A Conceptual Framework for the Spruce Budworm Early Intervention Strategy: Can Outbreaks be Stopped?
Abstract
:1. Introduction
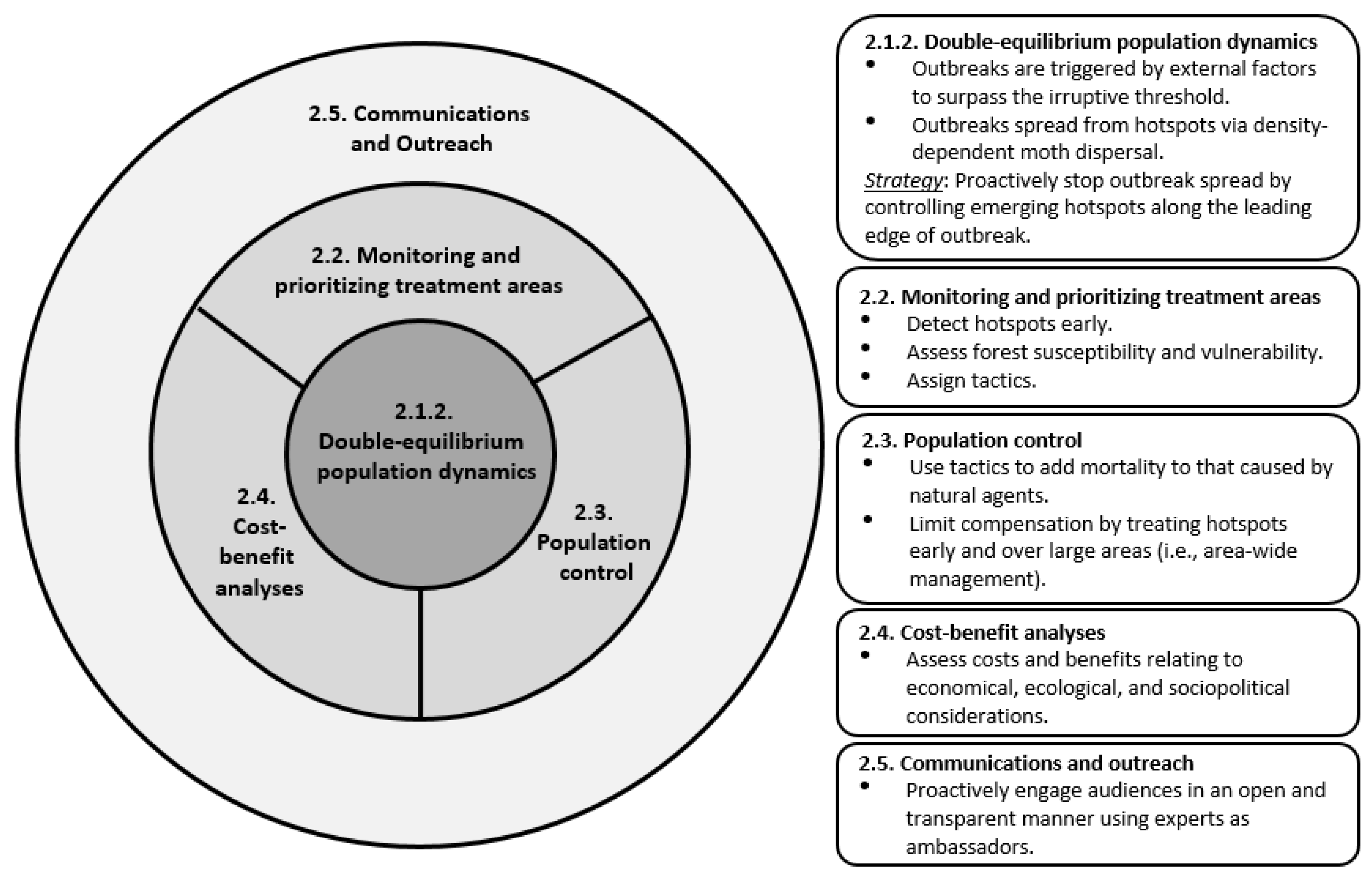
2. Conceptual Framework
2.1. Population Dynamics
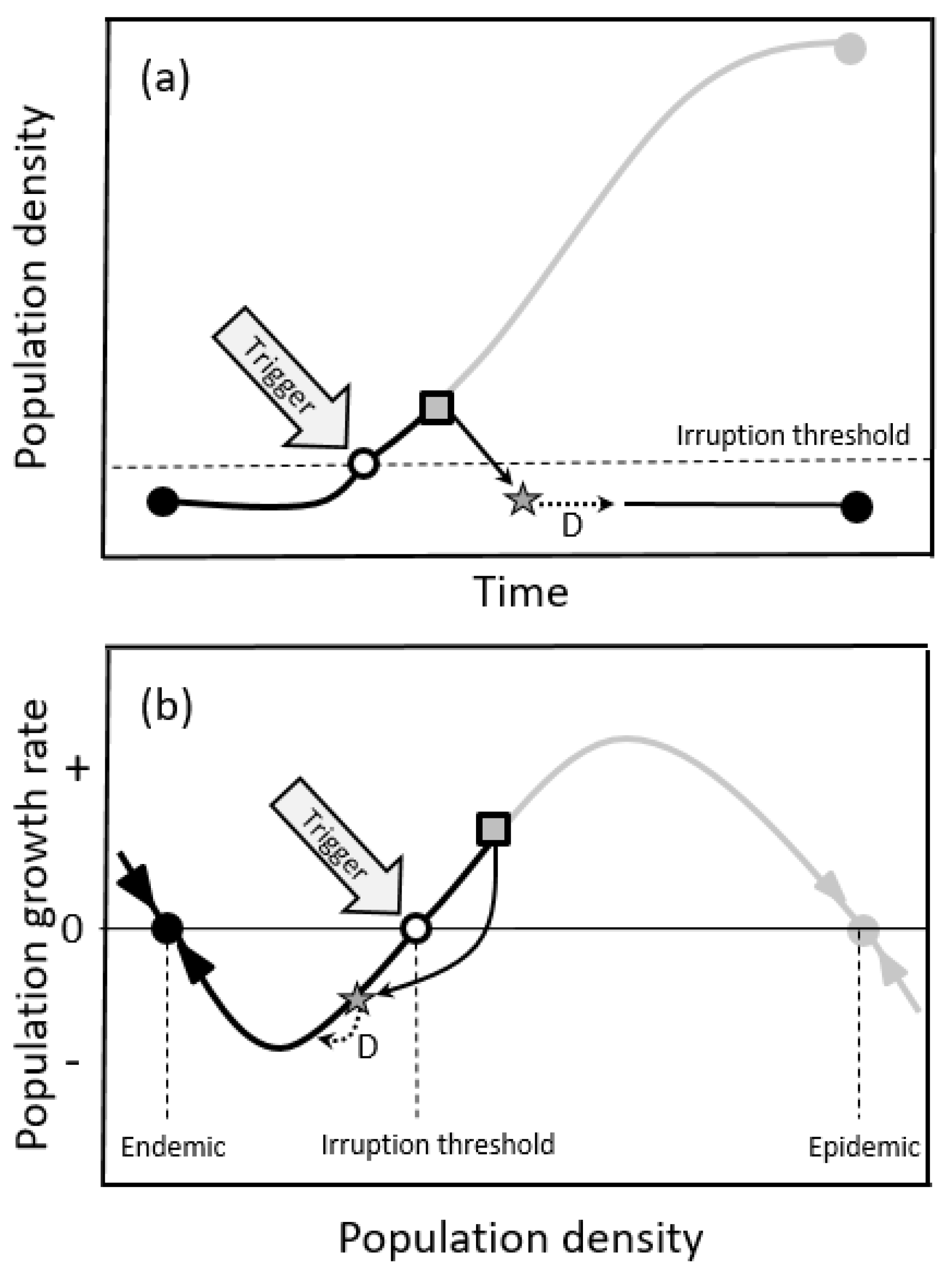
2.1.1. Oscillatory Dynamics
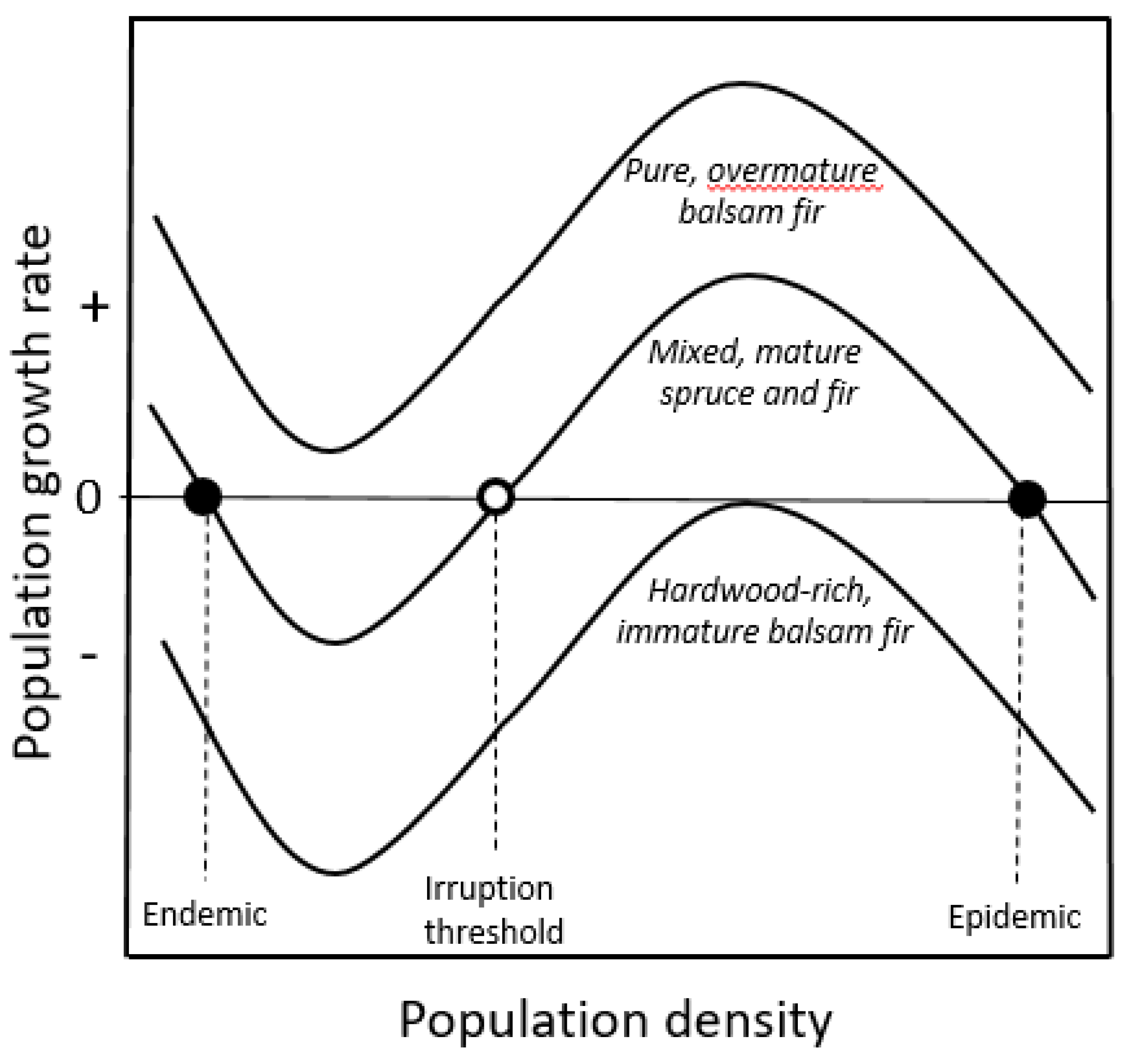
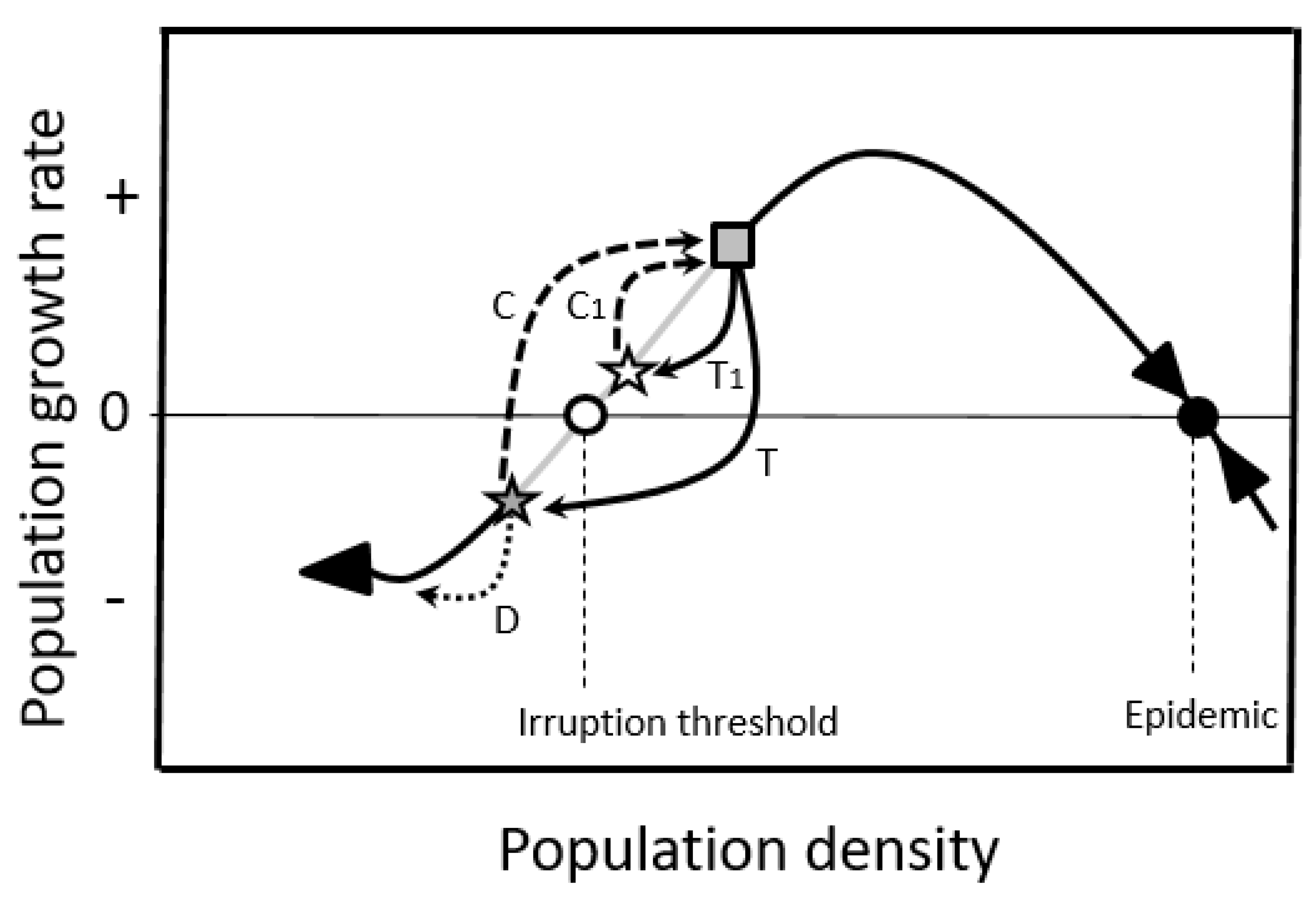
2.1.2. Double-Equilibrium Dynamics and EIS
2.2. Monitoring and Prioritizing Treatment Areas
2.2.1. Detecting Hotspots
2.2.2. Assessing Forest Susceptibility
2.2.3. Selecting and Assigning Control Tactics
2.3. Population Control
2.3.1. Enhance Additive Mortality
2.3.2. Limit Compensation
2.4. Costs and Benefits
2.4.1. Economic
2.4.2. Ecological
2.4.3. Sociopolitical
2.5. Communication and Outreach
2.5.1. Communications Strategy
2.5.2. Key Messages
2.5.3. Outreach Tactics
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Risser, P.G. Landscape ecology: State of the art. In Landscape Heterogeneity and Disturbance; Turner, M.G., Ed.; Springer: New York, NY, USA, 1987; pp. 3–14. [Google Scholar]
- Sturtevant, B.R.; Cooke, B.J.; Kneeshaw, D.D.; MacLean, D.A. Modelling insect disturbance across forested landscapes: Insights from spruce budworm. In Simulation Modelling of Forest Landscape Disturbances; Perera, A.H., Sturtevant, B.R., Buse, L.J., Eds.; Springer International Publishing: Geneva, Switzerland, 2015; pp. 93–134. [Google Scholar]
- Pureswaran, D.; Johns, R.C.; Heard, S.B.; Quiring, D. Paradigms in eastern spruce budworm (Lepidoptera: Tortricidae) population ecology: A century of debate. Environ. Entomol. 2016, 45, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Webb, F.E. Studies of Aerial Spraying Against the Spruce Budworm in New Brunswick: A Preliminary Report on the 1952 Upsalquitch Project; Interim Report; Forest Biology Laboratory: Fredericton, NB, Canada, 1952; pp. 1–9. [Google Scholar]
- Kettela, E.G. Aerial spraying for protection of forests infested by spruce budworm. For. Chron. 1975, 51, 141–142. [Google Scholar] [CrossRef]
- MacDonald, D.R.; Webb, F.E. Insecticides and the spruce budworm. Mem. Entomol. Soc. Can. 1963, 95, 288–310. [Google Scholar] [CrossRef]
- Randall, A.P. Evidence of DDT resistance in populations of spruce budworm, Choristoneura fumiferana (Clem.), from DDT-sprayed areas of New Brunswick. Can. Entomol. 1965, 97, 1281–1293. [Google Scholar] [CrossRef]
- Van Frankenhuyzen, K. Development and current status of Bacillus thuringiensis for control of defoliating forest insects. In Forest Insect Pests in Canada; Armstrong, J.A., Ives, W.G.H., Eds.; Canadian Forest Service: Ottawa, ON, Canada, 1995; pp. 315–325. [Google Scholar]
- Durkin, P. Control/Eradication Agents for the Gypsy Moth: Human Health and Ecological Risk Assessment for Bacillus thuringiensis var. Kurstaki (B.T.K.); Syracuse Environment Research Associates, Inc.: Fayetteville, NY, USA, 2004; p. 152. [Google Scholar]
- Durkin, P.; Klotzbach, J. Control/Eradication Agents for the Gypsy Moth - Human Health and Ecological Risk Assessment for Tebufenozide (Mimic); Syracuse Environment Research Associates, Inc.: Fayetteville, NY, USA, 2004; p. 161. [Google Scholar]
- Fuentealba, A.; Dupont, A.; Hébert, C.; Berthiaume, R.; Quezada-García, R.; Bauce, É. Comparing the efficacy of various aerial spraying scenarios using Bacillus thuringiensis to protect trees from spruce budworm defoliation. For. Ecol. Manag. 2019, 432, 1013–1021. [Google Scholar] [CrossRef]
- Ministère des Forêts, de la Faune et des Parcs. Aires Infestées par la Tordeuse des Bourgeons de L’épinette au Québec en 2015—Version 1.0; Gouvernement du Québec, Direction de la Protection des Forêts: Quebec, QC, Canada, 2015.
- MacLean, D.A.; Amirault, P.; Amos-Binks, L.; Carleton, D.; Hennigar, C.; Johns, R.C.; Régnière, J. Positive results of an Early Intervention Strategy to suppress a spruce budworm outbreak after five years. Forests 2019, 10, 448. [Google Scholar] [CrossRef]
- Sharov, A.A.; Leonard, D.; Liebhold, A.M.; Roberts, E.A.; Dickerson, W. “Slow the spread”: A national program to contain the gypsy moth. J. For. 2002, 100, 30–36. [Google Scholar]
- Bomford, M.; O’Brien, P. Eradication or control for vertebrate pests? Wildl. Soc. Bull. 1995, 23, 249–255. [Google Scholar]
- Sandercock, B.K.; Nilsen, E.B.; Brøseth, H.; Pedersen, H.C. Is hunting mortality additive or compensatory to natural mortality? Effects of experimental harvest on the survival and cause-specific mortality of willow ptarmigan. J. Anim. Ecol. 2011, 80, 244–258. [Google Scholar] [CrossRef]
- Royama, T. Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol. Monogr. 1984, 54, 429–462. [Google Scholar] [CrossRef]
- Royama, T.; Eveleigh, E.S.; Morin, J.R.B.; Pollock, S.J.; McCarthy, P.C.; McDougall, G.A.; Lucarotti, C.J. Mechanisms underlying spruce budworm outbreak processes as elucidated by a 14-year study in New Brunswick, Canada. Ecol. Monogr. 2017, 87, 600–631. [Google Scholar] [CrossRef]
- Régnière, J.; Lysyk, T.J. Population dynamics of the spruce budworm, Choristoneura fumiferana. In Forest Insects Pests in Canada; Armstrong, J.A., Ives, W.G.H., Eds.; Natural Resources Canada, Canadian Forest Service Publication: Ottawa, ON, Canada, 1995; pp. 95–105. [Google Scholar]
- Williams, D.W.; Liebhold, A.M. Spatial synchrony of spruce budworm outbreaks in eastern North America. Ecology 2000, 81, 2753–2766. [Google Scholar] [CrossRef]
- Royama, T.; MacKinnon, W.E.; Kettela, E.G.; Carter, N.E.; Hartling, L.K. Analysis of spruce budworm outbreak cycles in New Brunswick, Canada, since 1952. Ecology 2005, 86, 1212–1224. [Google Scholar] [CrossRef]
- Greenbank, D.O. The role of climate and dispersal in the initiation of outbreaks of the spruce budworm in New Brunswick. I. The role of climate. Can. J. Zool. 1956, 34, 453–476. [Google Scholar] [CrossRef]
- Boulanger, Y.; Fabry, F.; Kilambi, A.; Pureswaran, D.S.; Sturtevant, B.R.; Saint-Amant, R. The use of weather surveillance radar and high-resolution three dimensional weather data to monitor a spruce budworm mass exodus flight. Agric. Forest Meteorol. 2017, 234, 127–135. [Google Scholar] [CrossRef]
- Régnière, J.; Nealis, V.G. Density dependence of egg recruitment and moth dispersal in spruce budworms. Forests 2019, 10, 706. [Google Scholar] [CrossRef]
- Cooke, B.J.; Nealis, V.G.; Régnière, J. Insect defoliators as periodic disturbances in northern forest ecosystems. In Plant Disturbance Ecology: The Process and the Response; Johnson, E.A., Miyanishi, K., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2007; pp. 487–526. [Google Scholar]
- Morris, R.F. The dynamics of epidemic spruce budworm populations. Mem. Entomol. Soc. Can. 1963, 95, 7–12. [Google Scholar] [CrossRef]
- Ludwig, D.; Jones, D.D.; Holling, C.S. Qualitative analysis of insect outbreak systems: The spruce budworm and forest. J. Anim. Ecol. 1978, 47, 315–332. [Google Scholar] [CrossRef]
- Régnière, J.; Cooke, B.J.; Béchard, A.; Dupont, A.; Therrien, P. Dynamics and management of rising outbreak spruce budworm populations. Forests 2019, 10, 748. [Google Scholar] [CrossRef]
- Bouchard, M.; Régnière, J.; Therrien, P. Bottom-up factors contribute to large-scale synchrony in spruce budworm populations. Can. J. For. Res. 2018, 48, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Régnière, J.; Delisle, J.; Pureswaran, D.; Trudel, R. Mate-finding Allee effect in spruce budworm population dynamics. Entomol. Exp. Appl. 2013, 146, 112–122. [Google Scholar] [CrossRef]
- Régnière, J.; Nealis, V.G. Ecological mechanisms of population change during outbreaks of the spruce budworm. Ecol. Entomol. 2007, 32, 461–477. [Google Scholar] [CrossRef]
- Liebhold, A.; Bascompte, J. The Allee effect, stochastic dynamics and the eradication of alien species. Ecol. Lett. 2003, 6, 133–140. [Google Scholar] [CrossRef]
- Cappuccino, N.; Lavertu, D.; Bergeron, Y.; Régnière, J. Spruce budworm impact, abundance and parasitism rate in a patchy landscape. Oecologia 1998, 114, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Eveleigh, E.S.; McCann, K.S.; McCarthy, P.C.; Pollock, S.J.; Lucarotti, C.J.; Morin, B.; McDougall, G.A.; Strongman, D.B.; Huber, J.T.; Umbanhowar, J.; et al. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 2007, 104, 16976–16981. [Google Scholar] [CrossRef] [Green Version]
- Marrec, R.; Pontbriand-Paré, O.; Legault, S.; James, P.M.A. Spatiotemporal variation in drivers of parasitoid metacommunity structure in continuous forest landscapes. Ecosphere 2018, 9, e02075. [Google Scholar] [CrossRef]
- Legault, S.; James, P.M.A. Parasitism rates of spruce budworm larvae: Testing the enemy hypothesis along a gradient of forest diversity measured at different spatial scales. Environ. Entomol. 2018, 47, 1083–1095. [Google Scholar] [CrossRef]
- Miller, C.A.; Kettela, E.G.; McDougall, G.A. A Sampling Technique for Overwintering Spruce Budworm and Its Applicability to Population Surveys; Canadian Forestry Service: Fredericton, NB, Canada, 1971; p. 11.
- Sanders, C.J. Monitoring spruce budworm population density with sex pheromone traps. Can. Entomol. 1988, 120, 175–183. [Google Scholar] [CrossRef]
- MacLean, D.A.; MacKinnon, W.E. Accuracy of aerial sketch-mapping of spruce budworm defoliation in New Brunswick. Can. J. For. Res. 1996, 26, 2099–2108. [Google Scholar] [CrossRef]
- Régnière, J.; Delisle, J.; Sturtevant, B.; Garcia, M.; St-Amant, R. Modeling migratory flight in the spruce budworm: Temperature Constraints. Forests 2019, 10, 802. [Google Scholar] [CrossRef]
- Rahimzadeh-Bajgiran, P.; Weiskittel, A.; Kneeshaw, D.; MacLean, D.A. Detection of annual spruce budworm defoliation and severity classification using Landsat imagery. Forests 2018, 9, 357. [Google Scholar] [CrossRef]
- Goodbody, T.R.H.; Coops, N.C.; Hermosilla, T.; Tompalski, P.; McCartney, G.; MacLean, D.A. Digital aerial photogrammetry for assessing cumulative spruce budworm defoliation and enhancing forest inventories at a landscape-level. ISPRS J. Photogramm. Remote Sens. 2018, 142, 1–11. [Google Scholar] [CrossRef]
- MacLean, D.A. Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks: A review and discussion. For. Chron. 1980, 56, 213–221. [Google Scholar] [CrossRef]
- Hennigar, C.R.; MacLean, D.A.; Quiring, D.T.; Kershaw, J.A. Differences in spruce budworm defoliation among balsam fir and white, red, and black spruce. For. Sci. 2008, 54, 158–166. [Google Scholar]
- Bognounou, F.; De Grandpré, L.; Pureswaran, D.S.; Kneeshaw, D. Temporal variation in plant neighborhood effects on the defoliation of primary and secondary hosts by an insect pest. Ecosphere 2017, 8, e01759. [Google Scholar] [CrossRef]
- Su, Q.; Needham, T.D.; MacLean, D.A. The influence of hardwood content on balsam fir defoliation by spruce budworm. Can. J. For. Res. 1996, 26, 1620–1628. [Google Scholar] [CrossRef]
- Campbell, E.M.; MacLean, D.A.; Bergeron, Y. The severity of budworm-caused growth reductions in balsam fir/spruce stands varies with the hardwood content of surrounding forest landscapes. For. Sci. 2008, 54, 195–205. [Google Scholar]
- Zhang, B.; MacLean, D.A.; Johns, R.C.; Eveleigh, E.S. Effects of hardwood content on balsam fir defoliation during the building phase of a spruce budworm outbreak. Forests 2018, 9, 530. [Google Scholar] [CrossRef]
- Robert, L.E.; Kneeshaw, D.; Sturtevant, B.R. Effects of forest management legacies on spruce budworm (Choristoneura fumiferana) outbreaks. Can. J. For. Res. 2012, 42, 463–475. [Google Scholar] [CrossRef]
- Robert, L.E.; Sturtevant, B.R.; Cooke, B.J.; James, P.M.; Fortin, M.J.; Townsend, P.A.; Wolter, P.T.; Kneeshaw, D. Landscape host abundance and configuration regulate periodic outbreak behavior in spruce budworm Choristoneura fumiferana. Ecography 2018, 41, 1556–1571. [Google Scholar] [CrossRef]
- Bouchard, M.; Auger, I. Influence of environmental factors and spatio-temporal covariates during the initial development of a spruce budworm outbreak. Landsc. Ecol. 2014, 29, 111–126. [Google Scholar] [CrossRef]
- Bauce, É.; Carisey, N.; Dupont, A.; van Frankenhuyzen, K. Bacillus thuringiensis subsp. kurstaki aerial Spray prescriptions for balsam fir stand protection against spruce budworm (Lepidoptera: Tortricidae). J. Econ. Entomol. 2004, 97, 97–1624. [Google Scholar] [CrossRef] [PubMed]
- van Frankenhuyzen, K.; Régnière, J. Multiple effects of tebufenozide on the survival and performance of the spruce budworm (Lepidoptera: Tortricidae). Can. Entomol. 2017, 149, 227–240. [Google Scholar] [CrossRef]
- Régnière, J.; Delisle, J.; Dupont, A.; Trudel, R. The impact of moth migration on apparent fecundity overwhelms mating disruption as a method to manage spruce budworm populations. Forests 2019, 10, 775. [Google Scholar] [CrossRef]
- Rhainds, M.; Kettela, E.G.; Silk, P.J. Thirty-five years of pheromone-based mating disruption studies with Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae). Can. Entomol. 2012, 144, 379–395. [Google Scholar] [CrossRef]
- Crawford, H.S.; Titterington, R.W.; Jennings, D.T. Bird predation and spruce budworm populations. J. For. 1983, 81, 433–478. [Google Scholar]
- Jennings, D.T.; Houseweart, M.W. Predation by eumenid wasps (Hymenoptera: Eumenidae) on spruce budworm (Lepidoptera: Tortricidae) and other lepidopterous larvae in spruce-fir forests of Maine. Ann. Entomol. Soc. Am. 1984, 77, 39–45. [Google Scholar] [CrossRef]
- Waage, J.K.; Hassell, M.P.; Godfray, H.C.J. The dynamics of pest-parasitoid-insecticide interactions. J. Appl. Ecol. 1985, 22, 825–838. [Google Scholar] [CrossRef]
- Nealis, V.; van Frankenhuyzen, K. Interactions between Bacillus thuringiensis Berliner and Apanteles fumiferanae Vier. (Hymenoptera: Braconidae), a parasitoid of the spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can. Entomol. 1990, 122, 585–594. [Google Scholar] [CrossRef]
- Nealis, V.; van Frankenhuyzen, K.; Cadogan, B.L. Conservation of spruce budworm parasitoids following application of Bacillus thuringiensis var. kurstaki Berliner. Can. Entomol. 1992, 124, 1085–1092. [Google Scholar] [CrossRef]
- Cooke, B.J.; Régnière, J. An objectoriented, process-based stochastic simulation model of Bacillus thuringiensis efficacy against spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Int. J. Pest Manag. 1996, 42, 291–306. [Google Scholar] [CrossRef]
- Quiring, D.; Flaherty, L.; Adams, G.; McCartney, A.; Miller, J.D.; Edwards, S. An endophytic fungus interacts with crown level and larval density to reduce the survival of eastern spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae), on white spruce (Picea glauca). Can. J. For. Res. 2019, 49, 221–227. [Google Scholar] [CrossRef]
- Williams, M.; Eveleigh, E.; Forbes, G.; Lamb, R.; Roscoe, L.; Silk, P. Evidence of a direct chemical plant defense role for maltol against spruce budworm. Entomol. Exp. Appl. 2019, 167, 755–762. [Google Scholar] [CrossRef]
- Larroque, J.; Legault, S.; Johns, R.; Lumley, L.; Cusson, M.; Renaut, S.; Levesque, R.C.; James, P.M.A. Temporal variation in spatial genetic structure during population outbreaks: Distinguishing among different potential drivers of spatial synchrony. Evol. Appl. 2019. [Google Scholar] [CrossRef]
- Dobesberger, E.J.; Lim, K.P.; Raske, A.G. Spruce budworm (Lepidoptera: Tortricidae) moth flight from New Brunswick to Newfoundland. Can. Entomol. 1983, 115, 1641–1645. [Google Scholar] [CrossRef]
- Elliott, N.C.; Onstad, D.W.; Brewer, M.J. History and ecological basis for areawide pest management. In Area-wide Pest Management: Theory and Implementation; Koul, O., Cuperus, G.W., Elliott, N., Eds.; CABI International: Oxford, UK, 2008; pp. 15–33. [Google Scholar]
- MacLean, D.A.; Ostaff, D.P. Patterns of balsam fir mortality caused by an uncontrolled spruce budworm outbreak. Can. J. Forest Res. 1989, 19, 1087–1095. [Google Scholar] [CrossRef]
- Hennigar, C.R.; Erdle, T.A.; Gullison, J.J.; MacLean, D.A. Re-examining wood supply in light of future spruce budworm outbreaks: A case study in New Brunswick. For. Chron. 2013, 89, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.Y.; Lantz, V.A.; Hennigar, C.R.; MacLean, D.A. Benefit-cost analysis of spruce budworm (Choristoneura fumiferana Clem.) control: Incorporating market and non-market values. J. Environ. Manag. 2012, 93, 104–112. [Google Scholar] [CrossRef]
- Chang, W.Y.; Lantz, V.A.; Hennigar, C.R.; MacLean, D.A. Economic impacts of spruce budworm (Choristoneura fumiferana Clem.) outbreaks and control in New Brunswick, Canada. Can. J. For. Res. 2012, 42, 490–505. [Google Scholar] [CrossRef]
- Cathro, J.; Mulkey, S.; Bradley, T. A bird’s eye view of small tenure holdings in British Columbia. J. Ecosyst. Manag. 2007, 8, 58–66. [Google Scholar]
- Liu, E.Y.; Lantz, V.; MacLean, D.A.; Hennigar, C. Economics of early intervention to suppress a potential spruce budworm outbreak on Crown land in New Brunswick, Canada. Forests 2019, 10, 481. [Google Scholar] [CrossRef]
- Belyea, R.M. Death and deterioration of balsam fir weakened by spruce budworm defoliation in Ontario. Can. Entomol. 1952, 84, 325–335. [Google Scholar] [CrossRef]
- Ostaff, D.P.; MacLean, D.A. Spruce budworm populations, defoliation, and changes in stand condition during an uncontrolled spruce budworm outbreak on Cape Breton Island, Nova Scotia. Can. J. For. Res. 1989, 19, 1077–1086. [Google Scholar] [CrossRef]
- Stillwell, M.A. Pathological aspects of severe spruce budworm attack. For. Sci. 1956, 2, 174–180. [Google Scholar]
- Ostaff, D. D. A Wood Quality Study of Dead and Dying Balsam Fir—The Incidence of Armillaria Root Rot; Technical Note 82; Environment Canada, Canadian Forestry Service, Maritimes Forest Research Centre: Fredericton, NB, Canada, 1983. [Google Scholar]
- Stocks, B.J. Fire potential in the spruce budworm-damaged forests of Ontario. For. Chron. 1987, 63, 8–14. [Google Scholar] [CrossRef]
- James, P.M.A.; Fortin, M.J.; Sturtevant, B.R.; Fall, A.; Kneeshaw, D. Modelling spatial interactions among fire, spruce budworm, and logging in the boreal forest. Ecosystems 2011, 14, 60–75. [Google Scholar] [CrossRef]
- James, P.M.A.; Robert, L.E.; Wotton, B.M.; Martell, D.L.; Fleming, R.A. Lagged cumulative spruce budworm defoliation affects the risk of fire ignition in Ontario, Canada. Ecol. Appl. 2017, 27, 532–544. [Google Scholar] [CrossRef]
- QMFFP: Québec Ministère des Forêts, de la Faune et des Parcs. Aires Infestées par la Tordeuse des Bourgeons de L’épinette au Québec en 2018; Gouvernement du Québec, Direction de la Protection des Forêts: Québec City, QC, Canada, 2018; p. 20. [Google Scholar]
- Carson, R. Silent Spring; Houghton Mifflin: New York, NY, USA, 1962. [Google Scholar]
- May, E. Budworm Battles; Four East Publications Ltd.: Halifax, NS, Canada, 1982. [Google Scholar]
- Armstrong, J.A.; Cook, C.A. Aerial Spray Applications on Canadian Forests: 1945–1990; Information Report; Forestry Canada: Ottawa, ON, Canada, 1993.
- Holmes, S.B.; MacQuarrie, C.J.K. Chemical control in forest pest management. Can. Entomol. 2016, 148, S270–S295. [Google Scholar] [CrossRef] [Green Version]
- Soin, T.; Smagghe, G. Endocrine disruption in aquatic insects: A review. Ecotoxicology 2007, 16, 83–93. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Gunn, J.M.; Thompson, D.G.; Pollard, H.G.; Faber, M.J. Zooplankton community responses to a novel forest insecticide, tebufenozide (RH-5992), in littoral lake enclosures. Can. J. Fish. Aquat. Sci. 1998, 55, 639–648. [Google Scholar] [CrossRef]
- Tassou, K.T.; Schulz, R. Low filed-relevant tebufenozide concentrations affect reproductions in Chironomus riparius (Diptera: Chironomidae) in a long-term toxicity test. Environ. Sci. Pollut. Res. 2013, 20, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Martel, V.; Johns, R.C.; Eveleigh, E.; McCann, K.; Pureswaran, D.; Sylvain, Z.; Morrison, A.; Morin, B.; Owens, E.; Hébert, C. Landscape level impacts of EIS on SBW, other herbivores and associated natural enemies (ACOA RD100 2.2.2). In Proceedings of the SERG-I Workshop, Fredericton, NB, Canada, 7–8 February 2017; pp. 112–118. [Google Scholar]
- Schowalter, T.D. Insect herbivore effects on forest ecosystem services. J. Sustain. For. 2012, 31, 518–536. [Google Scholar] [CrossRef]
- Postel, S.L.; Thompson, B.H., Jr. Watershed protection: Capturing the benefits of nature’s water supply services. Nat. Resour. Forum 2005, 29, 98–108. [Google Scholar] [CrossRef]
- Dhar, A.; Parrott, L.; Heckbert, S. Consequences of mountain pine beetle outbreak on forest ecosystem services in western Canada. Can. J. For. Res. 2016, 46, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Redding, T.; Winkler, R.; Teti, P.; Spittlehouse, D.; Boon, S.; Rex, J.; Dubé, S.; Moore, R.D.; Wei, A.; Carver, M.; et al. Mountain pine beetle and watershed hydrology. BCJ. Ecosys. Manag. 2008, 9, 33–50. [Google Scholar]
- Venier, L.A.; Holmes, S.B. A review of the interaction between forest birds and eastern spruce budworm. Environ. Rev. 2010, 18, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Crewe, J.; Johnstone, J.F. Plant Use in Vuntut Gwitchin Territory; Vuntut Gwitchin First Nation: Old Crow, YT, Canada, 2008; pp. 1–49. [Google Scholar]
- Kanowski, P.J.; Williams, K.J.H. The reality of imagination: Integrating the material and cultural values of old forests. For. Ecol. Manag. 2009, 258, 341–346. [Google Scholar] [CrossRef]
- Nowak, D.J.; Hirabayashi, S.; Bodine, A.; Greenfield, E. Tree and forest effects on air quality and human health in the United States. Environ. Pollut. 2014, 193, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.Y.; Lantz, V.A.; MacLean, D.A. Public attitudes about forest pest outbreaks and control: Case studies in two Canadian provinces. For. Ecol. Manag. 2009, 257, 1333–1343. [Google Scholar] [CrossRef]
- Two-Eyed Seeing. Available online: http://www.integrativescience.ca/Principles/TwoEyedSeeing/ (accessed on 12 August 2019).
- Miller, A. Conventional problem solving. In Environmental Problem Solving: Psychosocial Barriers to Adaptive Change, 1st ed.; Alexander, D.E., Ed.; Springer: New York, NY, USA, 1999; pp. 82–123. [Google Scholar]
- Healthy Forest Partnership. Available online: http://www.healthyforestpartnership.ca (accessed on 12 August 2019).
- Simard, I.; Morin, H.; Lavoie, C. A millennial-scale reconstruction of spruce budworm abundance in Saguenay, Quebec, Canada. Holocene 2006, 16, 31–37. [Google Scholar] [CrossRef]
- Budworm Tracker Program. Available online: http://healthyforestpartnership.ca/budworm-tracker/about-the-program/ (accessed on 23 September 2019).
- Régnière, J.; St-Amant, R.; Duval, P. Predicting insect distributions under climate change from physiological responses: Spruce budworm as an example. Biol. Invasions 2012, 14, 1571–1586. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; De Grandpré, L.; Paré, D.; Taylor, A.; Barrette, M.; Morin, H.; Régnière, J.; Kneeshaw, D. Climate-induced changes in host tree–insect phenology may drive ecological state-shift in boreal forests. Ecology 2015, 96, 1480–1491. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Neau, M.; Marchand, M.; De Grandpré, L.; Kneeshaw, D. Phenological synchrony between eastern spruce budworm and its host trees increases with warmer temperatures in the boreal forest. Ecol. Evol. 2018, 9, 576–586. [Google Scholar]
- Schindler, D.W. Replication versus realism: The need for ecosystem-scale experiments. Ecosystems 1998, 1, 323–334. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johns, R.C.; Bowden, J.J.; Carleton, D.R.; Cooke, B.J.; Edwards, S.; Emilson, E.J.S.; James, P.M.A.; Kneeshaw, D.; MacLean, D.A.; Martel, V.; et al. A Conceptual Framework for the Spruce Budworm Early Intervention Strategy: Can Outbreaks be Stopped? Forests 2019, 10, 910. https://doi.org/10.3390/f10100910
Johns RC, Bowden JJ, Carleton DR, Cooke BJ, Edwards S, Emilson EJS, James PMA, Kneeshaw D, MacLean DA, Martel V, et al. A Conceptual Framework for the Spruce Budworm Early Intervention Strategy: Can Outbreaks be Stopped? Forests. 2019; 10(10):910. https://doi.org/10.3390/f10100910
Chicago/Turabian StyleJohns, Robert C., Joseph J. Bowden, Drew R. Carleton, Barry J. Cooke, Sara Edwards, Erik J. S. Emilson, Patrick M. A. James, Dan Kneeshaw, David A. MacLean, Véronique Martel, and et al. 2019. "A Conceptual Framework for the Spruce Budworm Early Intervention Strategy: Can Outbreaks be Stopped?" Forests 10, no. 10: 910. https://doi.org/10.3390/f10100910
APA StyleJohns, R. C., Bowden, J. J., Carleton, D. R., Cooke, B. J., Edwards, S., Emilson, E. J. S., James, P. M. A., Kneeshaw, D., MacLean, D. A., Martel, V., Moise, E. R. D., Mott, G. D., Norfolk, C. J., Owens, E., Pureswaran, D. S., Quiring, D. T., Régnière, J., Richard, B., & Stastny, M. (2019). A Conceptual Framework for the Spruce Budworm Early Intervention Strategy: Can Outbreaks be Stopped? Forests, 10(10), 910. https://doi.org/10.3390/f10100910




