Exploring the Sensitivity of Subtropical Stand Aboveground Productivity to Local and Regional Climate Signals in South China
Abstract
1. Introduction
2. Materials and Methods
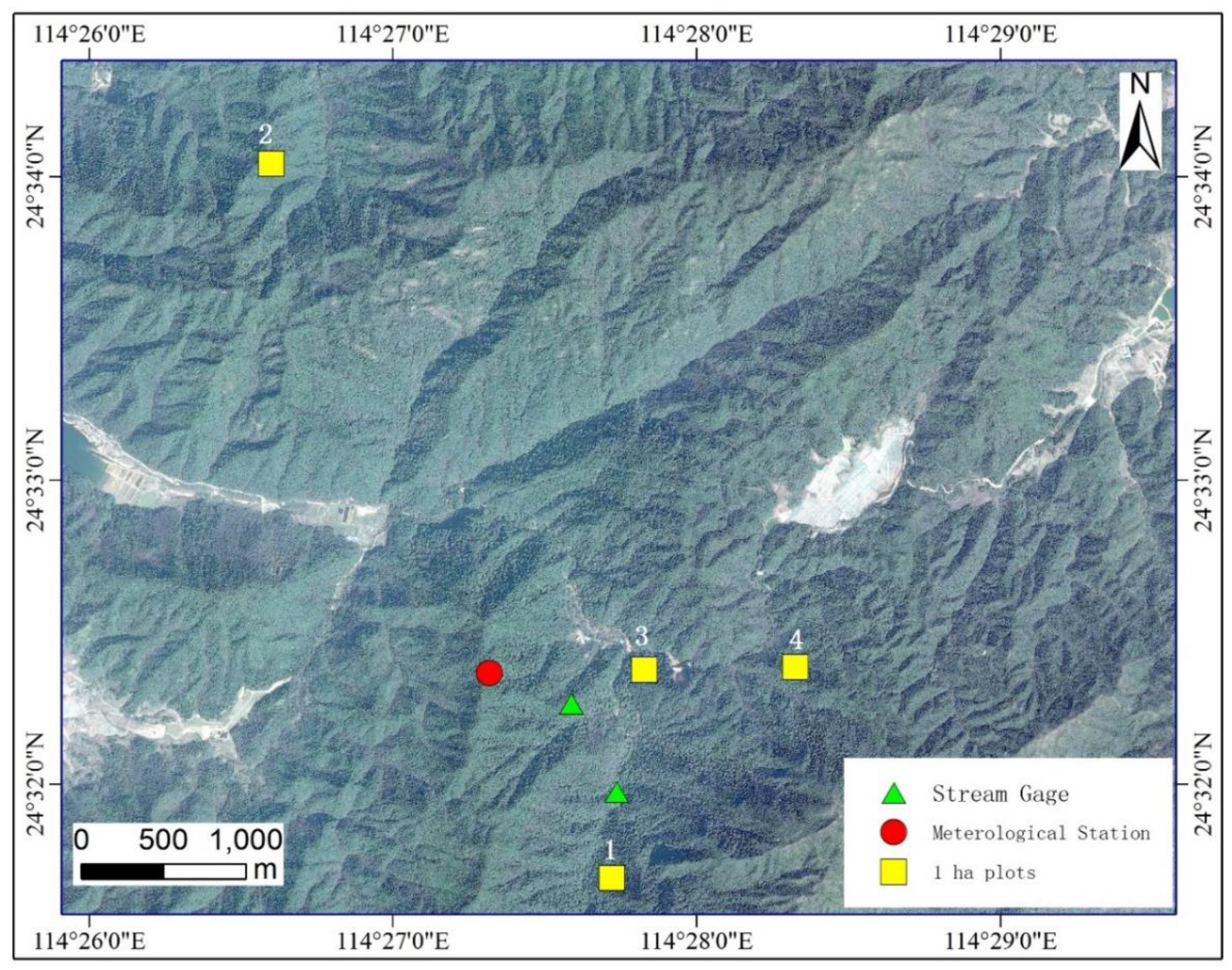
2.1. Research Site
2.2. Research Design
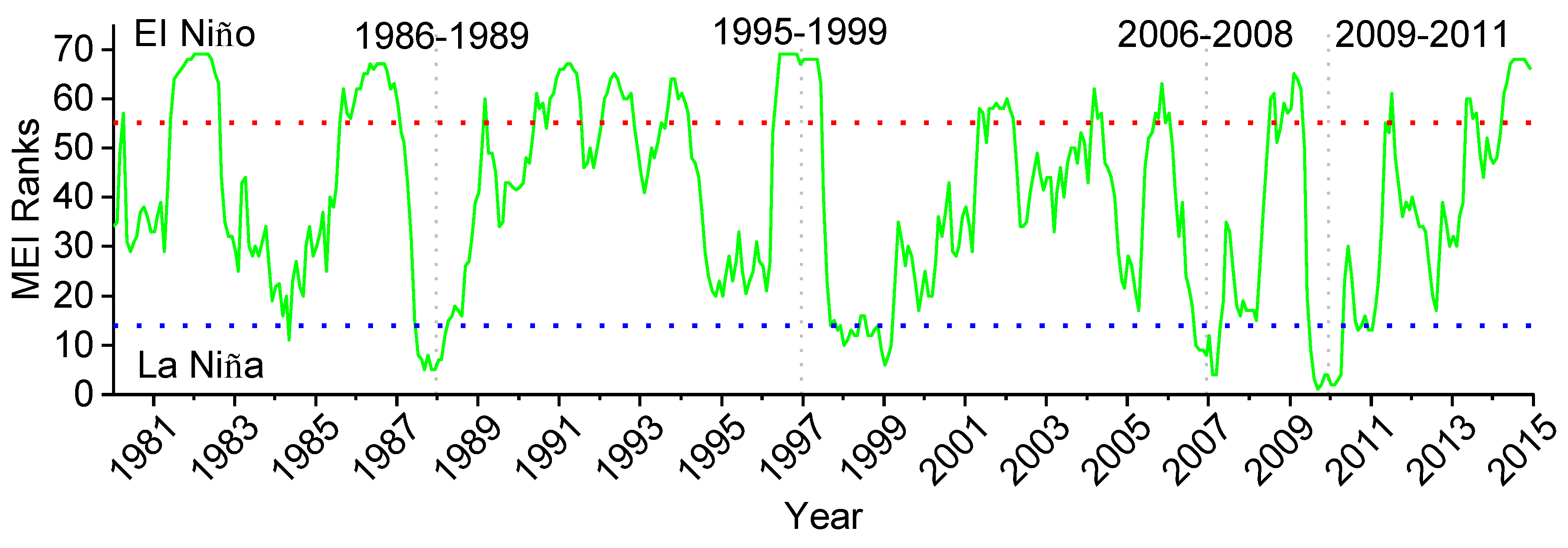
2.3. Multivariate ENSO Index
2.4. Climate-Productivity Analysis
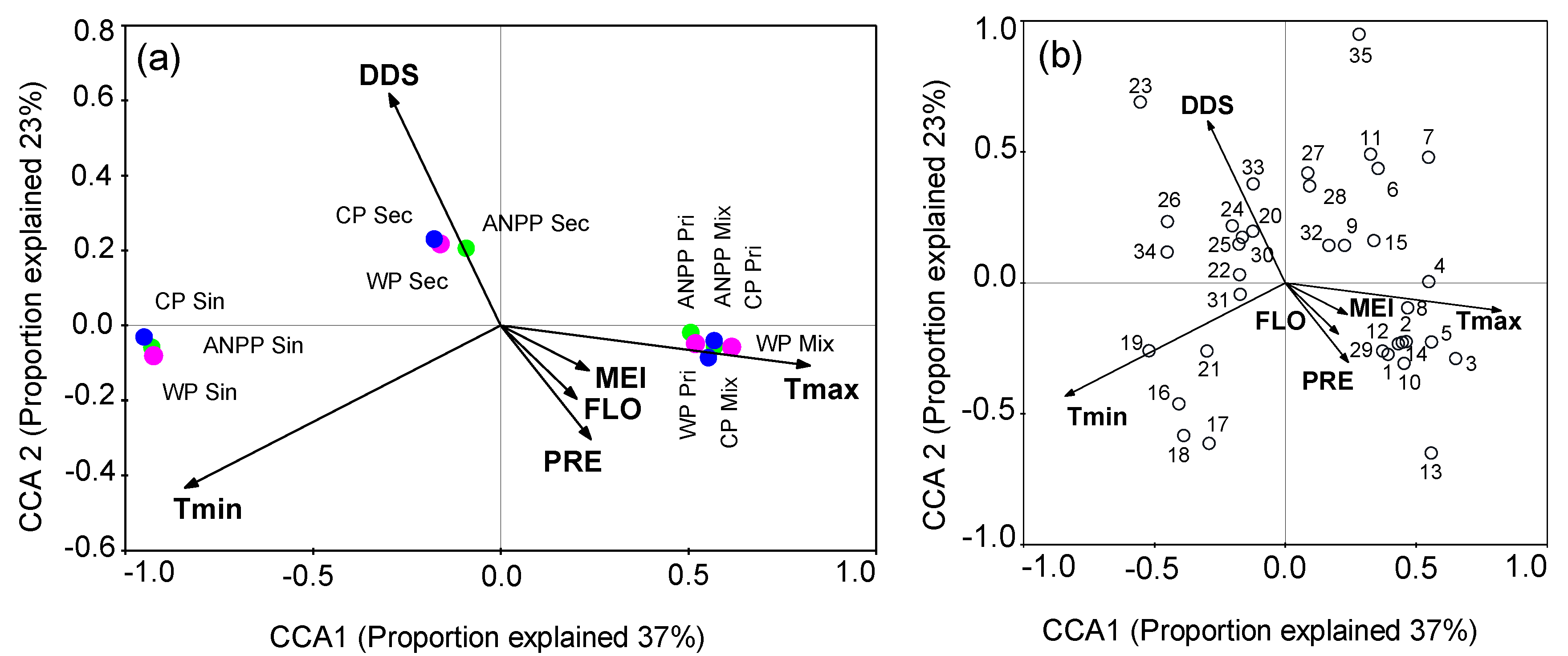
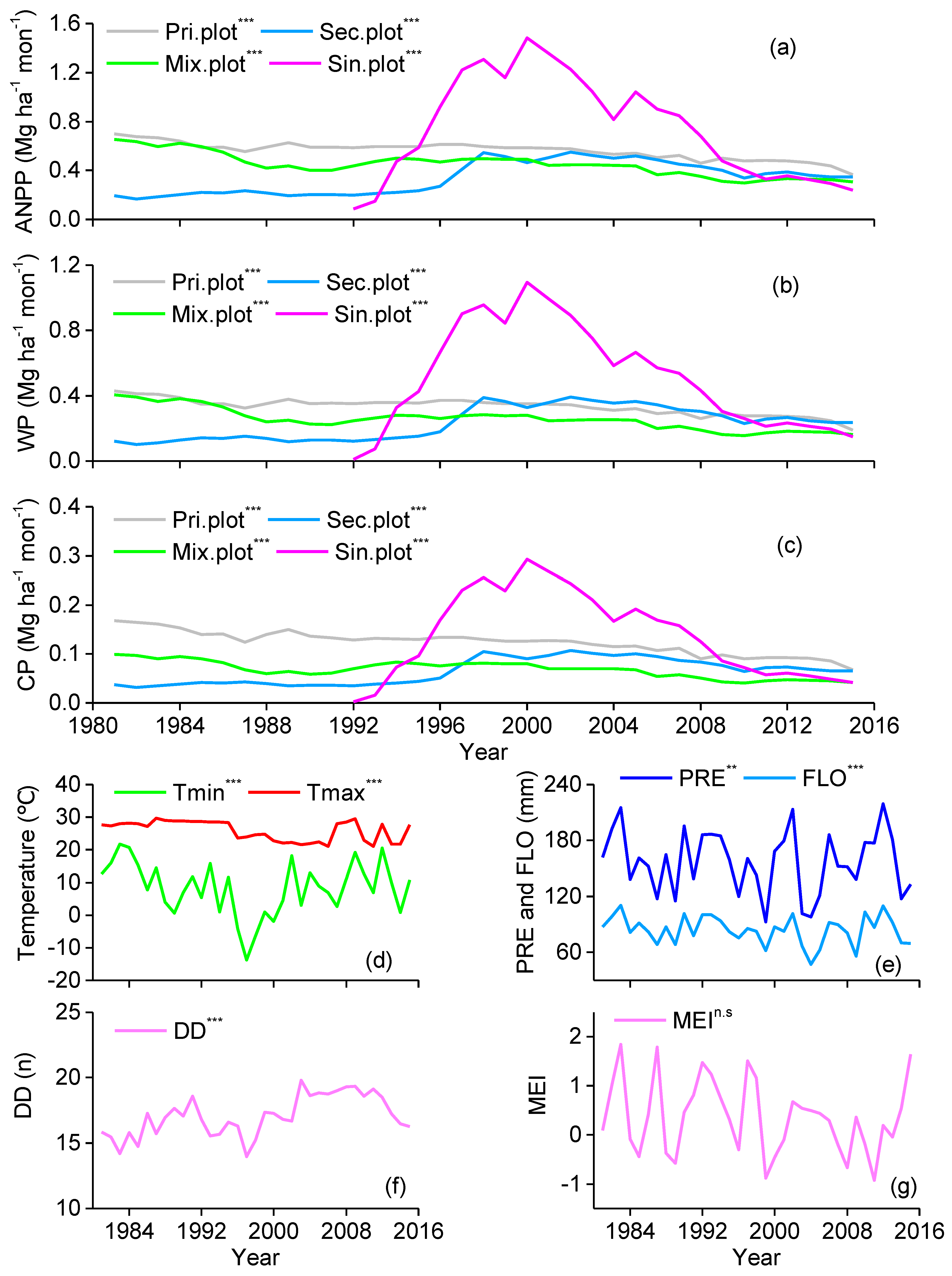
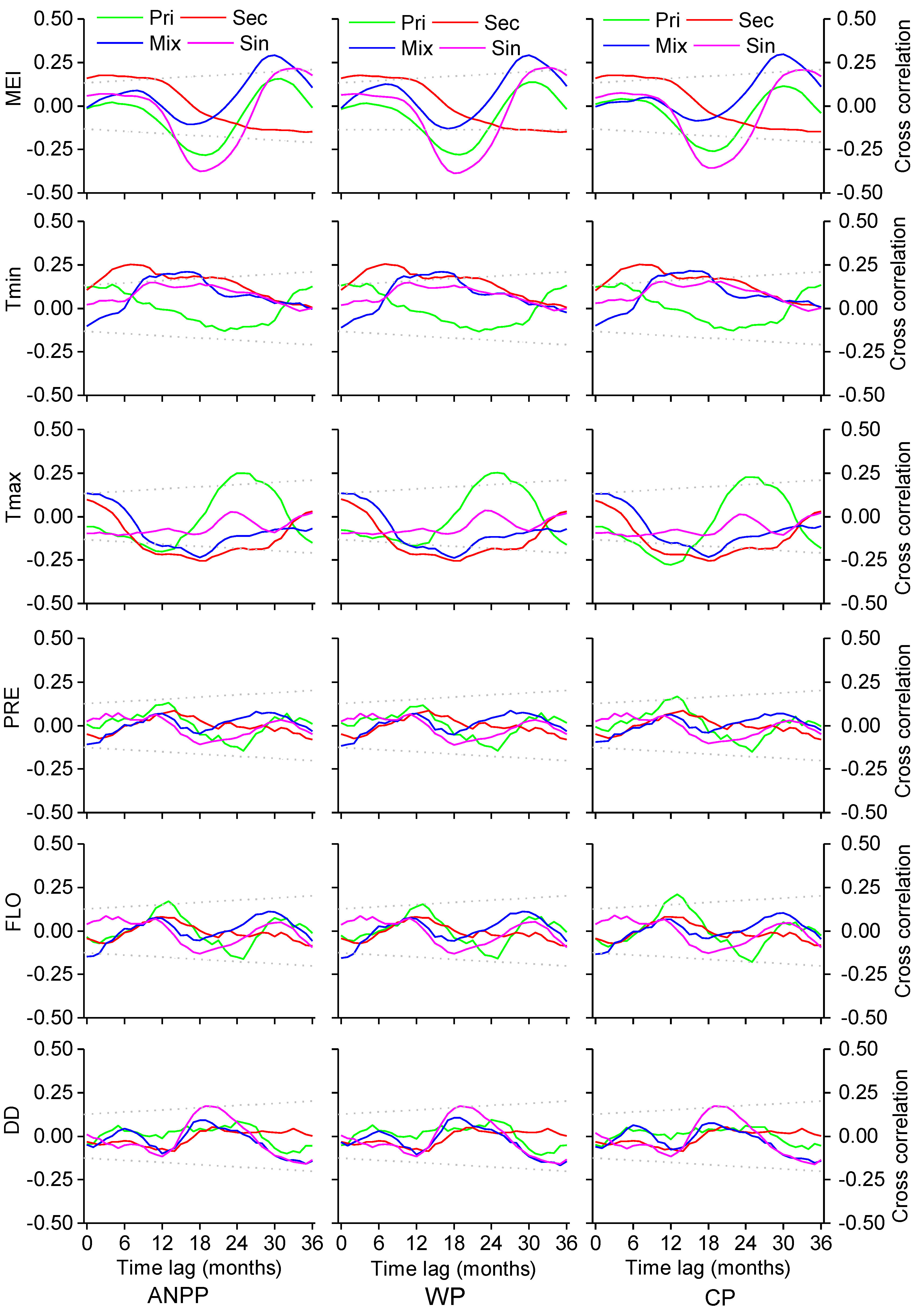
3. Results
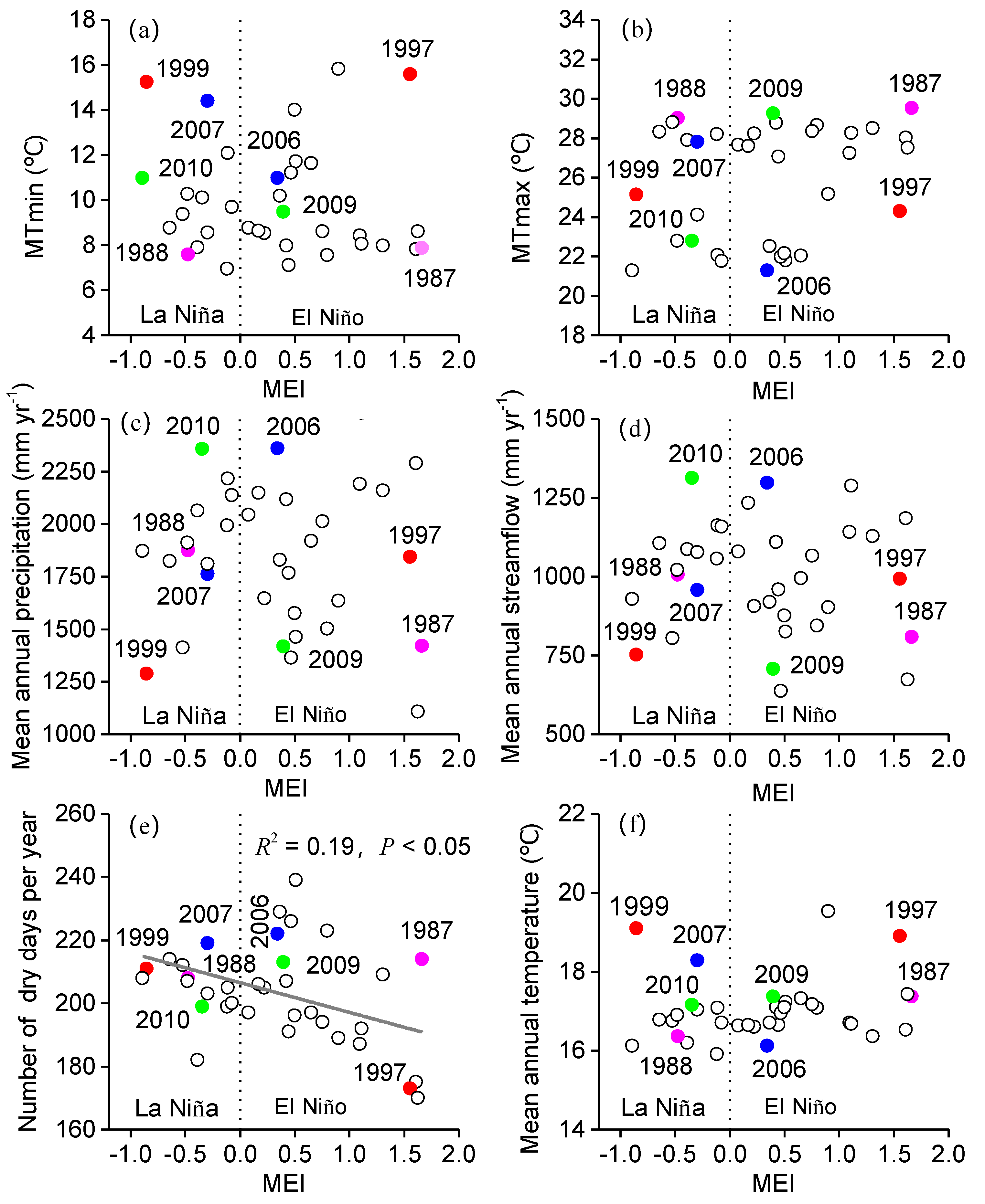
3.1. Influence of MEI on Local Climate
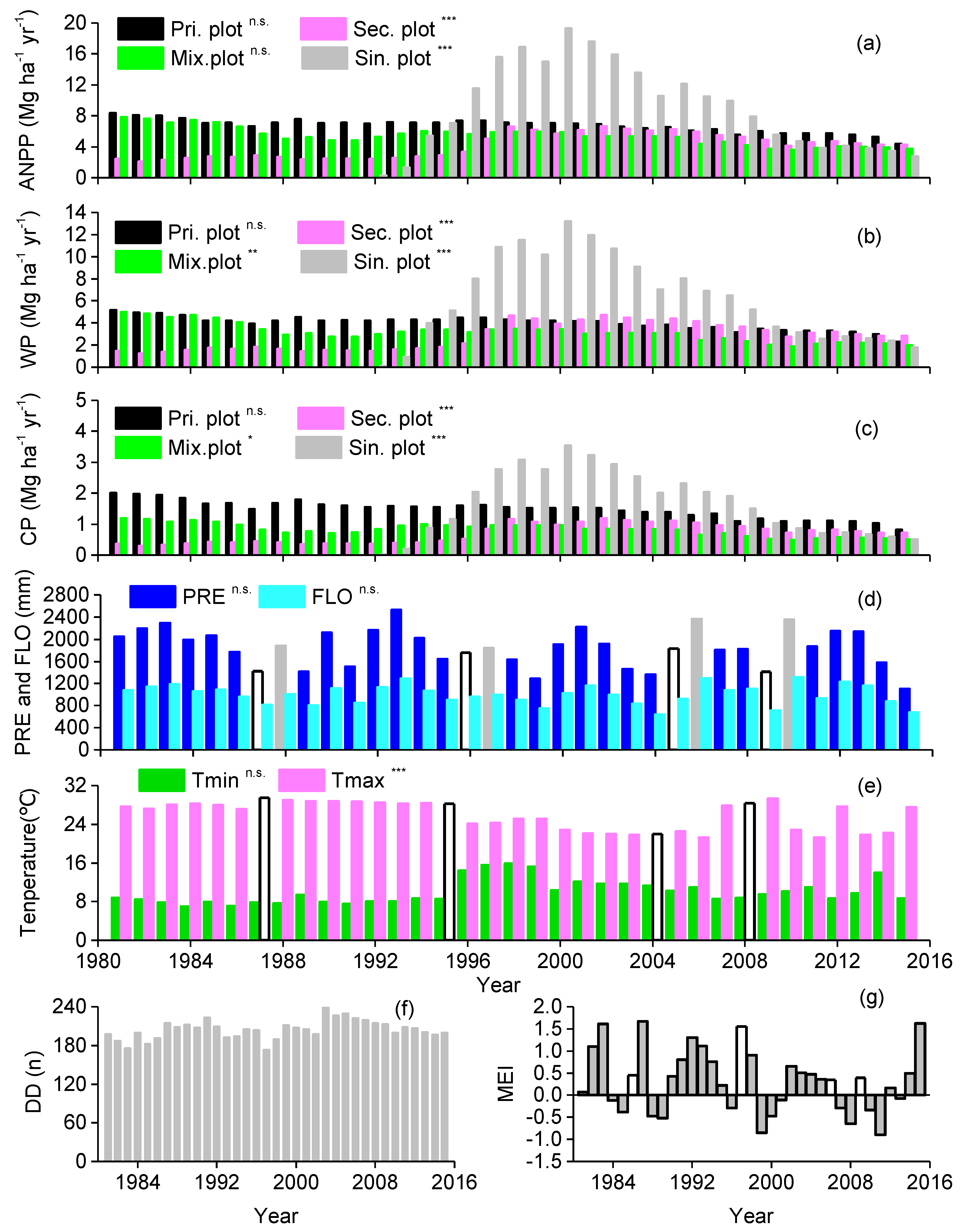
3.2. Correlations between Forest Aboveground Productivity and Climatic Drivers
4. Discussion
4.1. Correlation between Subtropical Mountain Productivity and Local Climatic Variation
4.2. Response of Subtropical Mountain Productivity to Large-Scale Climate Anomalies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kira, T. Forest ecosystems of east and southeast Asia in a global perspective. Ecol. Res. 1991, 6, 185–200. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, Y.; Schaefer, D.; Yu, G.; Liang, N.; Song, Q. An old-growth subtropical Asian evergreen forest as a large carbon sink. Atmos. Environ. 2011, 45, 1548–1554. [Google Scholar] [CrossRef]
- Feroz, S.M.; Enamul Kabir, M.; Hagihara, A. Species composition, diversity and stratification in subtropical evergreen broadleaf forests along a latitudinal thermal gradient in the Ryukyu Archipelago, Japan. Glob. Ecol. Conserv. 2015, 4, 63–72. [Google Scholar] [CrossRef]
- Wu, Z.Y. Vegetation of China; Science Press: Beijing, China, 1980; p. 825. [Google Scholar]
- Anderegg, W.R.L.; Martinez-Vilalta, J.; Cailleret, M.; Camarero, J.J.; Ewers, B.E.; Galbraith, D.; Gessler, A.; Grote, R.; Huang, C.; Levick, S.R.; et al. When a Tree Dies in the Forest: Scaling Climate-Driven Tree Mortality to Ecosystem Water and Carbon Fluxes. Ecosystems 2016, 19, 1133–1147. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Chapin, F.R.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; p. 32. [Google Scholar]
- Shah, S.K.; Mehrotra, N. Tree–ring studies of Toona ciliata from subtropical wet hill forests of Kalimpong, eastern Himalaya. Dendrochronologia 2017, 46, 46–55. [Google Scholar] [CrossRef]
- Carón, M.M.; De Frenne, P.; Ortega-Baes, P.; Quinteros, A.; Verheyen, K. Regeneration responses to climate and land-use change of four subtropical tree species of the southern Central Andes. For. Ecol. Manag. 2018, 417, 110–121. [Google Scholar] [CrossRef]
- Phillips, J.; Marion, D.A.; Yocum, C.; Mehlhope, S.H.; Olson, J.W. Geomorphological impacts of a tornado disturbance in a subtropical forest. Catena 2015, 125, 111–119. [Google Scholar] [CrossRef]
- Guisasola, R.; Tang, X.; Bauhus, J.; Forrester, D.I. Intra- and inter-specific differences in crown architecture in Chinese subtropical mixed-species forests. For. Ecol. Manag. 2015, 353, 164–172. [Google Scholar] [CrossRef]
- Crabbe, R.A.; Dash, J.; Rodriguez-Galiano, V.F.; Janous, D.; Pavelka, M.; Marek, M.V. Extreme warm temperatures alter forest phenology and productivity in Europe. Sci. Total Environ. 2016, 563–564, 486–495. [Google Scholar]
- Luo, D.; Huang, J.; Jiang, X.; Ma, Q.; Liang, H.; Guo, X.; Zhang, S. Effect of climate and competition on radial growth of Pinus massoniana and Schima superba in China’s subtropical monsoon mixed forest. Dendrochronologia 2017, 46, 24–34. [Google Scholar] [CrossRef]
- Wang, X.; Kent, M.; Fang, X. Evergreen broad-leaved forest in Eastern China: Its ecology and conservation and the importance of resprouting in forest restoration. For. Ecol. Manag. 2007, 245, 76–87. [Google Scholar] [CrossRef]
- Li, W. Degradation and restoration of forest ecosystems in China. For. Ecol. Manag. 2004, 201, 33–41. [Google Scholar]
- Lieffers, V.J.; Macmillan, R.B.; Macpherson, D.; Branter, K.; Stewart, J.D. Semi-natural and intensive silvicultural systems for the boreal mixedwood forest. For. Chron. 1996, 72, 286–292. [Google Scholar] [CrossRef]
- Pretzsch, H. Diversity and Productivity in Forests: Evidence from Long-Term Experimental Plots, Forest Diversity and Function; Springer: Berlin Heidelberg, Germany, 2005; pp. 41–64. [Google Scholar]
- Pretzsch, H.; Schutze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Lombardi, F.; Battipaglia, G.; Palombo, C.; Altieri, S.; La Porta, N.; Marchetti, M.; Tognetti, R. Growth dynamics, climate sensitivity and water use efficiency in pure vs. mixed pine and beech stands in Trentino (Italy). For. Ecol. Manag. 2018, 409, 707–718. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snall, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Froberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Pretzsch, H. Facilitation and competition in mixed-species forests analysed along an ecological gradient. Nova Acta Leopoldina 2013, 114, 255–266. [Google Scholar]
- De Ridder, M.; Van den Bulcke, J.; Van Acker, J.; Beeckman, H. Tree-ring analysis of an African long-lived pioneer species as a tool for sustainable forest management. For. Ecol. Manag. 2013, 417–426. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Y.; Tian, H. A dendroclimatic reconstruction of May–June mean temperature variation in the Heng Mounatins, north China, since 1767 AD. Quat. Int. 2013, 283, 3–10. [Google Scholar] [CrossRef]
- Arsalani, M.; Azizi, G.; Bräuning, A. Dendroclimatic reconstruction of May-June maximum temperatures in the central Zagros Mountains, western Iran. Int. J. Climatol. 2015, 35, 408–416. [Google Scholar] [CrossRef]
- Nadi, M.; Bazrafshan, J.; Pourtahmasi, K.; Bräuning, A. Tree-ring based reconstruction of the joint deficit index in Javan-Roud Region, Kermanshah (Iran). Int. J. Climatol. 2017, 37, 420–429. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Yu, S.L.; Zhang, T.W. Reconstructed temperature for Yong’an, Fujian, Southeast China: Linkages to the Pacific Ocean climate variability. Glob. Planet Chang. 2012, 86–87, 11–19. [Google Scholar] [CrossRef]
- Volland-Voigt, F.; Bräuning, A.; Ganzhi, O.; Peters, T.; Maza, H. Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees 2011, 25, 39–48. [Google Scholar] [CrossRef]
- Fichtler, E.C.D.A. Age and long-term growth of trees in an old-growth tropical rain forest, based on analyses of tree rings and C-14. Biotropica 2003, 306–317. [Google Scholar] [CrossRef]
- Dünisch, O.; Montóia, V.R.; Bauch, J. Dendroecological investigations on Swietenia macrophylla King and Cedrela odorata L. (Meliaceae) in the central Amazon. Trees 2003, 17, 244–250. [Google Scholar]
- Toïgo, M.; Vallet, P.; Perot, T.; Bontemps, J.; Piedallu, C.; Courbaud, B. Overyielding in mixed forests decreases with site productivity. J. Ecol. 2015, 103, 502–512. [Google Scholar] [CrossRef]
- Forrester, D.I.; Albrecht, A.T. Light absorption and light-use efficiency in mixtures of Abies alba and Picea abies along a productivity gradient. For. Ecol. Manag. 2014, 328, 94–102. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Cavard, X.; Laganière, J.; Reich, P.B.; Bergeron, Y.; Paré, D.; Yuan, Z. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2013, 101, 210–219. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Meng, S.; Liu, Q. Long-term response of living forest biomass to extensive logging in subtropical China. J. For. Res. 2018. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Klinka, K. Aboveground productivity of western hemlock and western redcedar mixed-species stands in southern coastal British Columbia. For. Ecol. Manag. 2003, 184, 55–64. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, C.; Jiang, T.; Wu, Y.; Det, T.V.; Universitet, U.; Sektionen, G.; Geovetenskaper, I.F.R.; Ra, L.O.V. Possible influence of ENSO on annual maximum streamflow of the Yangtze River, China. J. Hydrol. 2007, 333, 265–274. [Google Scholar] [CrossRef]
- Bao, B.; Ren, G. Climatological characteristics and long-term change of SST over the marginal seas of China. Cont. Shelf Res. 2014, 77, 96–106. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Lebrija-Trejos, E.; Zuidema, P.A.; Martínez-Ramos, M. Climate-growth analysis for a Mexican dry forest tree shows strong impact of sea surface temperatures and predicts future growth declines. Glob. Chang. Biol. 2010, 16, 2001–2012. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Palmer, J.; Ummenhofer, C.C.; Kyaw, N.N.; Krusic, P. Three centuries of Myanmar monsoon climate variability inferred from teak tree rings. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Pucha-Cofrep, D.; Peters, T.; Bräuning, A. Wet season precipitation during the past century reconstructed from tree-rings of a tropical dry forest in Southern Ecuador. Glob. Planet Chang. 2015, 133, 65–78. [Google Scholar] [CrossRef]
- Locosselli, G.M.; Schöngart, J.; Ceccantini, G. Climate/growth relations and teleconnections for a Hymenaea courbaril (Leguminosae) population inhabiting the dry forest on karst. Trees 2016, 30, 1127–1136. [Google Scholar] [CrossRef]
- Hofhansl, F.; Kobler, J.; Ofner, J.; Drage, S.; Pölz, E.M.; Wanek, W. Sensitivity of tropical forest aboveground productivity to climate anomalies in SW Costa Rica. Glob. Biogeochem. Cycles 2014, 28, 1437–1454. [Google Scholar] [CrossRef]
- Liu, X.Z.; Xiao, Z.Y.; Ma, J.Z. Scientific Survey and Study on the Forest Ecosystem in Jiangxi Nature Reserve; China Forestry Publishing House: Beijing, China, 2002; pp. 14–53. [Google Scholar]
- Toshio, T.; Li, C.; Goro, I.; Li, W.; Shigenobu, T.; Kyozo, C.; Shigeo, K. A study on the evergreen broad-leaved forest in the Jiulian Mountain of Jiangxi province. Resour. Sci. 2001, 23, 15–35. [Google Scholar]
- Zhou, H.; Meng, S.; Liu, Q. Diameter Growth, Biological Rotation Age and Biomass of Chinese Fir in Burning and Clearing Site Preparations in Subtropical China. Forests 2016, 7, 177. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Q. Climate and hydrology characteristics of subtropical evergreen broadleaved forest in Jiulianshan. Resour. Sci. 2018, 40, 125–136. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Yang, Q.; Li, M.; Wang, B.; Li, R.; Wang, C. Dynamics of biomass and net primary productivity in succession of south subtropical forests in Southwest Guangdong. Chin. J. Appl. Ecol. 2003, 14, 2136–2140. [Google Scholar]
- Sun, R.; Zhu, Q. Distribution and Seasonal Change of Net Primary Productivity in China from April, 1992 to March, 1993. Acta Geogr. Sin. 2000, 55, 36–45. [Google Scholar]
- Cui, L.; Shi, J.; Tang, P.; Gao, Z. Seasonal change of terrestrial net primary productivity in China. Prog. Geogr. 2005, 24, 8–16. [Google Scholar]
- Wolter, K.; Timlin, M.S. Monitoring ENSO in COADS with a Seasonally Adjusted Principal Component Index. In Proceedings of the 17th Climate Diagnostics Workshop; NOAA/NMC/CAC, NSSL, Oklahoma Climate Survey, CIMMS and the School of Meteorology, University of Oklahoma: Norman, Oklahoma, 1993; pp. 52–57. [Google Scholar]
- Wolter, K.; Timlin, M.S. El Niño/Southern Oscillation behaviour since 1871 as diagnosed in an extended multivariate ENSO index (MEI.ext). Int. J. Climatol. 2011, 31, 1074–1087. [Google Scholar] [CrossRef]
- IBM, Corp. IBM SPSS Statistics for Windows, version 24.0; IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- Clark, D.A.; Clark, D.B. Climate-Induced Annual Variation in Canopy Tree Growth in a Costa Rican Tropical Rain Forest. J. Ecol. 1994, 82, 865–872. [Google Scholar] [CrossRef]
- He, M.; Yang, B.; Brauning, A. Tree growth–climate relationships of Juniperus tibetica along an altitudinal gradient on the southern Tibetan Plateau. Trees 2013, 27, 429–439. [Google Scholar] [CrossRef]
- Buckley, B.M.; Palakit, K.; Duangsathaporn, K.; Sanguantham, P.; Prasomsin, P. Decadal scale droughts over northwestern Thailand over the past 448 years: Links to the tropical Pacific and Indian Ocean sectors. Clim. Dyn. 2007, 29, 63–71. [Google Scholar] [CrossRef]
- Vlam, M.; Baker, P.J.; Bunyavejchewin, S.; Zuidema, P.A. Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 2014, 174, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.B.; Clark, D.A.; Oberbauer, S.F. Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Glob. Chang. Biol. 2010, 16, 747–759. [Google Scholar] [CrossRef]
- Clark, D.A.; Piper, S.C.; Keeling, C.D.; Clark, D.B. Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proc. Natl. Acad. Sci. USA 2003, 100, 5852–5857. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.X.; Davies, S.J.; Ashton, P.S.; Bunyavejchewin, S.; Supardi, M.N.; Kassim, A.R.; Tan, S.; Moorcroft, P.R. Variability in solar radiation and temperature explains observed patterns and trends in tree growth rates across four tropical forests. Proc. Biol. Sci. 2012, 279, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- Savva, Y.; Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Vaganov, E.A.; Modrzynski, J. Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains, Poland. Trees (Berlin) 2006, 20, 735–746. [Google Scholar] [CrossRef]
- MäKinen, H.; NöJd, P.; Kahle, H.; Neumann, U.; Tveite, B.; Mielik Inen, K.; RöHle, H.; Spiecker, H. Large-scale climatic variability and radial increment variation of Picea abies (L.) Karst. in central and northern Europe. Trees 2003, 17, 173–184. [Google Scholar]
- Helama, S.; Lindholm, M.; Meriläinen, J.O.U.K.; Timonen, M.; Eronen, M. Multicentennial ring-width chronologies of Scots pine along a north-south gradient across Finland. Tree-Ring Res. 2005, 61, 21–32. [Google Scholar] [CrossRef]
- Lloyd, J.; Farquhar, G.D. Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 1811–1817. [Google Scholar] [CrossRef]
- Galbraith, D.; Levy, P.E.; Sitch, S.; Huntingford, C.; Cox, P.; Williams, M.; Meir, P. Multiple mechanisms of Amazonian forest biomass losses in three dynamic global vegetation models under climate change. New Phytol. 2010, 187, 647–665. [Google Scholar] [CrossRef] [PubMed]
- Durán, S.M.; Sánchez Azofeifa, G.A.; Rios, R.S.; Gianoli, E. The relative importance of climate, stand variables and liana abundance for carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 939–949. [Google Scholar] [CrossRef]
- Meir, P.; Metcalfe, D.B.; Costa, A.C.; Fisher, R.A. The fate of assimilated carbon during drought: Impacts on respiration in Amazon rainforests. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Farquhar, G.D. The CO2 dependence of photosynthesis, plant growth responses to elevated atmospheric CO2 concentrations and their interaction with soil nutrient status. I. General Principles and Forest Ecosystems. Funct. Ecol. 1996, 10, 4–32. [Google Scholar] [CrossRef]
- Atkin, O.K.; Bruhn, D.; Hurry, V.M.; Tjoelker, M.G. Evans Review No. 2: The hot and the cold: Unravelling the variable response of plant respiration to temperature. Funct. Plant Biol. 2005, 32, 87–105. [Google Scholar] [CrossRef]
- Brienen, R.J.; Zuidema, P.A. Relating tree growth to rainfall in Bolivian rain forests: A test for six species using tree ring analysis. Oecologia 2005, 146, 1–12. [Google Scholar] [CrossRef]
- Gebrekirstos, A.; Mitlöhner, R.; Teketay, D.; Worbes, M. Climate–growth relationships of the dominant tree species from semi-arid savanna woodland in Ethiopia. Trees 2008, 22, 631–641. [Google Scholar] [CrossRef]
- Couralet, C.; Sterck, F.J.; Sass-Klaassen, U.; Van Acker, J.; Beeckman, H. Species-Specific Growth Responses to Climate Variations in Understory Trees of a Central African Rain Forest. Biotropica 2010, 42, 503–511. [Google Scholar] [CrossRef]
- Trouet, V.; Coppin, P.; Beeckman, H. Annual Growth Ring Patterns in Brachystegia spiciformis Reveal Influence of Precipitation on Tree Growth. Biotropica 2010, 38, 375–382. [Google Scholar] [CrossRef]
- Li, C.; Li, Z. A preliminary study on the physical properties, moisture condition, and water retention ability of the soil under the evergreen broad-leaved forest of the Jiulian Mountain in Jiangxi province. J. Nat. Resour. 1991, 6, 370–379. [Google Scholar]
- Wagner, F.; Rossi, V.; Stahl, C.; Bonal, D.; Hérault, B. Asynchronism in leaf and wood production in tropical forests: A study combining satellite and ground-based measurements. Biogeosciences 2013, 10, 7307–7321. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Malhi, Y.; Amézquita, F.F.; Doughty, C.E.; SilvaEspejo, J.E.; Girardin, C.A.J.; Metcalfe, D.B.; Aragão, L.E.O.C.; HuaracaQuispe, L.P.; Alzamora-Taype, I.; Eguiluz-Mora, L.; et al. The productivity, metabolism and carbon cycle of two lowland tropical forest plots in south-western Amazonia, Peru. Plant Ecol. Divers. 2014, 7, 85–105. [Google Scholar] [CrossRef]
- Broadhead, J.S.; Ong, C.K.; Black, C.R. Tree phenology and water availability in semi-arid agroforestry systems. For. Ecol. Manag. 2003, 180, 61–73. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Belowground drought response of European beech: Fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Sprunger, C.D.; Oates, L.G.; Jackson, R.D.; Robertson, G.P. Plant community composition influences fine root production and biomass allocation in perennial bioenergy cropping systems of the upper Midwest, USA. Biomass Bioenergy 2017, 105, 248–258. [Google Scholar] [CrossRef]
- Li, Y.; Bao, W.; Bongers, F.; Chen, B.; Chen, G.; Guo, K.; Jiang, M.; Lai, J.; Lin, D.; Liu, C.; et al. Drivers of tree carbon storage in subtropical forests. Sci. Total Environ. 2018, 654, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Girardin, C.J.; Silva Espejob, J.; Doughty, C.; Huasco, W.H.; Metcalfe, D.; Durand-Baca, L.; Marthews, T.; Aragao, L.O.C.; Farfán-Rios, W.; García-Cabrera, K. Productivity and carbon allocation in a tropical montane cloud forest in the Peruvian Andes. Plant Ecol. Divers. 2014, 7, 107–123. [Google Scholar] [CrossRef]
- Doughty, C.E.; Malhi, Y.; Araujo-Murakami, A.; Metcalfe, D.B.; Silva-Espejo, J.E.; Arroyo, L.; Heredia, J.P.; Pardo-Toledo, E.; Mendizabal, L.M.; Rojas-Landivar, V.D.; et al. Allocation trade-offs dominate the response of tropical forest growth to seasonal and interannual drought. Ecology 2014, 95, 2192–2201. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Xu, J.; Shi, Y.; Fang, H.; Zhou, G.; Zhou, Y.; Tao, J.; Ji, B.; Li, C.; et al. Vegetation carbon stocks driven by canopy density and forest age in subtropical forest ecosystems. Sci. Total Environ. 2018, 631–632, 619–626. [Google Scholar] [CrossRef]
- Vieilledent, G.; Gardi, O.; Grinand, C.; Burren, C.; Andriamanjato, M.; Camara, C.; Gardner, C.J.; Glass, L.; Rasolohery, A.; Rakoto Ratsimba, H.; et al. Bioclimatic envelope models predict a decrease in tropical forest carbon stocks with climate change in Madagascar. J. Ecol. 2016, 104, 703–715. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Wang, Q.; Zhang, Y. How temperature, precipitation and stand age control the biomass carbon density of global mature forests. Glob. Ecol. Biogeogr. 2014, 23, 323–333. [Google Scholar] [CrossRef]
- Xu, B.; Pan, Y.; Plante, A.F.; Johnson, A.; Cole, J.; Birdsey, R. Decadal change of forest biomass carbon stocks and tree demography in the Delaware River Basin. For. Ecol. Manag. 2016, 374, 1–10. [Google Scholar] [CrossRef]
- Wang, G.; Guan, D.; Xiao, L.; Peart, M.R. Forest biomass-carbon variation affected by the climatic and topographic factors in Pearl River Delta, South China. J. Environ. Manag. 2019, 232, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; He, N. Forest carbon storage along the north-south transect of eastern China: Spatial patterns, allocation, and influencing factors. Ecol. Indic. 2016, 61, 960–967. [Google Scholar] [CrossRef]
- Malhi, Y.; Girardin, C.A.J.; Goldsmith, G.R.; Doughty, C.E.; Salinas, N.; Metcalfe, D.B.; Huaraca Huasco, W.; Silva-Espejo, J.E.; Del Aguilla-Pasquell, J.; Farfán Amézquita, F.; et al. The variation of productivity and its allocation along a tropical elevation gradient: A whole carbon budget perspective. New Phytol. 2017, 214, 1019–1032. [Google Scholar] [CrossRef] [PubMed]

| Month | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEBLF (%) | 2.4 | 3.1 | 6.7 | 5.4 | 7.9 | 12.9 | 14.1 | 15.9 | 14.1 | 7.8 | 5.8 | 3.7 |
| PECF (%) | 1.1 | 1.5 | 3.8 | 4.9 | 8.7 | 13.8 | 19.2 | 17.0 | 16.0 | 7.7 | 4.1 | 2.1 |
| PMBCF (%) | 1.8 | 2.3 | 5.3 | 5.2 | 8.3 | 13.3 | 16.7 | 16.5 | 15.1 | 7.7 | 5.0 | 2.9 |
| ANPP | ||||||||
| Primary Forest | Secondary Forest | Mixed Forest | Single-Species Forest | |||||
| Variation | Estimates | p Value | Estimates | p Value | Estimates | p Value | Estimates | p Value |
| Constant | −0.25520 | 0.000 | 0.22554 | 0.000 | −0.33853 | 0.000 | 0.19981 | 0.035 |
| MEI | −0.00850 | 0.276 | −0.00876 | 0.249 | 0.00079 | 0.921 | −0.04457 | 0.213 |
| Tmin | 0.02359 | 0.000 | 0.02653 | 0.000 | 0.02242 | 0.000 | 0.07922 | 0.000 |
| Tmax | 0.02206 | 0.000 | −0.00695 | 0.000 | 0.02210 | 0.000 | −0.01006 | 0.228 |
| PRE | 0.00063 | 0.003 | −0.00002 | 0.909 | 0.00045 | 0.038 | −0.00063 | 0.436 |
| FLO | −0.00127 | 0.003 | −0.00001 | 0.982 | −0.00100 | 0.022 | 0.00079 | 0.622 |
| DD | 0.00126 | 0.319 | 0.00208 | 0.091 | 0.00024 | 0.852 | −0.00086 | 0.872 |
| R2 p value | 0.879 0.000 | 0.798 0.000 | 0.866 0.000 | 0.725 0.000 | ||||
| Wood Production | ||||||||
| Primary Forest | Secondary Forest | Mixed Forest | Single-Species Forest | |||||
| Variation | Estimates | p Value | Estimates | p Value | Estimates | p Value | Estimates | p Value |
| Constant | −0.15887 | 0.000 | 0.20828 | 0.000 | −0.24726 | 0.000 | 0.17117 | 0.031 |
| MEI | −0.00498 | 0.306 | −0.00664 | 0.226 | 0.00122 | 0.819 | −0.02950 | 0.635 |
| Tmin | 0.01382 | 0.000 | 0.01847 | 0.000 | 0.01301 | 0.000 | 0.05370 | 0.000 |
| Tmax | 0.01346 | 0.000 | −0.00674 | 0.000 | 0.01434 | 0.000 | −0.00742 | 0.000 |
| PRE | 0.00040 | 0.002 | −0.00003 | 0.843 | 0.00029 | 0.043 | −0.00041 | 0.075 |
| FLO | −0.00080 | 0.002 | 0.00000 | 0.998 | −0.00063 | 0.028 | 0.00045 | 0.104 |
| DD | 0.00071 | 0.364 | 0.00113 | 0.204 | 0.00057 | 0.512 | −0.00132 | 0.895 |
| R2 p value | 0.870 0.000 | 0.776 0.000 | 0.845 0.000 | 0.722 0.000 | ||||
| Canopy Production | ||||||||
| Primary Forest | Secondary Forest | Mixed Forest | Single-Species Forest | |||||
| Variation | Estimates | p Value | Estimates | p Value | Estimates | p Value | Estimates | p Value |
| Constant | −0.07115 | 0.000 | 0.09443 | 0.000 | −0.04961 | 0.000 | 0.04743 | 0.030 |
| MEI | −0.00163 | 0.408 | −0.00224 | 0.189 | 0.00048 | 0.711 | −0.00900 | 0.175 |
| Tmin | 0.00476 | 0.000 | 0.00393 | 0.000 | 0.00350 | 0.000 | 0.01466 | 0.000 |
| Tmax | 0.00561 | 0.000 | −0.00233 | 0.000 | 0.00328 | 0.000 | −0.00243 | 0.117 |
| PRE | 0.00015 | 0.004 | −0.00002 | 0.631 | 0.00007 | 0.040 | −0.00013 | 0.403 |
| FLO | −0.00031 | 0.004 | 0.00003 | 0.774 | −0.00016 | 0.021 | 0.00017 | 0.563 |
| DD | 0.00024 | 0.455 | −0.00044 | 0.110 | 0.00002 | 0.935 | −0.00001 | 0.994 |
| R2 p value | 0.851 0.000 | 0.633 0.000 | 0.854 0.000 | 0.719 0.000 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Luo, Y.; Zhou, G.; Yu, J.; Shah, S.; Meng, S.; Liu, Q. Exploring the Sensitivity of Subtropical Stand Aboveground Productivity to Local and Regional Climate Signals in South China. Forests 2019, 10, 71. https://doi.org/10.3390/f10010071
Zhou H, Luo Y, Zhou G, Yu J, Shah S, Meng S, Liu Q. Exploring the Sensitivity of Subtropical Stand Aboveground Productivity to Local and Regional Climate Signals in South China. Forests. 2019; 10(1):71. https://doi.org/10.3390/f10010071
Chicago/Turabian StyleZhou, Hua, Yang Luo, Guang Zhou, Jian Yu, Sher Shah, Shengwang Meng, and Qijing Liu. 2019. "Exploring the Sensitivity of Subtropical Stand Aboveground Productivity to Local and Regional Climate Signals in South China" Forests 10, no. 1: 71. https://doi.org/10.3390/f10010071
APA StyleZhou, H., Luo, Y., Zhou, G., Yu, J., Shah, S., Meng, S., & Liu, Q. (2019). Exploring the Sensitivity of Subtropical Stand Aboveground Productivity to Local and Regional Climate Signals in South China. Forests, 10(1), 71. https://doi.org/10.3390/f10010071





