feces of Armadillidium vulgare (Isopoda: Oniscidea) Produced from the Same Litter Affected by Temperature and Litter Quality. Forests. 2019; 10(11):939. https://doi.org/10.3390/f10110939
Chicago/Turabian StyleŠpaldoňová, Alexandra, and Jan Frouz. 2019. "Decomposition of Forest Litter and Feces of Armadillidium vulgare (Isopoda: Oniscidea) Produced from the Same Litter Affected by Temperature and Litter Quality" Forests 10, no. 11: 939. https://doi.org/10.3390/f10110939
APA StyleŠpaldoňová, A., & Frouz, J. (2019). Decomposition of Forest Litter and Feces of Armadillidium vulgare (Isopoda: Oniscidea) Produced from the Same Litter Affected by Temperature and Litter Quality. Forests, 10(11), 939. https://doi.org/10.3390/f10110939






