Abstract
Eogystia hippophaecola Hua, Chou, Fang et Chen (Lepidoptera: Cossidae) is an important borer pest of the sea buckthorn forest (Hippophae rhamnoides L.) in China. Its larvae, which are highly cold tolerant, mainly overwinter in sea buckthorn roots. Heat shock proteins (Hsps) are important molecular chaperones that have been linked to cold tolerance in insects. In this study, we cloned the open reading frames (ORFs) of two Hsp90 genes from E. hippophaecola, EhHsp90-1 and EhHsp90-2, and analyzed their expression under cold stress by qRT-PCR. EhHsp90-1 and EhHsp90-2 are 2154 and 2346 bp in length, respectively, encoding 717 and 781 amino acids. The deduced amino acid sequences contain the conserved signature sequences of the Hsp90 family and the C-terminus characteristic sequence of cytoplasmic or endoplasmic reticulum Hsp90 protein. Phylogenetic analysis revealed the amino acid sequences of EhHsp90-1 and EhHsp90-2 were very similar to the corresponding proteins from Lepidoptera. Under various low-temperature treatments lasting 2 h, EhHsp90-1 and EhHsp90-2 exhibited similar expression patterns, increasing first and then decreasing. At −5 °C, EhHsp90-1 was significantly up-regulated after 12 h, whereas EhHsp90-2 was up-regulated after just 1 h and reached its highest level at 2 h; however, the overall degree of upregulation was greater for EhHsp90-1. Subsequently, the expression level of EhHsp90-2 fluctuated with time. Our results suggest that the two Hsp90s play important roles in E. hippophaecola larvae response to cold stress, but that their response times and the magnitudes of their responses to low-temperature stress differed significantly, providing a theoretical basis for further studying the molecular mechanism of cold tolerance in E. hippophaecola larvae.
1. Introduction
Insects are poikilotherms, i.e., their body temperatures are susceptible to environmental conditions. Accordingly, environmental temperature is one of the most important factors affecting insects’ many biological processes. Previous studies show that in addition to insects’ genetic basis, insects also adopt different physiological and biochemical strategies to respond to low-temperature stress [1]. Among these strategies, there are many genes and their encoded protein products associate with tolerance to low temperatures in insects, including heat shock proteins (Hsps) [2,3], antifreeze proteins [4], trehalose synthase [5], chitinase [6], ice-nucleating protein [7], and the DCA [8] and FST proteins [9]. The induction of heat shock proteins is one of the most extensively studied areas, and is thought to be a key determinant to improve insect cold tolerance.
Heat shock proteins are important molecular chaperones and when organisms are exposed to heat, cold, or some other stresses, they synthesize a set of proteins to participate in folding and unfolding, aggregation, degradation, and transport of proteins induced by the stress [10,11,12,13]. The Hsps are a group of highly conserved proteins that are divided into several families based on their molecular mass (kDa) [11]. Among the different families of Hsps, the heat shock proteins 90 (Hsp90) family is one of the most abundant proteins in all eukaryotes and prokaryotes. Conserved structured of Hsp90 are characterized by the N-terminal ATPase domain and the C-terminal polypeptide-binding domain [14]. Consequently, the role of Hsp90 as stress markers has been well recognized in many organisms [15]. Furthermore, Hsp90 mRNA or protein have enhanced in response to heat shock [16,17,18,19,20], heavy metal pollution [20,21,22], diapause [23,24,25,26], proteotoxic stresses [27], starvation stresses [20], pesticide stresses [17,21], and so on. Eukaryotic Hsp90 can be classified into five types, of which cytoplasmic Hsp90 (Cy-Hsp90) and endoplasmic reticulum Hsp90 (ER-Hsp90) are the most widespread [28,29]. Recent research has focused on the important role of Cy-Hsp90 in the insect response to various environmental stresses [30,31], revealing that Hsp90 in Drosophila melanogaster Meigen, Chilo suppressalis Warker, and Spodoptera exigua Huebner, as well as other insects, are significantly up-regulated under cold stress [18,31,32]. However, the ER-Hsp90 response to cold stress has not been as widely studied. Upregulation of ER-Hsp90 is an important marker of the ER stress response [33], and ER-Hsp90 plays an important role in insect adaptation and the innate immune system [34,35].
Eogystia hippophaecola Hua, Chou, Fang et Chen (Lepidoptera: Cossidae) [36,37] is one of the most serious borer pests of sea buckthorn (Hippophae rhamnoides L.) in China. The larvae bore mainly into the sea buckthorn backbone and roots, feeding on those parts and leading to the death of whole plants, thereby seriously damaging the ecological and economic benefits of sea buckthorn forests [38]. According to an overwintering survey, four-year-old larvae are highly cold tolerant, with zero winter mortality [39]. In previous work, we showed that sHsp and Hsp70 [40] from E. hippophaecola play important roles under low-temperature stress. However, very little research has been performed on E. hippophaecola Hsp90. Based on the E. hippophaecola transcriptome, we identified Hsp90 homologs and cloned their ORFs. In addition, using qRT-PCR, we studied their expression patterns under low-temperature stress. Our results provide a theoretical reference for deeper exploration of the molecular mechanisms of cold tolerance in E. hippophaecola, laying the foundation for further studies of Hsp90 gene function.
2. Materials and Methods
2.1. Insects
Larvae of E. hippophaecola were collected from the sea buckthorn forest in Jianping, Liaoning, China (42°1′ N, 119°37′ E) in September 2018. The larvae were stored in a plastic bucket and maintained on fresh sea buckthorn roots. The soil from around the sea buckthorn root was kept in the plastic bucket to retain humidity. To prevent the larvae from escaping, the mouth of the bucket was sealed with a stainless steel mesh. In the laboratory, head shell width was measured to distinguish larvae of different ages [39], and then the larvae were placed in a 25 °C incubator for experimental use.
2.2. Cold Stress Treatments
The 13–15 instar larvae were randomly selected for the experiment and each larva was placed in a 50 ml perforated centrifuge tube, then they were immediately transferred to different cold temperatures from the common culturing temperature. Treatments at different temperatures were performed as follows: 13–15 instar larvae were treated at five temperatures (25 °C, 5 °C, 0 °C, −5 °C, and −10 °C) for 2 h, and larvae treated at 25 °C were regarded as the control group. Treatments for different times were performed as follows: 13–15 instar larvae were placed at −5 °C for 1, 2, 6, 12, or 24 h; larvae treated for 0 h were used as the control group. Each treatment was repeated three times. The treated larvae were immediately frozen in liquid nitrogen and placed in a −80 °C refrigerator for later use.
2.3. Isolation of Total RNA and Synthesis of First-Strand cDNA
Total RNA from treated larvae were isolated using the TRIzol reagent (number 15596018; Invitrogen, Carlsbad, CA, USA) and the RNeasy Plus Mini Kit (number 74134; Qiagen, Hilden, Germany), and then stored at −80 °C. The concentration, purity, and quantity of the RNA were assessed by agarose gel electrophoresis and the NanoDrop 8000 (Thermo Scientific, Waltham, MA, USA). First-strand cDNA was synthesized from 1 µg of total RNA using the PrimeScript RT Reagent Kit (number RR047Q; TaKaRa, Dalian, China), and then stored at −20 °C until use.
2.4. Cloning of EhHsp90 ORFs
Based on the larval transcriptome data established previously in our laboratory [41], we screened Hsp90 homologs and predicted the ORF regions using an ORF finder (https://www.ncbi.nlm.nih.gov/orffinder, 22 April 2019). The complete ORFs were named EhHsp90-1 and EhHsp90-2. Primers were designed against two full-length sequences using Primer Premier 5.0, and the sequences of all primers used in this study are summarized in supplementary material 1. PCR reactions were conducted as follows: 35 cycles of 98 °C for 10 s, 53 °C for 15 s (EhHsp90-1) or 55 °C for 5 s (EhHsp90-2), 72 °C for 5 s, and 1 min at 72 °C.
The amplified PCR products were confirmed in a 1.0% agarose gel (Oxoid, Hants, UK)and then the purified PCR products were cloned into the pEASY-Blunt Simple Cloning Vector (number CB111-01; TransGen Biotech, Beijing, China). The ligated product was transformed into Trans1-T1 Phage Resistant Chemically Competent Cells (number CD501-01, TransGen Biotech, Beijing, China. Then, the positive colonies were identified using blue and white selection and sequenced at RuiBiotech Co., Ltd (Beijing, China). After alignment, the ORF sequences were obtained.
2.5. Bioinformatics Analysis
The sequencing results were confirmed by homologous sequence alignment in GenBank, and then ligated using DNAMAN and converted into amino acid sequences. The physicochemical and chemical properties of the EhHsp90 gene sequences were analyzed using ProtParam (http://web.expasy.org/protparam/) (19 May 2019). Amino acid sequences were analyzed for hydrophilicity, hydrophobicity, and transmembrane structures using ProtScale (http://web.expasy.org/protscale/) (19 May 2019). The presence of signal peptides were predicted using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) (19 May 2019). The domains of the proteins were predicted and analyzed using ScanProsite (http://prosite.expasy.org/scanprosite/) (22 May 2019) and SMART (http://smart.embl-heidelberg.de/smart/) (22 May 2019). In addition, for subsequent analysis, we screened the NCBI (http://www.nibi.nlm.nih.gov/) (22 May 2019) database for sequences homologous to EhHsp90-1 and EhHsp90-2. The multiple sequence alignments of deduced amino acid sequences were carried out online using MAFFT (Version 7) from the website (https://mafft.cbrc.jp/alignment/server/index.html) (22 October 2019). The phylogenetic tree was constructed by the maximum likelihood (ML) and neighbor-joining (NJ) methods [42,43], and the reliability of the tree was tested by bootstrap analysis with 1000 replications using the MEGA-X software [44].
2.6. Expression of EhHsp90 under Different Treatments
Specific primers (supplementary material 1) for qRT-PCR were designed based on the sequences of ORFs using Primer Premier 5.0 [45]. The E. hippophaecola actin gene was used as a reference [40,41,46] and we used Bestkeeper to evaluate the std value (<1) of actin gene under different temperatures which indicated that actin gene was stable [47]. The qRT-PCR was carried out on a Bio-Rad CFX96 PCR machine (Hercules, CA) in a reaction mixture of 12.5 μL consisting of 6.25 μL of SYBR Premix Ex Taq II (2×) (Takara, Dalian, China), 0.5 μL of each primer, 1 μL of cDNA template, and 4.25 μL of ddH2O. The reactions were conducted as follows: 95 °C for 30 s, 40 cycles of 5 s at 95 °C, 30 s at 60 °C, and 15 s at 95 °C; the Ct value was read at the end of the reaction. Each qRT-PCR reaction was performed in three biological replicates, and each sample was technically repeated three times. The relative expression levels of Hsps were calculated by the 2−∆∆Ct method [48]. The Ct mean and standard error of each treatment were calculated using the Bio-Rad CFX Manager (version 3.1.1517.0823) software [49], and the relative expression levels of EhHsp90 were statistically analyzed by one-way analysis of variance (ANOVA) and Tukey’s test using spss 18.0 software (spss, Inc., Chicago, IL, USA). Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Characterization of EhHsp90
The ORFs of two Hsp90 clones were named EhHsp90-1 (GenBank accession number: MN565559) and EhHsp90-2 (GenBank accession number: MN565560). The gene sequences and their deduced amino acid sequences are shown in Figure 1 and Figure 2, respectively. The sequence characteristics are shown in Table 1 The protein encoded by EhHsp90-1 is unstable, whereas the protein encoded by EhHsp90-2 is relatively stable; both are hydrophilic. In addition, the C-terminus of EhHsp90-1 has the common characteristic motif (EEVD) of all eukaryotic Hsp90 proteins, while the C-terminus of EhHsp90-2 has an ER retention sequence (HDEL). For EhHsp90-2, these is an extra ~20aa with signal peptide that only exists in the N-terminal (Figure 2).

Figure 1.
Nucleotide and deduced amino acid sequence of EhHsp90-1. The initiation codon (atg) is shown in bowed text, and the termination codon (taa) is shown in bowed text and marked with an asterisk. The signature sequences of the Hsp90 family are marked with gray background, and the Cy-Hsp90 carboxyl terminal region is marked with a red background. The wavy line indicates the ATPase domain.
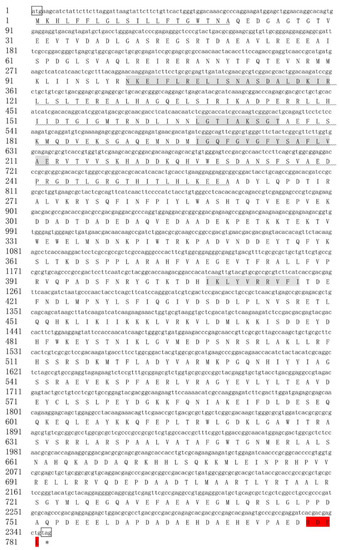
Figure 2.
Nucleotide and deduced amino acid sequence of the EhHsp90-2. The initiation codon (atg) is shown in bowed text, and the termination codon (taa) is shown in bowed text and marked with an asterisk. The signature sequences of the Hsp90 family are marked with gray background, and the ER-Hsp90 carboxyl terminal region is marked with a red background. The signal peptide sites are underlined, and the wavy line indicates the ATPase domain.

Table 1.
Sequence characteristics of EhHsp90-1 and EhHsp90-2.
3.2. Phylogenetic Analysis of EhHsp90
Sequence similarity analysis revealed that EhHsp90-1 and EhHsp90-2 were 46% identical. Additionally, multiple sequence alignments indicated that the deduced amino acid sequences of E. hippophaecola Hsp90s show high homology to their corresponding sequences in other insects (Figure 3). EhHsp90-1 shares 97% similarity with Cy-Hsp90s from Argynnis paphia Linnaeus (ADM26735.1), and 96% similarity with Cy-Hsp90 from Mythimna separata Walker (ADM26740.1), Bombyx mori Linnaeus (NP_001036876.1) and Plutella xylostella Linnaeus (NP_001296043.1). EhHsp90-2 shares 87% similarity with ER-Hsp90s from Helicoverpa armigera Hübner (ATB54999.1), Trichoplusia ni Hübner (XP_026745125.1) and 84% similarity with Antheraea pernyi Guérin-Méneville (APX61061.1) and 80% similarity with ER-Hsp90s from Pieris rapae Linnaeus (XP_022121909.1).
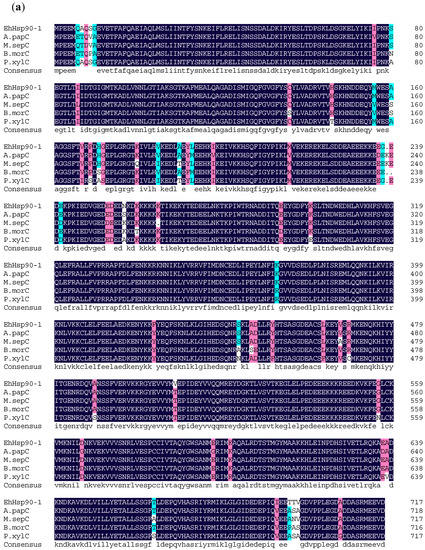
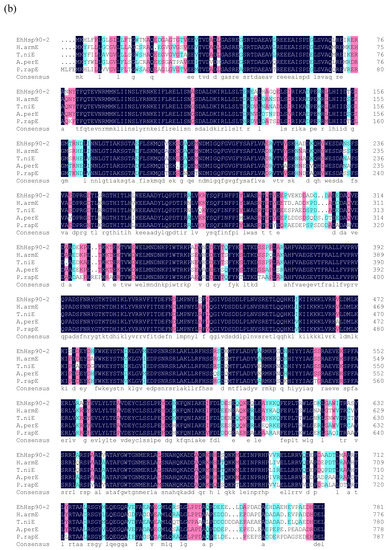
Figure 3.
Multiple sequence alignments of the amino acid sequences of EhHsp90-1 (a) and EhHsp90-2 (b) with other insect Hsp90. Abbreviations: A.papC (Argynnis paphia Cy-Hsp90), M.sepC (Mythimna separate Cy-Hsp90), B.morC (Bombyx mori Cy-Hsp90), P.xylC (Plutella xylostella Cy-Hsp90), H.armE (Helicoverpa armigera Er-Hsp90), T.niE (Trichoplusia ni Er-Hsp90), A.perE (Antheraea pernyi Er-Hsp90), and P.rapE (Pieris rapae Er-Hsp90).
Thirty-nine full-length hsp90 amino acid sequences from Lepidoptera, Diptera, Hemiptera, and Coleoptera were employed for these genetic analysis (Figure 4). The phylogenetic tree used ML and NJ were distinctly divided into two groups: Cy-Hsp90s and ER-Hsp90s. Every group was further divided into four orders: Lepidoptera, Diptera, Hemiptera, and Coleoptera. E. hippophaecola Hsp90s belong to different classes of Hsp90, and cluster with Hsp90 from Lepidoptera. Moreover, branches of the same family insects cluster well in each order of insects.
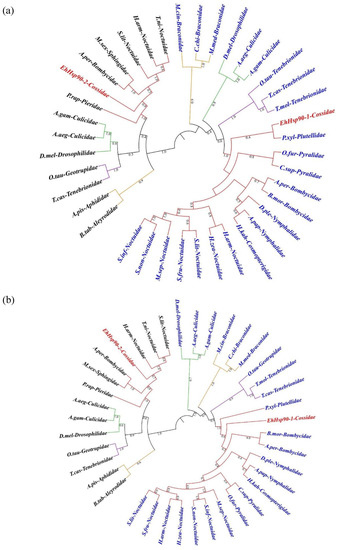
Figure 4.
ML (a) and NJ (b) phylogenetic trees showing phylogenetic analysis of Cy-Hsp90s (blue texts) and ER-Hsp90s (black texts). EhHsp90-1 and EhHsp90-2 are shown in red texts. Different orders of insects are represented by branches in different colors: Red-Lepidoptera; Green-Diptera; Yellow-Hymenoptera; and Purple-Coleoptera). The label consists of the abbreviation of specie name and family name. Details about the gene information and the abbreviations of labels used in this analysis are described in supplementary material 2.
3.3. Expression of EhHsp90 under Different Low-Temperature Treatments
The expression levels of the two Hsp90 after 2 h treatments at various low-temperature treatments are shown in Figure 5. The relative expression of EhHsp90-1 was the highest at 0 °C but did not differ significantly from the control; however, the expression at −10 °C was significantly lower than at −5 °C (p = 0.04). In addition, the relative expression of EhHsp90-2 was the highest (1.75-fold) at −5 °C and significantly higher than at 5 °C (p = 0.01), and there was also a significant decrease between −10 °C and −5 °C (p = 0.03). Thus, the levels of EhHsp90-1 and EhHsp90-2 first increased, and then decreased, with decreasing temperature.
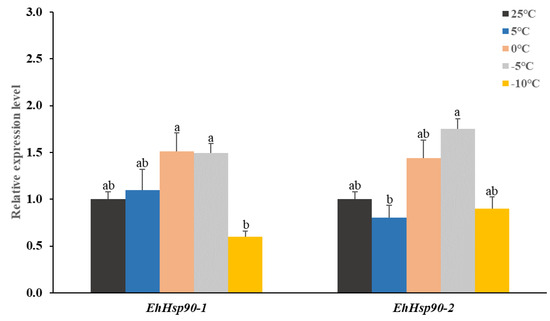
Figure 5.
Relative expression levels of EhHsp90-1 and EhHsp90-2 after exposure to different temperatures; a temperature of 25 °C served as a control. Data in the figure are means ± SE, and the different letters above the error bars indicated a significant difference by one-way ANOVA, followed by Tukey’s test (p < 0.05).
3.4. Expression of EhHsp90 under Different Treatment Times
The expression levels of the two Hsp90 in larvae exposed to −5 °C for various times are shown in Figure 6. Expression of EhHsp90-1 was the highest at −5 °C for 12 h, 3.44 times higher than in the control, and remained significantly higher at 24 h (p = 0.04). However, the expression pattern of EhHsp90-2 differed: the level of EhHsp90-2 was up-regulated after 1 h and reached a maximum at 2 h. But was significantly lower at 6 h than at 2 h (p = 0.01). After that, the expression level of EhHsp90-2 fluctuated with time. However, the overall degree of upregulation was greater for EhHsp90-1. Thus, under cold stress, the expression patterns of EhHsp90-1 and EhHsp90-2 differed over time.
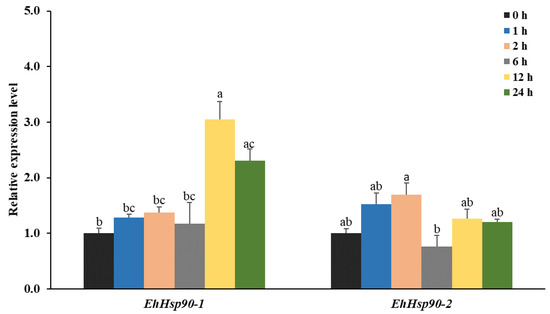
Figure 6.
Relative expression levels of EhHsp90-1 and EhHsp90-2 in larvae treated at −5 °C for the indicated times. The 0 h treatment served as a control. Data in the figure are means ± SE. Different letters above the error bars indicate a significant difference, as determined by one-way ANOVA followed by Tukey’s test (p < 0.05).
4. Discussion
4.1. Characterization and Phylogenetic Analysis of EhHsp90
Environmental temperature is a key factor affecting the growth and development of insects. Hsps play important roles in various stress responses, especially in heat or cold adaptation in insects, and studies have shown that Hsps contribute to the improvement of insect cold tolerance [15,23,24]. In this study, we cloned two Hsp90 genes, EhHsp90-1 and EhHsp90-2, from the larvae of E. hippophaecola. Sequence analysis found both of the genes contain the characteristics motifs of the Hsp90 families. The presence of EEVD that binding to many cochaperones with small helical TRP domains at the EhHsp90-1 C-terminal indicates that EhHsp90-1 is a cytoplasmic Hsp [50,51,52], while the C-terminus of the EhHsp90-2 has a conserved HDEL motif which is an endoplasmic reticulum targeting sequence [28]. In addition, the ATPase domain was also found at the N-terminus of EhHsp90-1 and EhHsp90-2, indicating that Hsp90 is a highly conserved, ATP-regulated dimeric molecular chaperone. For EhHsp90-2, beside these domains, these is an extra ~20 aa with Gln-rich signal peptide that only exists in the N-terminal of endoplasmic reticulum Hsp90 not in that of cytoplasmic Hsp90 (Figure 2). Under the guidance of signal peptide, the newly synthesized protein enters the endoplasmic reticulum cavity, which also means that EhHsp90-2 is located differently from EhHsp90-1.
As expected, two E. hippophaecola hsp90s share high sequence similarities with their counterparts from other insects, and include conserved signature motifs that facilitate interaction with other proteins and enhance their chaperone function (Figure 3) [52,53]. Phylogenetic analysis revealed that Cy-Hsp90s and ER-Hsp90s were clearly divided into two groups, and they evolved separately from a common ancestor but Cy-Hsp90 originated earlier than ER-Hsp90, indicating that these two molecular chaperones may be under differential selections during their evolution [28,54]. The EhHsp90-1 and EhHsp90-2 were in the different groups, and they clustered together with Hsp90 from Lepidoptera, respectively. However, because little is known about cold resistance genes and no Hsp sequence information is in GenBank from Cossidae insects, the EhHsp90s may be clustered with different family of Lepidoptera in ML and NJ methods. In addition, the two groups showed that Lepidoptera, Diptera, Hemiptera, and Coleoptera are were well segregates from each other, and even the different family were also well separated, supporting that Hsp90 protein allows good phylogenetic analysis [25,55].
4.2. Expression of EhHsp90 under Different Treatments
The 4-year-old larvae of E. hippophaecola are highly cold tolerant and understanding how E. hippophaecola responds to cold stress is crucially important to understand how they withstand extreme environmental temperatures [18]. In the Hsps families, up-regulation of Hsp90 gene plays an important role in resisting cold stress [56]. This study revealed the expression patterns of EhHsp90s under cold stress. When larvae were exposed to various cold temperatures for 2 h, expression levels of both EhHsp90-1 and EhHsp90-2 were up-regulated, indicating that EhHsp90s had a relationship with cold temperature changes and perhaps play important roles in surviving temperatures stress for E. hippophaecola larvae. In addition, EhHsp90-1 and EhHsp90-2 had similar expression patterns at different low temperatures, with the expression levels of both proteins reaching the highest point at −5 °C, however the expression level was significantly lower at −10 °C than at −5 °C. In general, the threshold temperature for Hsps induction is correlated with the typical temperatures at which species live [57] and Hsps are subjected to strict regulation by multiple molecular mechanisms [58]. Thus, it is possible that the ability of Hsp90s as molecular chaperones to completely mitigate the damage resulting from extreme cold stress and increase cold tolerance of insects are limited [18]. In our study, the downregulation of EhHsp90s at −10 °C below the supercooling point of −4 ± 0.5 °C of E. hippophaecola larvae [59] may not be induced or expressions of Hsp90s were affected by the regulator (HSF1 or σ32) [60]. Secondly, we observed no significant difference in the expression level of EhHsp90-1 treated at low temperature for 2 h, potentially because this did not provide sufficient time for induction. This was also reflected in experiments in which larvae were exposed to −5 °C for various times. Above 12 h, the expression level of EhHsp90-1 was significantly up-regulated. The observation that EhHsp90-1 is significantly expressed after long-term low-temperature stress is consistent with the highest expression of Hsp90 in M. separata after 12 h of low-temperature induction [61]. However, the temporal profile of EhHsp90-2 expression at −5 °C differed from that of EhHsp90-1. EhHsp90-2 was up-regulated after 1 h, reaching a maximum at 2 h, but the overall degree of upregulation was lower than that of EhHsp90-1. Subsequently, the expression level fluctuates with the extension of time. It may be related to the localization of the EhHsp90-2 in the ER. ER-Hsp90 is a widely expressed chaperone protein, also known as glucose-regulated protein (GRP94). When the body is consuming a great deal of energy, expression of ER-Hsp90 is rapidly induced [62]. Hence, EhHsp90-2 may exhibit short-term stress-dependent expression during larval adaptation to low temperatures, which consumes large amounts of energy. In addition, the expression levels of EhHsp90-1 and EhHsp90-2 were down-regulated after 24 h of low-temperature stress relative to 12 h. With the extension of the low temperature time, the accumulation of Hsp90 continues, however the large amount of expression of Hsp90 requires energy substances. At this time, insects down-regulate Hsp90 in order to maintain the basic biological processes, but the expression level is still higher than that of Hsp90 under normal [63]. Therefore, the EhHsp90s plays an important role in response to cold stress. Our comprehension of Hsp90s in response to low temperatures stress has a reference for understanding the capability of E. hippophaecola larvae to cold tolerance. However, further experiments are needed to explore the details of the mechanisms of Hsp90 genes in cold tolerance of E. hippophaecola larvae.
5. Conclusions
In this study, we successfully cloned two Hsp90 genes of E. hippophaecola for the first time, which contain the conserved signature sequences of the Hsp90 family. The phylogenetic analysis showed that proteins encoded by EhHsp90-1 and EhHsp90-2 are cytoplasmic and endoplasmic reticulum Hsp90 proteins, respectively, and that their amino acid sequences were very similar to the corresponding proteins from Lepidoptera. The expression patterns of EhHsp90-1 and EhHsp90-2 under cold stress showed that their response times and the magnitudes of their responses to low-temperature stress differed significantly. These results suggest that the two Hsp90s play important roles in E. hippophaecola larvae response to cold stress, and provide a theoretical basis for further studying the molecular mechanism of cold tolerance in E. hippophaecola larvae.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/10/11/1039/s1, Supplementary Material 1: Primer sequences used in this study. Supplementary Material 2: Details about the gene information and the abbreviations of labels used for phylogenetic analyses. Supplementary Material 3: The Ct values of actin gene.
Author Contributions
Conceptualization, Q.W.; methodology, Q.W. and J.T.; validation, Q.W., J.T., Y.L., Y.X., X.L., and S.Z.; formal analysis, Q.W.; resources, J.T. and S.Z.; data curation, Q.W. and J.T.; writing—original draft preparation, Q.W.; writing—review and editing, Q.W.; project administration, J.T. and S.Z.
Funding
This research was funded by “the Fundamental Research Funds for the Central Universities” (NO. 2016ZCQ07).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations:
| ORF | Open Reading Frame |
| PCR | Polymerase Chain Reaction |
| qRT-PCR | Quantitative real-time PCR |
References
- Joanisse, D.R.; Storey, K.B. Oxidative stress and antioxidants in stress and recovery of cold-hardy insects. J. Insect Biochem. Mol. Biol. 1998, 28, 23–30. [Google Scholar] [CrossRef]
- Matz, J.M.; LaVoi, K.P.; Moen, R.J.; Blake, M.J. Cold-induced heat shock protein expression in rat aorta and brown adipose tissue. Physiol. Behav. 1996, 60, 1369–1374. [Google Scholar] [CrossRef]
- Sejerkilde, M.; Sorensen, J.G.; Loeschcke, V. Effects of cold- and heat hardening on thermal resistance in Drosophila melanogaster. J. Insect Physiol. 2003, 49, 719–726. [Google Scholar] [CrossRef]
- DeVries, A.L. Glycoproteins as biological antifreeze agents in antarctic fishes. Science 1971, 172, 1152–1155. [Google Scholar] [CrossRef]
- Neufeld, D.S.; Leader, L.P. Freezing survival by isolated Malpighian tubules of the New Zealand alpine weta Hemideina maori. J. Exp. Biol. 1998, 201, 227–236. [Google Scholar]
- Cai, Q.L.; Lu, X.Y.; Ma, J. Molecular cloning and expression profiling of a cold stress-related chitinase gene Mpcht19 in the desert beetle Microdera punctipennis (Coleoptera: Tenebrionidae). Acta Entomol. Sin. 2017, 60, 286–296. [Google Scholar]
- Maki, L.R.; Galyan, E.L.; Chang-Chien, M.M.; Caldwell, D.R. Ice nucleation induced by pseudomonas syringae. Appl. Microbiol. 1974, 28, 456–459. [Google Scholar]
- Goto, S.G.; Kimura, M.T. Heat- and cold-shock responses and temperature adaptations in subtropical and temperate species of Drosophila. J. Insect Physiol. 1998, 44, 1233–1239. [Google Scholar] [CrossRef]
- Goto, S.G. A novel gene that is up-regulated during recovery from cold shock in Drosophila melanogaster. Gene 2001, 270, 259–264. [Google Scholar] [CrossRef]
- Parsell, D.A.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes—Integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef]
- Beissinger, M.; Buchner, J. How chaperones fold proteins. Biol. Chem. 1998, 379, 245–259. [Google Scholar]
- Young, J.C.; Agashe, V.R.; Siegers, K.; Hartl, F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 2004, 5, 781–791. [Google Scholar] [CrossRef]
- Qiao, L.; Wu, J.X.; Qin, D.Z.; Liu, X.C.; Lu, Z.C.; Lv, L.Z.; Pan, Z.L.; Chen, H.; Li, G.W. Gene expression profiles of heat shock proteins 70 and 90 from Empoasca onukii (Hemiptera: Cicadellidae) in response to temperature stress. J. Insect Sci. 2015, 15, 49. [Google Scholar] [CrossRef]
- Sonoda, S.; Ashfaq, M.; Tsumuki, H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch. Insect Biochem. Physiol. 2006, 62, 80–90. [Google Scholar] [CrossRef]
- Feng, H.; Wang, L.; Liu, Y.; He, L.; Li, M.; Lu, W.; Xue, C. Molecular characterization and expression of a heat shock protein gene (HSP90) from the carmine spider mite, Tetranychus cinnabarinus (Boisduval). J. Insect Sci. 2010, 10, 112. [Google Scholar] [CrossRef]
- Jiang, X.; Zhai, H.; Wang, L.; Luo, L.; Sappington, T.W.; Zhang, L. Cloning of the heat shock protein 90 and 70 genes from the beet armyworm, Spodoptera exigua, and expression characteristics in relation to thermal stress and development. Cell Stress Chaperones 2012, 17, 67–80. [Google Scholar] [CrossRef]
- Li, H.B.; Du, Y.Z. Molecular cloning and characterization of an Hsp90/70 organizing protein gene from Frankliniella occidentalis (Insecta: Thysanoptera, Thripidae). Gene 2013, 520, 148–155. [Google Scholar] [CrossRef]
- Wang, H.; Li, K.; Zhu, J.Y.; Fang, Q.; Ye, G.Y.; Wang, H.; Li, K.; Zhu, J.Y. Cloning and expression pattern of heat shock protein genes from the endoparasitoid wasp, Pteromalus puparum in response to environmental stresses. Arch. Insect Biochem. Physiol. 2012, 79, 247–263. [Google Scholar] [CrossRef]
- Sonoda, S.; Tsumuki, H. Induction of heat shock protein genes by chlorfenapyr in cultured cells of the cabbage armyworm, Mamestra brassicae. Pestic. Biochem. Physiol. 2007, 89, 185–189. [Google Scholar] [CrossRef]
- Shu, Y.; Du, Y.; Wang, J. Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kayukawa, T.; Monteiro, A.; Ishikawa, Y. The expression of the HSP90 gene in response to winter and summer diapauses and thermal-stress in the onion maggot, Delia antiqua. Insect Mol. Biol. 2010, 14, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Li, A.; Yocum, G.D.; Robich, R.M.; Hayward, S.A.; Denlinger, D.L. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 2007, 104, 11130–11137. [Google Scholar] [CrossRef]
- Gkouvitsas, T.; Kontogiannatos, D.; Kourti, A. Cognate Hsp70 gene is induced during deep larval diapause in the moth Sesamia nonagrioides. Insect Mol. Biol. 2009, 18, 253–264. [Google Scholar] [CrossRef]
- Zhang, Q.; Denlinger, D.L. Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J. Insect Physiol. 2010, 56, 138–150. [Google Scholar] [CrossRef]
- Jones, C.; Anderson, S.; Singha, U.K.; Chaudhuri, M. Protein phosphatase 5 is required for Hsp90 function during proteotoxic stresses in Trypanos. Brucei. Parasitol. Res. 2008, 102, 835–844. [Google Scholar] [CrossRef]
- Csermely, P.; Schnaider, T.; Soti, C.; Prohaszka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genom. 2006, 7, 156. [Google Scholar]
- Boher, F.; Trefault, N.; Piulachs, M.D. Biogeographic origin and thermal acclimation interact to determine survival and hsp90 expression in Drosophila species submitted to thermal stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 391–396. [Google Scholar] [CrossRef]
- Colinet, H.; Lee, S.F.; Hoffmann, A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J. 2010, 277, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Fukumoto, K.; Izumi, Y.; Yoshida, H.; Tsumuki, H. Cloning of heat shock protein genes (hsp90 and hsc70) and their expression during larval diapause and cold tolerance acquisition in the rice stem borer, Chilo suppressalis Walker. Arch. Insect Biochem. Physiol. 2006, 63, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Dersh, D.; Argon, Y. GRP94 in ER quality control and stress responses. Semin. Cell Dev. Biol. 2010, 21, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Eletto, D.; Argon, Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta. 2012, 1823, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, B.; Dai, J.; Srivastava, P.K.; Zammit, D.J.; Lefrancois, L.; Li, Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 2007, 26, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.Z.; Zhou, Y.; Fang, D.Q.; Chen, S.L. Chinese Cossidae; Beijing Tianze Press: Beijing, China, 1990. [Google Scholar]
- Yakovlev, R.V. A revision of carpenter moths of the genus Holcocerus Staudinger, 1884(s. l.). Eversmannia 2006, 1, 1–104. [Google Scholar]
- Zong, S.X.; Jia, F.Y.; Luo, Y.Q.; Zhi-Chun, X.U.; Zhang, L.S. Harm characteristics and population dynamics of Holcocerus hippophaecolus. J. Beijing For. Univ. 2005, 27, 70–74. [Google Scholar]
- Zong, S.X. Studies on the Bio-ecological Characteristics of Seabuckthorn Carpenter Moth: Holcocerus hippophaecolus (Lepidoptera: Cossidae). Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2006. [Google Scholar]
- Li, W.B.; Cui, M.M.; Hu, P.; Tao, J.; Zong, S.X. Cloning and Expression Analysis of the Heat-Shock Protein 70 Gene in Eogystia hippophaecolus (Lepidoptera: Cossidae). Ann. Entomol. Soc. Am. 2017, 110, 528–535. [Google Scholar]
- Cui, M.; Hu, P.; Wang, T.; Tao, J.; Zong, S. Differential transcriptome analysis reveals genes related to cold tolerance in seabuckthorn carpenter moth, Eogystia hippophaecolus. PLoS ONE 2017, 12, e0187105. [Google Scholar] [CrossRef]
- Schmidt, H.A.; Strimmer, K.; Vingron, M.; Haeseler, A.V. TREE-PUZZLE: Maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 2002, 18, 502–504. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, S. Primer Premier 5. Biotech. Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Hu, P.; Tao, J.; Cui, M.; Gao, C.; Lu, P.; Luo, Y. Antennal transcriptome analysis and expression profiles of odorant binding proteins in Eogystia hippophaecolus (Lepidoptera: Cossidae). BMC Genom. 2016, 17, 651. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Gupta, R.S. Phylogenetic analysis of the 90 kDa heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol. 1995, 12, 1063–1073. [Google Scholar]
- Ramya, T.N.; Surolia, N.; Surolia, A. 15-Deoxyspergualin modulates Plasmodium falciparum heat shock protein function. Biochem. Biophys. Res. Commun. 2006, 348, 585–592. [Google Scholar] [CrossRef]
- Pearl, L.H.; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 2006, 75, 271–294. [Google Scholar] [CrossRef]
- Daugaard, M.; Rohde, M.; Jaattela, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Wu, G.X.; Ye, G.Y.; Hu, C. Heat shock protein genes (hsp20, hsp75 and hsp90) from Pieris rapae: Molecular cloning and transcription in response to parasitization by Pteromalus puparum. Insect Sci. 2013, 20, 183–193. [Google Scholar] [CrossRef]
- Cheng, W.; Li, D.; Wang, Y.; Liu, Y.; Zhu-Salzman, K. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis Mosellana. J. Insect Physiol. 2016, 95, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Denlinger, D.L. Heat-shock protein 90 is down-regulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Insect Mol. Biol. 2000, 9, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Sømme, L. Cold Hardiness in Terrestrial Arthropods. Eur. J. Entomol 1999, 96, 1–10. [Google Scholar]
- Tian, B.; Xu, L.; Tao, W.; Zong, S. Comparison of the supercooling capacity in three species of Cossidae overwintering larvae. Plant Prot. 2017, 43, 113–118. [Google Scholar]
- Bashir, S.; Bashir, H.; Shuja Qadri, S. Heat Shock and the Heat Shock Proteins: An Overview. Int. J. Med. Sci. Public Health 2013, 2, 489–494. [Google Scholar]
- Yang, J.; Baloch, N.A.; Dong, F.; University, N.A. cDNA Cloning and Induction of Heat Shock Protein Hsp90 from Myth. Sep. Chin. J. Biol. Control 2017, 33, 623–630. [Google Scholar]
- Zhang, Y.; Gu, S.; Li, C.; Sang, M.; Wu, W.; Yun, X.; Hu, X.; Li, B. Identification and characterization of novel ER-based hsp90 gene in the red flour beetle, Tribolium castaneum. Cell Stress Chaperones 2014, 19, 623–633. [Google Scholar] [CrossRef]
- Silbermann, R.; Tatar, M. Reproductive Costs of Heat Shock Protein in Transgenic Drosophila Melanogaster. Evolution 2000, 54, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).