Nitrogen Addition Alleviates Microbial Nitrogen Limitations and Promotes Soil Respiration in a Subalpine Coniferous Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Nitrogen Application Design
2.2. Soil Respiration Measurement
2.3. Soil Sampling and Analyses
2.4. Data Processing and Statistical Analysis
3. Results
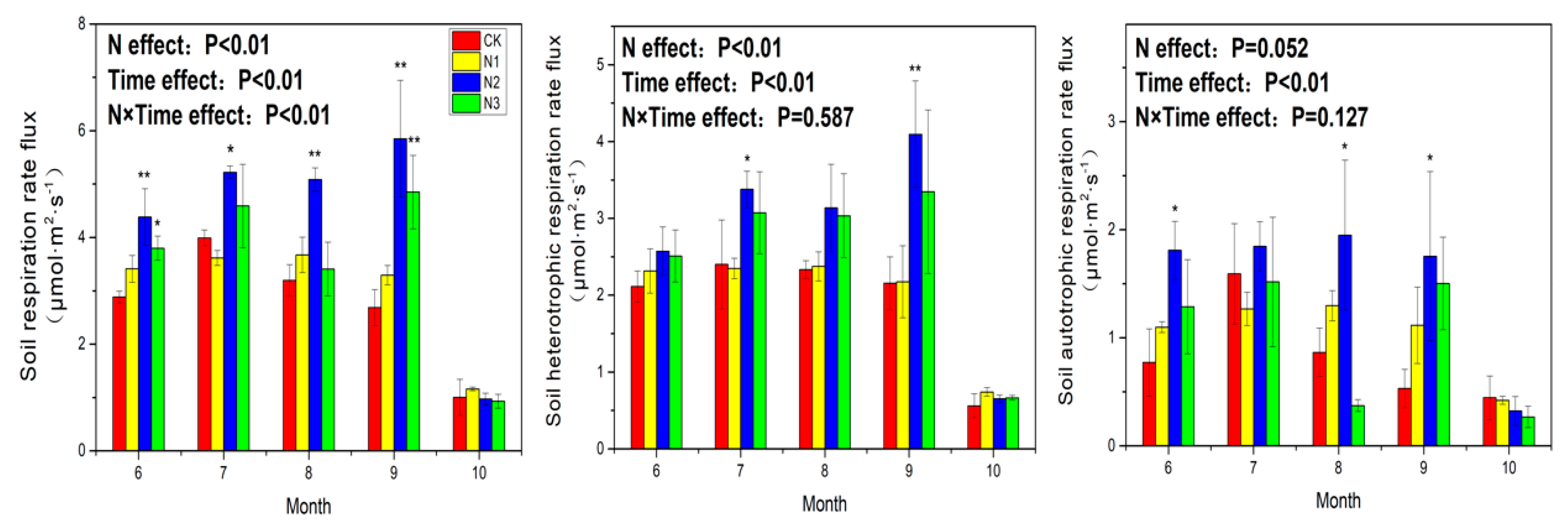
3.1. Soil Respiration
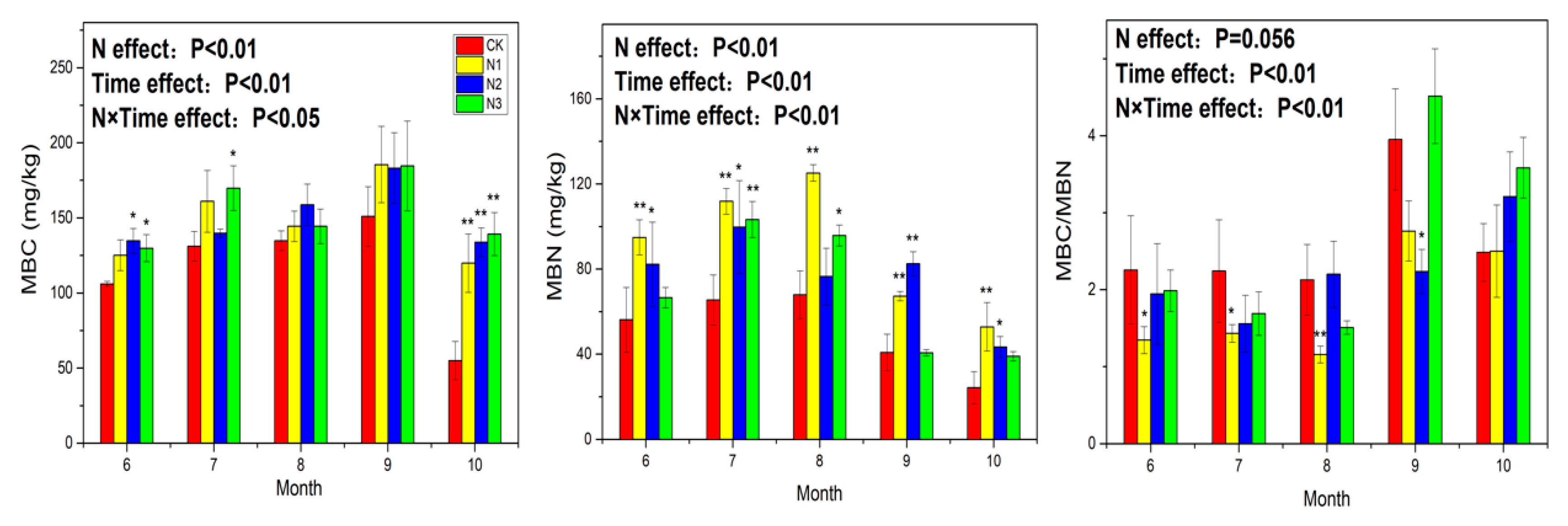
3.2. Soil Microbial Biomass Carbon and Nitrogen
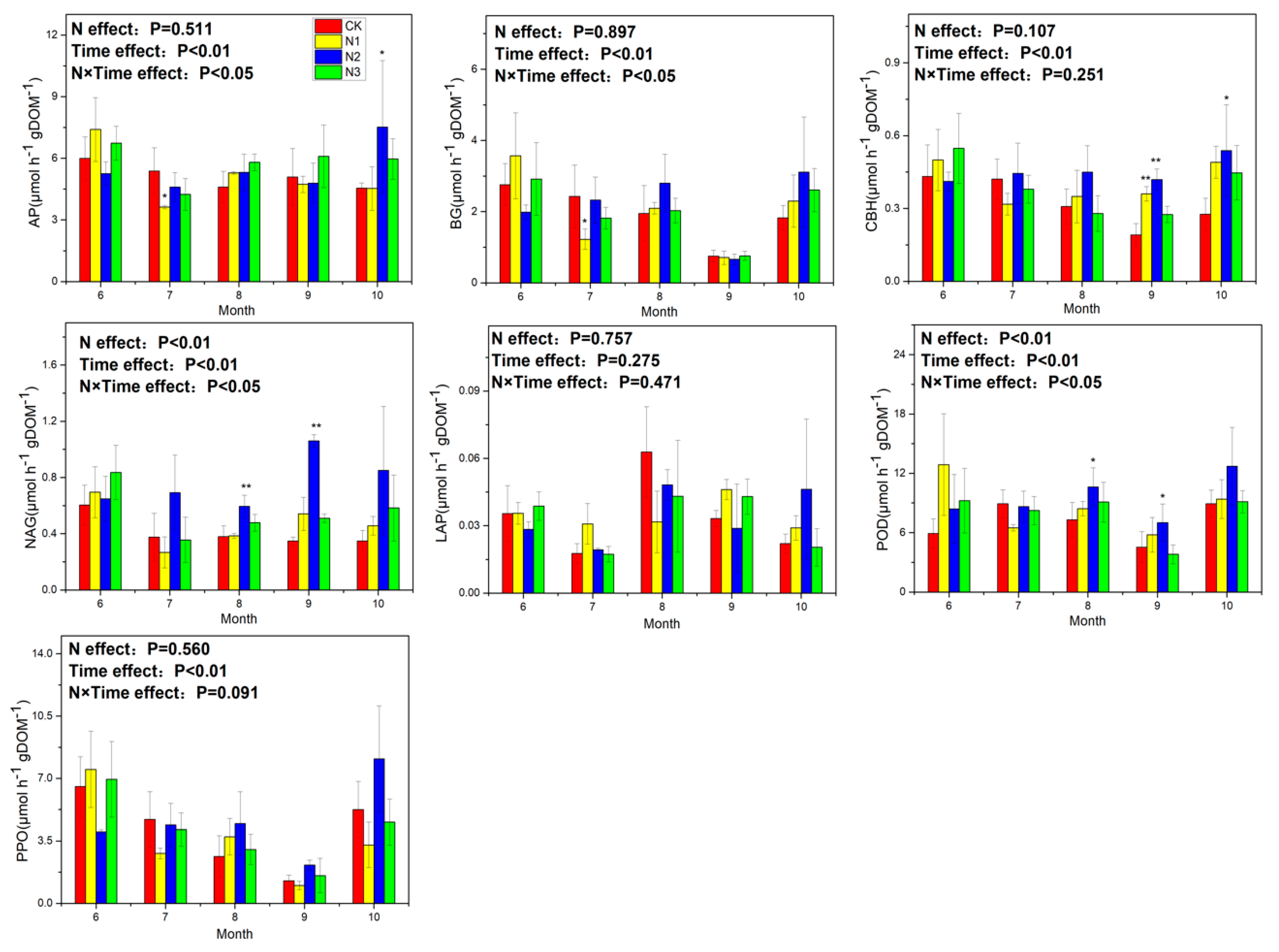
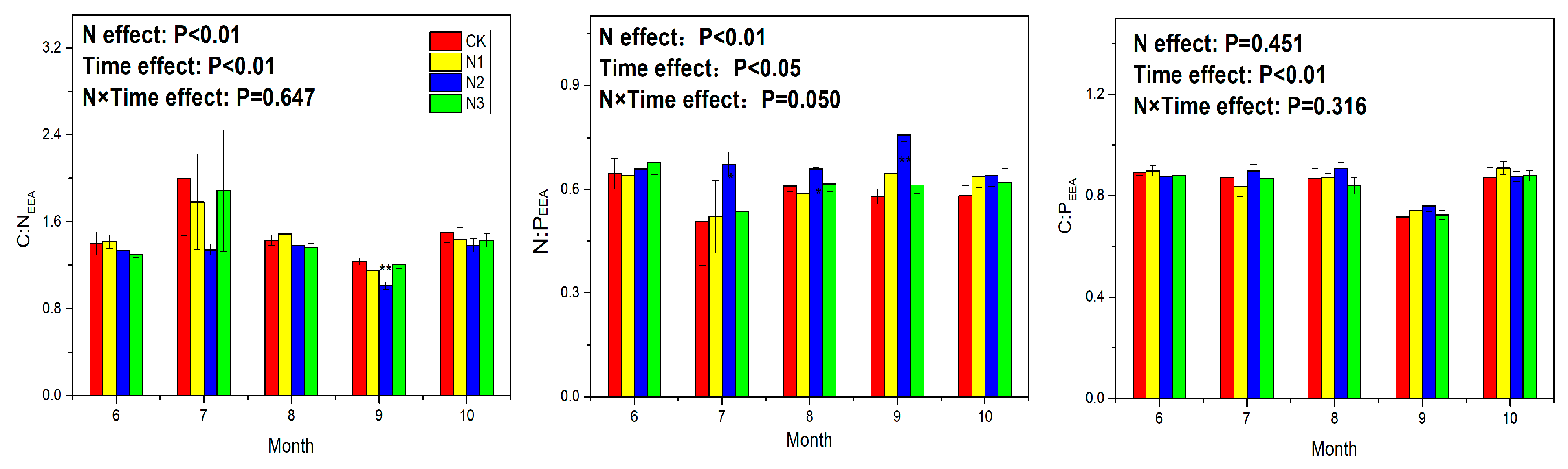
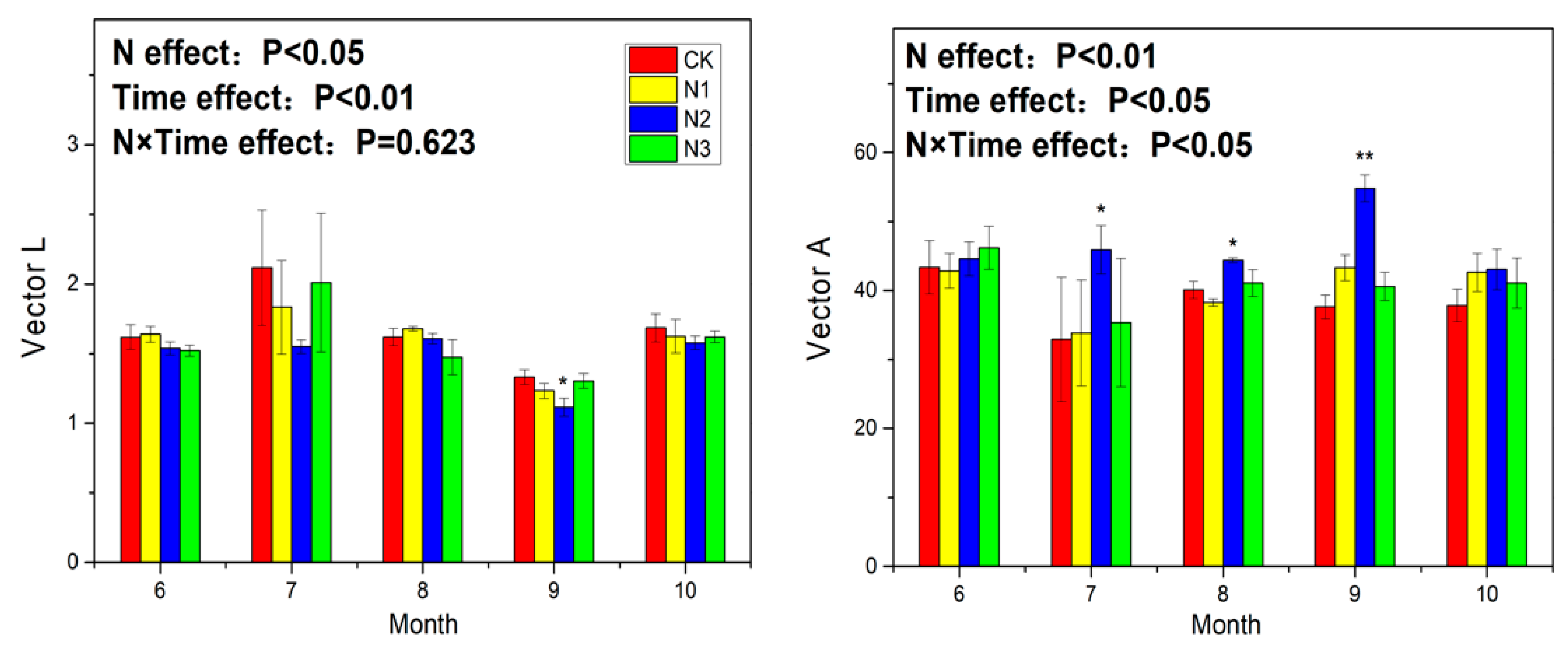
3.3. Soil Enzyme Activity and Ecoenzymatic Stoichiometry
3.4. Soil Physicochemical Properties
3.5. Soil Microbial PLFA Content
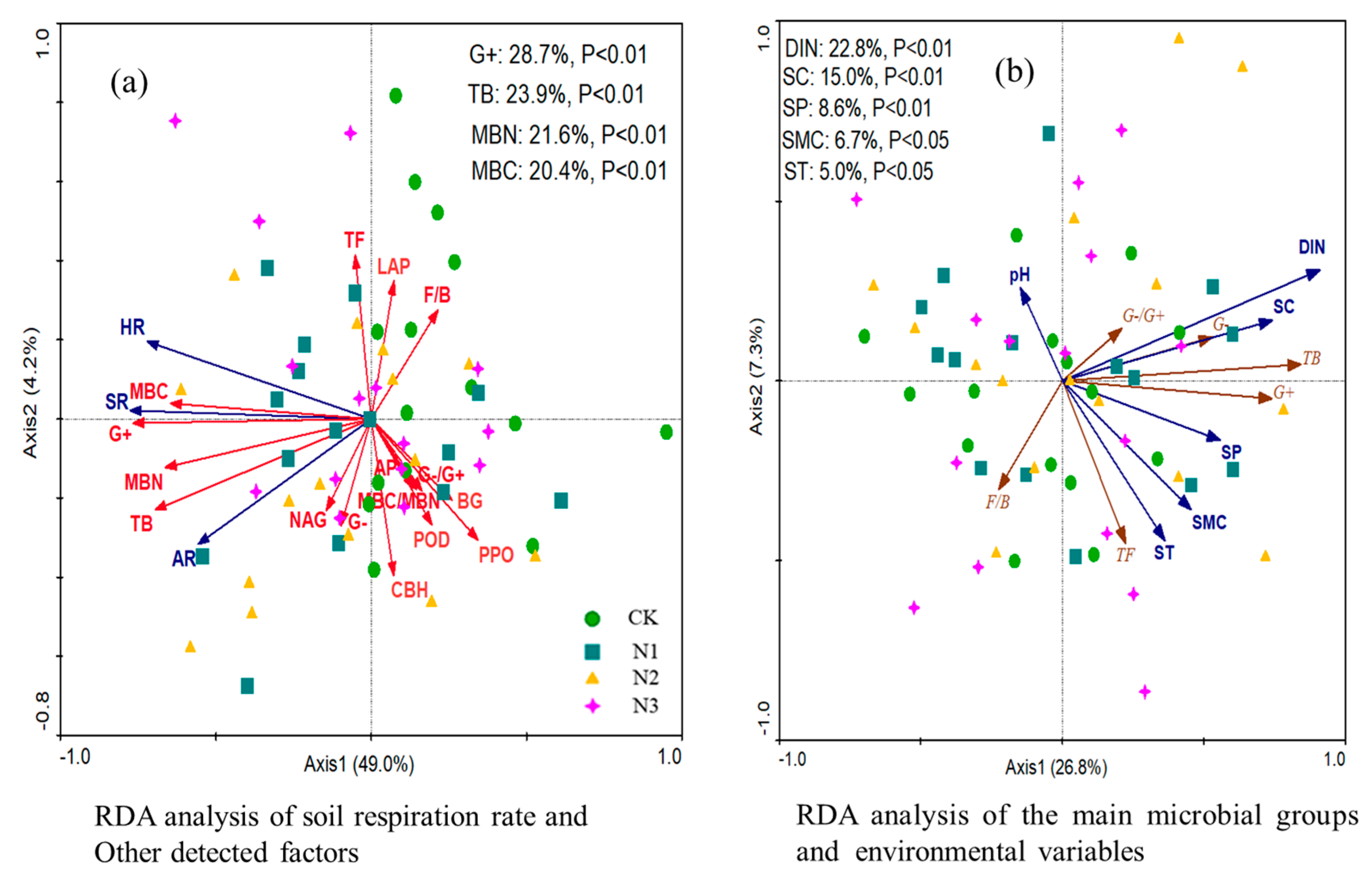
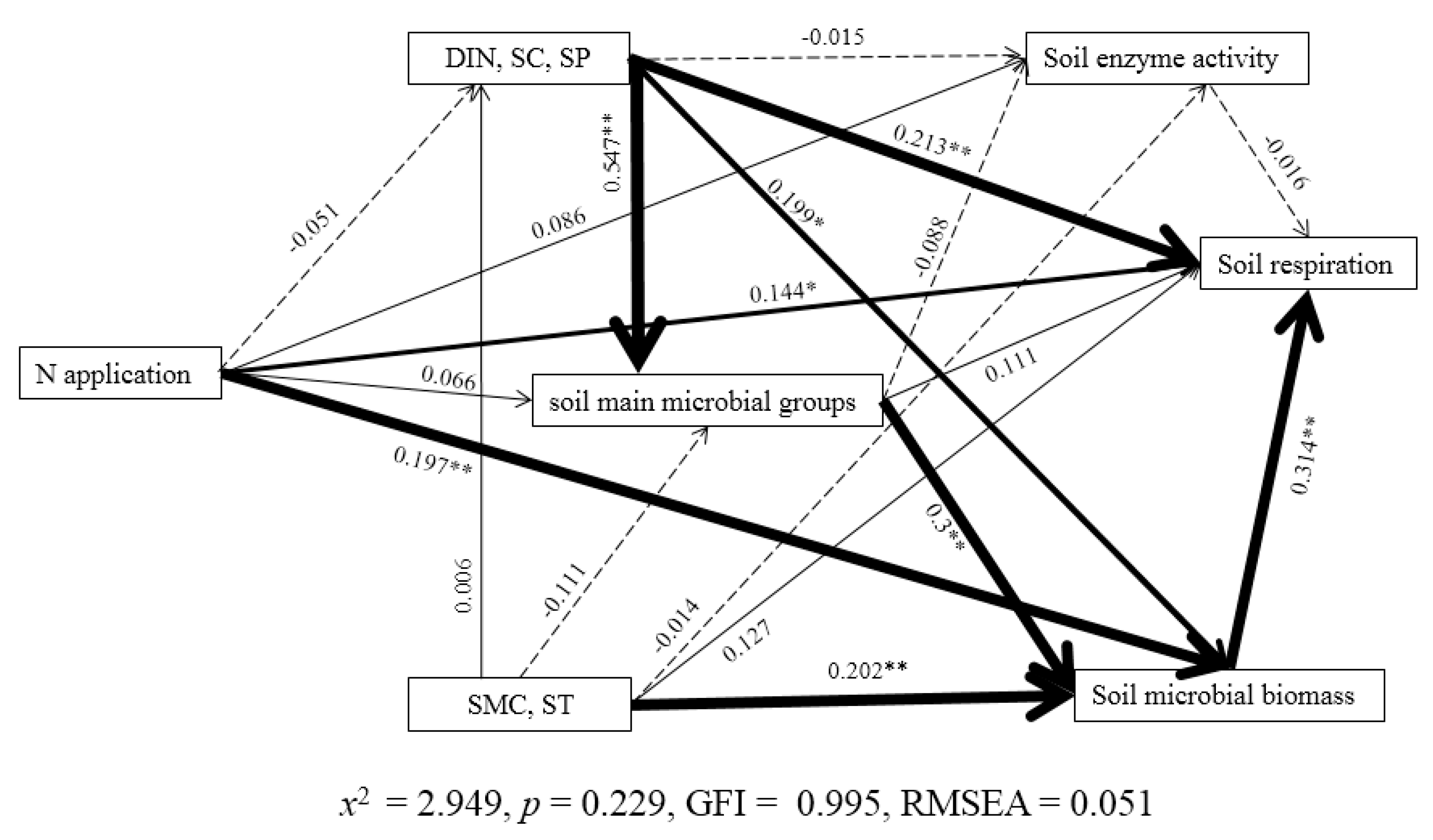
3.6. Relationships among Variables
4. Discussion
4.1. Response of Soil Respiration to N Application
4.2. Response of Soil Microbial Biomass to N Application
4.3. Response of Soil Enzyme Activity to N Application
4.4. Response of Ecoenzymatic Stoichiometry to N Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Townsend, A.R.; Jan Willem, E.; Mateete, B.; Zucong, C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Rivas-Ubach, A.; Janssens, I.A. The Human-Induced Imbalance between C, N and P in Earth’s Life System. Glob. Chang. Biol. 2015, 18, 3–6. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2012, 10, 1714–1721. [Google Scholar] [CrossRef]
- Zhu, J.; He, N.; Wang, Q.; Yuan, G.; Wen, D.; Yu, G.; Jia, Y. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Chang. Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Xie, J.; Lyu, M.; Zheng, Y.; You, Z.; Fan, Y.; Lin, C.; Chen, G.; Chen, Y.; et al. Nitrogen Addition Affects Soil Respiration Primarily through Changes in Microbial Community Structure and Biomass in a Subtropical Natural Forest. Forests 2019, 10, 495. [Google Scholar] [CrossRef]
- López-Aizpún, M.; Arango-Mora, C.; Santamaría, C.; Lasheras, E.; Santamaría, J.M.; Ciganda, V.S.; Cárdenas, L.M.; Elustondo, D. Atmospheric ammonia concentration modulates soil enzyme and microbial activity in an oak forest affecting soil microbial biomass. Soil Biol. Biochem. 2018, 116, 378–387. [Google Scholar] [CrossRef]
- Chen, D.M.; Li, J.J.; Lan, Z.C.; Hu, S.J.; Bai, Y.F. Soil acidification exerts a greater control on soil respiration than soil nitrogen availability in grasslands subjected to long-term nitrogen enrichment. Funct. Ecol. 2016, 30, 658–669. [Google Scholar] [CrossRef]
- Ye, C.; Chen, D.; Hall, S.J.; Pan, S.; Yan, X.; Bai, T.; Guo, H.; Zhang, Y.; Bai, Y.; Hu, S. Reconciling multiple impacts of nitrogen enrichment on soil carbon: Plant, microbial and geochemical controls. Ecol. Lett. 2018, 21, 1162–1173. [Google Scholar] [CrossRef]
- Zong, N.; Shi, P.L.; Chai, X.; Jiang, J.; Zhang, X.Z.; Song, M.H. Responses of ecosystem respiration to nitrogen enrichment and clipping mediated by soil acidification in an alpine meadow. Pedobiologia 2017, 60, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Song, B.; Ma, F.; Tian, D.; Li, Y.; Yan, T.; Quan, Q.; Zhang, F.; Li, Z.; Wang, B.; et al. Nitrogen addition reduces soil respiration but increases the relative contribution of heterotrophic component in an alpine meadow. Funct. Ecol. 2019, 1–15. [Google Scholar] [CrossRef]
- Sun, S.; Wu, Y.; Zhang, J.; Wang, G.; DeLuca, T.H.; Zhu, W.; Li, A.; Duan, M.; He, L. Soil warming and nitrogen deposition alter soil respiration, microbial community structure and organic carbon composition in a coniferous forest on eastern Tibetan Plateau. Geoderma 2019, 353, 283–292. [Google Scholar] [CrossRef]
- Cusack, D.F.; Torn, M.S.; McDowell, W.H.; Silver, W.L. The response of heterotrophic activity and carbon cycling to nitrogen additions and warming in two tropical soils. Glob. Chang. Biol. 2010, 16, 2555–2572. [Google Scholar] [CrossRef]
- He, T.X.; Wang, Q.K.; Wang, S.L.; Zhang, F.Y. Nitrogen Addition Altered the Effect of Belowground C Allocation on Soil Respiration in a Subtropical Forest. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.H.; Zhou, Z.Y.; Zhang, Y.Y. Inorganic Nitrogen Addition Affects Soil Respiration and Belowground Organic Carbon Fraction for a Pinus tabuliformis Forest. Forests 2019, 10, 369. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Z.L.; Zhuang, D.H.; Wang, J.; Xie, S.S.; Liu, G.B. Urea fertilization decreases soil bacterial diversity, but improves microbial biomass, respiration, and N-cycling potential in a semiarid grassland. Biol. Fertil. Soils 2019, 55, 229–242. [Google Scholar] [CrossRef]
- Song, Y.Y.; Song, C.C.; Meng, H.N.; Swarzenski, C.M.; Wang, X.W.; Tan, W.W. Nitrogen additions affect litter quality and soil biochemical properties in a peatland of Northeast China. Ecol. Eng. 2017, 100, 175–185. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Deng, Q.; Zhou, G.; Liu, J.; Liu, S.; Duan, H.; Zhang, D. Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China. Biogeosciences 2010, 7, 315–328. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Zeng, X.; Moore, D.J.P.; Zeng, X. Soil microbial respiration from observations and Earth System Models. Environ. Res. Lett. 2013, 8, 034034. [Google Scholar]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Jandl, R. Carbon losses due to soil warming: Do autotrophic and heterotrophic soil respiration respond equally? Glob. Chang. Biol. 2009, 15, 901–913. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J. Follstad. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795. [Google Scholar] [CrossRef] [PubMed]
- Nadelhoffer, K.J.; Emmett, B.A.; Gundersen, P.; Kjønaas, O.J.; Koopmans, C.J.; Schleppi, P.; Tietema, A.; Wright, R.F. Nitrogen deposition makes a minor contribution to carbon sequestration in temperate forests. Nature 1999, 398, 145–148. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhou, X.H.; Zhang, B.C.; Lu, M.; Luo, Y.Q.; Liu, L.L.; Li, B. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis. Glob. Chang. Biol. 2014, 20, 2332–2343. [Google Scholar] [CrossRef]
- Jian, S.Y.; Li, J.W.; Chen, J.; Wang, G.S.; Mayes, M.A.; Dzantor, K.E.; Hui, D.F.; Luo, Y.Q. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Dong, C.C.; Wang, W.; Liu, H.Y.; Xu, X.T.; Zeng, H. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol. Indic. 2019, 101, 453–464. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.J.; Zhao, J.; Zhang, W.; Xiao, K.C.; Wang, K.L. Nitrogen addition aggravates microbial carbon limitation: Evidence from ecoenzymatic stoichiometry. Geoderma 2018, 329, 61–64. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Liu, Y.H.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.A.; Chen, H.Y.H.; Ruan, H.H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.; Fu, Y.; Chen, X.P.; Song, X.Y. Short-term effects of nitrogen deposition on soil respiration components in two alpine coniferous forests, southeastern Tibetan Plateau. J. For. Res. 2019, 30, 1029–1041. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.; Li, R.H.; Dai, H.Z.; Luo, S.H. Short-term simulated nitrogen deposition increases carbon sequestration in a Pleioblastus amarus plantation. Plant Soil 2011, 340, 383–396. [Google Scholar] [CrossRef]
- Dolman, A.J.; Law, B.E.; Papale, D.; Schulze, E.; Matteucci, G.; Janssens, I.A.; Subke, J.; Grace, J.; Tang, J.; Reichstein, M. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar]
- Liu, W.; Lü, X.; Xu, W.; Shi, H.; Hou, L.; Li, L.; Yuan, W. Effects of water and nitrogen addition on ecosystem respiration across three types of steppe: The role of plant and microbial biomass. Sci. Total Environ. 2018, 619, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ye, C.; Zhang, H.; Pan, S.; Ji, Y.; Li, Z.; Liu, M.; Zhou, X.; Du, G.; Hu, F. Long-term nitrogen & phosphorus additions reduce soil microbial respiration but increase its temperature sensitivity in a Tibetan alpine meadow. Soil Biol. Biochem. 2017, 113, 26–34. [Google Scholar]
- Zhang, J.Y.; Ai, Z.M.; Liang, C.T.; Wang, G.L.; Xue, S. Response of soil microbial communities and nitrogen thresholds of Bothriochloa ischaemum to short-term nitrogen addition on the Loess Plateau. Geoderma 2017, 308, 112–119. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Reynolds, H.; Long, T.M. Rapid assay for amidohydrolase (urease) activity in environmental samples. Soil Biol. Biochem. 2001, 32, 2095–2097. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Jicha, T.M.; Kolka, R.K.; Llp, L.; Sebestyen, S.D.; Seifertmonson, L.R. Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types. Biogeochemistry 2014, 120, 203–224. [Google Scholar] [CrossRef]
- Grace, J.B.; Anderson, T.M.; Olff, H.; Scheiner, S.M. On the specification of structural equation models for ecological systems. Ecol. Monogr. 2010, 80, 67–87. [Google Scholar] [CrossRef]
- Wang, Q.K.; Xiao, F.M.; Zhang, F.Y.; Wang, S.L. Labile soil organic carbon and microbial activity in three subtropical plantations. Forestry 2013, 86, 569–574. [Google Scholar] [CrossRef]
- Stone, M.M.; Plante, A.F.; Casper, B.B. Plant and nutrient controls on microbial functional characteristics in a tropical Oxisol. Plant Soil 2013, 373, 893–905. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Wang, J.; Tian, D.; Li, Y.; He, N.; Niu, S. Soil and climate determine differential responses of soil respiration to nitrogen and acid deposition along a forest transect. Eur. J. Soil Biol. 2019, 93, 562–573. [Google Scholar] [CrossRef]
- Zheng, S.; Bian, H.F.; Quan, Q.; Xu, L.; Chen, Z.; He, N.P. Effect of nitrogen and acid deposition on soil respiration in a temperate forest in China. Geoderma 2018, 329, 82–90. [Google Scholar] [CrossRef]
- Wei, H.; Chen, X.M.; He, J.H.; Huang, L.T.; Shen, W.J. Warming but Not Nitrogen Addition Alters the Linear Relationship Between Microbial Respiration and Biomass. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Huang, G.; Li, L.; Su, Y.G.; Li, Y. Differential seasonal effects of water addition and nitrogen fertilization on microbial biomass and diversity in a temperate desert. Catena 2018, 161, 27–36. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Mcnulty, S.; Fernandez, I.J.; Boggs, J.; Schlesinger, W.H. Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For. Ecol. Manag. 2006, 222, 459–468. [Google Scholar] [CrossRef]
- Carrara, J.E.; Walter, C.A.; Hawkins, J.S.; Peterjohn, W.T.; Averill, C.; Brzostek, E.R. Interactions among plants, bacteria, and fungi reduce extracellular enzyme activities under long-term N fertilization. Glob. Chang. Biol. 2018, 24, 2721–2734. [Google Scholar] [CrossRef]
- Argiroff, W.A.; Zak, D.R.; Upchurch, R.A.; Salley, S.O.; Grandy, A.S. Anthropogenic N deposition alters soil organic matter biochemistry and microbial communities on decaying fine roots. Glob. Chang. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Stark, S.; Mannisto, M.K.; Eskelinen, A. Nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 2014, 383, 373–385. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, H.; Liu, T.; Mao, P.; Zhang, W.; Shao, Y.; Fu, S. An increase in precipitation exacerbates negative effects of nitrogen deposition on soil cations and soil microbial communities in a temperate forest. Environ. Pollut. 2017, 235, 293. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar]
- Ma, Y.; Zhu, B.; Sun, Z.; Zhao, C.; Yan, Y.; Piao, S. The effects of simulated nitrogen deposition on extracellular enzyme activities of litter and soil among different-aged stands of larch. J. Plant Ecol. 2014, 7, 240–249. [Google Scholar] [CrossRef]
- Jing, X.; Chen, X.; Tang, M.; Ding, Z.; Jiang, L.; Li, P.; Ma, S.; Tian, D.; Xu, L.; Zhu, J. Nitrogen deposition has minor effect on soil extracellular enzyme activities in six Chinese forests. Sci. Total Environ. 2017, 607, 806–815. [Google Scholar] [CrossRef]
- Keuskamp, J.A.; Feller, I.C.; Laanbroek, H.J.; Verhoeven, J.T.A.; Hefting, M.M. Short- and long-term effects of nutrient enrichment on microbial exoenzyme activity in mangrove peat. Soil Biol. Biochem. 2015, 81, 38–47. [Google Scholar] [CrossRef]
- Zak, D.R.; Sinsabaugh, R.L. Nitrogen Deposition Modifies Soil Carbon Storage through Changes in Microbial Enzymatic Activity. Ecol. Appl. 2004, 14, 1172–1177. [Google Scholar]
- Deforest, J.L.; Zak, D.R.; Pregitzer, K.S.; Burton, A.J. Atmospheric nitrate deposition and the microbial degradation of cellobiose and vanillin in a northern hardwood forest. Soil Biol. Biochem. 2004, 36, 965–971. [Google Scholar] [CrossRef]
- Weand, M.P.; Arthur, M.A.; Lovett, G.M.; McCulley, R.L.; Weathers, K.C. Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol. Biochem. 2010, 42, 2161–2173. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B.A. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Gong, S.; Tao, Z.; Rui, G.; Cao, H.; Wei, S. Response of soil enzyme activity to warming and nitrogen addition in a meadow steppe. Soil Res. 2015, 53, 242–252. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Q.; Wang, Z.; Zheng, H.; Chen, Y.; Chen, X.; Wang, L.; Li, H.; Zhang, J. Nitrogen Addition Alleviates Microbial Nitrogen Limitations and Promotes Soil Respiration in a Subalpine Coniferous Forest. Forests 2019, 10, 1038. https://doi.org/10.3390/f10111038
Liu Y, Chen Q, Wang Z, Zheng H, Chen Y, Chen X, Wang L, Li H, Zhang J. Nitrogen Addition Alleviates Microbial Nitrogen Limitations and Promotes Soil Respiration in a Subalpine Coniferous Forest. Forests. 2019; 10(11):1038. https://doi.org/10.3390/f10111038
Chicago/Turabian StyleLiu, Yang, Qianmei Chen, Zexi Wang, Haifeng Zheng, Yamei Chen, Xian Chen, Lifeng Wang, Hongjie Li, and Jian Zhang. 2019. "Nitrogen Addition Alleviates Microbial Nitrogen Limitations and Promotes Soil Respiration in a Subalpine Coniferous Forest" Forests 10, no. 11: 1038. https://doi.org/10.3390/f10111038
APA StyleLiu, Y., Chen, Q., Wang, Z., Zheng, H., Chen, Y., Chen, X., Wang, L., Li, H., & Zhang, J. (2019). Nitrogen Addition Alleviates Microbial Nitrogen Limitations and Promotes Soil Respiration in a Subalpine Coniferous Forest. Forests, 10(11), 1038. https://doi.org/10.3390/f10111038




