Abstract
Moso bamboo is one of the fastest-growing plants in the world. The objective of this study was to investigate the impact of converting secondary broadleaf evergreen forests (CK) to Moso bamboo plantations, and the impact of different management strategies, including no disturbance (M0), extensive management (M1), and intensive management (M2), on the soil organic carbon (SOC) sequestration potential, and relevant characteristics of the soil bacterial community. Our results showed that, in comparison with CK, M0 and M1 had significantly higher SOC and recalcitrant organic materials (aliphatic and aromatic compounds), and a lower C mineralization rate, whereas M2 had the opposite effects. The conversion from CK to Moso bamboo plantation significantly decreased the relative abundance of Acidobacteria in both the topsoil and subsoil soil layers. Compared with CK, M0 led to the enrichment of bacteria such as Alphaproteobacteria, Chloroflexi, and Bacteroidetes, which are involved in the decomposition of organic matter and the formation of humus and are, therefore, potentially beneficial for increasing the SOC. Furthermore, the ratio of the microbial biomass C (MBC) to total organic C (TOC), C mineralization rate, and bacterial diversity increased from M0 to M2, i.e., with an increase in the disturbance intensity. These findings indicate that the conversion of secondary broadleaf forest to bamboo forest alter the soil bacterial community structure. Reducing disturbance in bamboo forest management strategies should be actively taken up to improve the SOC, and maintain sustainable development in the forest industry.
1. Introduction
Bamboo forests are distributed in tropical and subtropical regions of the world, and cover a total area of 31.5 million ha, accounting for approximately 0.8% of the global forest area [1]. The growth pattern of bamboos differs from that of woody plants, which is related to the fact that the former possess a wooden vascular bundle structure, while the latter have a clear growth ring. Moso bamboo (Phyllostachys pubescens Mazel) is widely distributed across subtropical regions of China, and constitutes approximately 74% of the total area under bamboo forest in these regions [2]. Moso bamboo is economically important because it can provide wood for construction and paneling, edible shoots, and other raw materials, such as bamboo vinegar and charcoal, all of which have agricultural, industrial, and domestic applications [3]. In addition, owing to its high growth rate (with height and diameter increasing by 10–20 m and 6–18 cm, respectively, within 60–70 days) and a high annual regrowth rate after harvesting, Moso bamboo plays an important role as a carbon sink [4]. Given the considerable ecological and commercial value of Moso bamboo forests, the area planted with this bamboo has been expanding rapidly in subtropical China, with a 3% annual rate of increase in recent decades [5]. This expansion has largely been at the expense of the original evergreen broadleaf forests. In some areas, such as Anji County in Zhejiang Province, which was selected as the region for the present investigation, Moso bamboo forest occupies more than 70% of the total forest area.
Land use change, such as the conversion of natural forests to plantation forest, has been found to induce differences in litter quality and quantity, soil characteristics and, consequently, the structure and functionality of microbial communities [6,7]. The conversion of native Pinus massoniana L. plantations to exotic eucalyptus plantations significantly altered the composition of vegetation and availability of soil resources, resulting in changes in soil microbial community composition and reduced carbon metabolism [8]. Tardy et al. [9] suggested that bacterial diversity follows a linear positive relationship with land management intensity and carbon (C) mineralization, suggesting a major role for bacteria in C transformations in the soil. Some researchers also showed that a gradual shift in the microbial community can alter SOC (soil organic carbon) mineralization, due to interactions between SOC chemical composition and microbial community composition [10].
Intensive management practices, such as those involving the application of fertilizers, tillage, and the removal of understory vegetation, have been used to maintain high productivity in Moso bamboo plantations. They invariably result in varying degrees of soil disturbance, and may have deleterious effects on the local environment (e.g., loss of biodiversity, increase in soil CO2 efflux, and decline in soil fertility) that lead to changes in the functional diversity of soil microbial communities and undermine any potential benefits [11,12,13]. By contrast, long-term conventional management of bamboo plantations does not appear to significantly alter soil microbial biomass and diversity [14]. Furthermore, some studies have also focused on the effects of the expansion of Moso bamboo forest into adjacent broadleaved forest and/or coniferous forests on the associated soil microbial communities [3,15]. Owing to the current lack of relevant comparative studies and long-term observations, however, the effects of the typical practices for converting broadleaved forest to Moso bamboo plantations, as well as the subsequent management practices, on bacterial communities and any mechanisms of soil C dynamics, remain poorly understood. The objectives of this study, therefore, were as follows: (1) to investigate the effects of different management strategies on soil properties, SOC mineralization, the chemical composition of SOC, and bacterial community structure and; (2) to evaluate the relationships of these parameters with SOC accumulation.
2. Materials and Methods
2.1. Study Area
The study area was located in Tianhuangping town (30°31′–30°14′ N, 119°37′–119°15′ E), Anji County, Zhejiang Province, China. The region has the climate characteristics of a subtropical monsoon region, with an altitude of 300–380 m, annual average temperature of 16 °C, annual average precipitation of 1300 mm, annual average sunshine of 1943 h, and frost-free period of 224–240 days. The soils in the region are classified as Ferric Luvisols, and are derived from silty sand and fine sand-mixed rock [16].
The total investigation area was about 12,000 ha, of which, nearly 90% was covered by Moso bamboo plantation. The majority of bamboo plantations were formed by the transplantation of mother bamboo in the late 1950s, when bamboo forests were preferred by the local government for their high biomass and bamboo shoots productivities. Prior to the conversion, most of the forest in this area was composed of natural secondary evergreen broadleaf trees. Only about 10% of the original secondary forest remained following conversion. In this study, we considered the natural secondary forest to be the control (CK). The main tree species in the natural secondary evergreen broadleaf forest consisted of Schima superba Gardner & Champ., Castanopsis sclerophylla Fagaceae, and Liquidambar formosana Hance. The canopy density, mean height, and mean diameter at breast height (DBH) of CK forests, were approximately 0.7–0.8, 10–12.5 m, and 12–16.5 cm, respectively.
On the basis of management intensity, the bamboo plantations under investigation could be divided into three categories: unmanaged (M0), extensively managed (M1), and intensively managed (M2). M0 bamboo forests had not undergone any management since the forest was converted, and had, therefore, gradually evolved into a mixed forest. Tree species mixed with bamboo in M0 were as in CK, and occupied approximately 40% of the forest area. The ranges of trunk density, height, and DBH of bamboo were 1570–1800 plants ha−1, 7.8–10.7 m, and 8–11 cm, respectively. M1 bamboo forests had not received much in the way of management practices, except for the selective harvest of bamboo trunks and shoots. Shrub species, such as Rubus corchorifolius L. f., Camellia fraternal Hance, and Trachelospermum jasminoides (Lindl.) Lem. covered approximately 90% of the soil surface in the bamboo forest. The ranges of trunk density, height, and DBH for M1 bamboo forests were 2100–2250 plants ha−1, 8.3–10.9 m, and 8–11 cm, respectively. The M2 bamboo forests were fertilized annually in mid- to late June. Fertilizers were applied to the soil surface, followed by tillage (to a depth of 15–25 cm). The fertilization process included the use of nitrogen (300–500 kg hm−2), phosphorus (50–200 kg hm−2), and potassium (100–250 kg hm−2). The ranges of trunk density, height, and DBH for M2 bamboo forest were 2700–3100 plants ha−1, 9.8–12.6 m, and 10–12 cm, respectively. Only about 10% of the soil surface was covered by herbs and shrubs under the bamboo canopy.
2.2. Soil Sampling and Analysis
In July 2017, three sites in the study area were chosen for investigation, based on the similarity of initial site conditions. Four comparable stands (M0, M1, M2, and CK) from each site with similar elevation, soil type, slope gradient, and aspect, were chosen for sampling. Three 20 × 20 m2 sampling plots in each stand were established. After thorough mixing of the samples, a portion of each fresh sample was air-dried. Its impurities were removed, and it was passed through a 2 mm sieve. These portions of samples were used to determine total organic carbon (TOC) and other soil properties. Another part of the fresh soil was stored in a refrigerator at 4 °C for the analysis of soil microbial biomass C (MBC) and indoor incubation, while the remaining portions were stored at −80 °C for subsequent DNA extraction.
2.3. Physicochemical and Biochemical Analyses of Soil
Soil pH was determined from soil suspensions (with a soil/water ratio of 1:2.5) using a glass combination electrode [17]. Soil bulk density was determined by the ring knife method [18]. TOC and total nitrogen were determined using an automated elemental analyzer (Perkin-Elmer 2400, PerkinElmer, Waltham, MA, USA) [19]. The contents of hot water-extractable dissolved organic C (DOC) and N (DON) were determined using a TOC analyzer (MultiN/C3100; Analytik Jena, Germany). Total phosphorus (TP) was determined colorimetrically using a UV spectrophotometer. MBC was analyzed using the chloroform fumigation extraction method, and values were calculated using a conversion factor of 0.45 [20]. Carbon mineralization was measured by pre-incubating remoistened soil samples for 10 days at room temperature. Then, from each incubated sample, 100 g of fresh moist soil was incubated in a 1000 mL sealed Mason jar in the dark at 24 °C, with 60% water holding capacity, for 50 days. The carbon dioxide released during incubation was determined by the alkali absorption method [21]. The cumulative C mineralization was expressed as mg CO2-C kg−1 of soil; C mineralization rate (g C g−1 SOC d−1) is given by the following equation:
C mineralization rate = cumulative C mineralization/(CSOC × t); where CSOC is the SOC content (g·kg−1), and t is the number of incubation days.
2.4. Fourier Transform Infrared Analysis
Fourier transform infrared (FTIR) spectroscopy was performed as described by Ellerbrock and Gerke [22]. First, the soil samples were pre-treated with hydrofluoric acid using the procedure detailed in Fang et al. [7], and then the treated samples were oven-dried at 70 °C. One hundred grams of KBr and 1 mg of HF-treated soil samples (<0.5 mm) were mixed in an agate mortar. The mixture was compressed into a translucent pellet using a hydraulic compressor. Using pure KBr spectra as the background, the pellet was characterized by infrared spectroscopy. The spectral measurement range was 4000–400 cm−1; resolution, 4 cm−1; and number of scans times, 16. According to the assignments of organic absorption peaks reported in the literature, the intensity (area) of each peak was calculated using the curve-fitting software Peakfit 4.11. The spectra were smoothed and deconvoluted after background correction using Peakfit 4.11 [23]. The hydrophobicity of soil organic matter (SOM) was determined by the ratio of aliphatic (hydrophobic) groups (in the range of 2980–2820 cm−1) to aromatic (hydrophilic) groups (in the range of 1634–1622 cm−1).
2.5. DNA Extraction, PCR Amplification, and Illumina Sequencing
For each soil sample, DNA was extracted from 0.5 g of each sample using a soil DNA Extraction Kit (Tiangen, Beijing, China), according to the manufacturer’s instructions. The V3 and V4 regions were amplified using the following primers: 5′-CCTACGGGNGGCWGCAG-3′ (forward) and 5′-GACTACHVGGGTATCTAATCC-3′ (reverse). PCR was conducted in 20 µL reaction volumes containing 1× reaction buffer (Takara, Dalian, China), 2 mM Mg2+, 0.2 mM dNTP, 0.1 µM of each primer, 1 U HotStarTaq polymerase (Takara, Dalian, China), and 2 µL of template DNA. The cycling program employed was as follows: 95 °C for 2 min; 35 cycles of 94 °C for 20 s, 55 °C for 40 s, and 72 °C for 1 min; followed by 72 °C for 2 min. To add specific tag sequences to each sample, the PCR was performed in a total volume of 20 µL containing 1× reaction buffer (Q5TM, NEB, Beijing, USA), 0.3 mM dNTP, 0.25 µM forward primer, 0.25 µM index primer, 1 U Q5TM DNA polymerase (NEB), and 1 µL of diluted template. The PCR conditions were as follows: pre-denaturation at 98 °C for 30 s; 11 cycles of denaturation at 98 °C for 10 s, annealing at 65 °C for 30 s, and extension at 72 °C for 30 s; and finally, extension at 72 °C for 5 min. The PCR products were visualized after electrophoresis, and extracted from the gel using a QIAquick Gel Extraction Kit (Qiagen, Shanghai, China). The DNA concentration of each PCR product was quantified using a UV–vis spectrophotometer (NanoDrop ND1000, Wilmington, DE, USA), and then equimolar amounts were pooled. Library preparation for next-generation Illumina MiSeq sequencing was conducted by a commercial company (G-BIO Inc., Hangzhou, China).
2.6. 16S rRNA Data Processing
PANDAseq was used for quality filtering and assembly of the two ends of each read into contigs, with the parameters of minimum and maximum length of 400 bp and 500 bp, respectively [24]. The chimeric reads were filtered using the “identity_chimeric_seqs.py” module of USEARCH in QIIME v.1.9 [25]. The chimera-filtered sequences from each sample were combined into a single file using the QIIME script add_qiime_labels.py. Operational taxonomic units (OTUs) were selected based on 97% identity using the open-reference OTU-picking workflow pipelines and the UCLUST algorithm [26] against the Greengenes reference database (http://greengenes.lbl.gov/), August 2013 release. OTUs with an abundance of less than 0.005% of the total number of sequences were discarded [27]. Alpha diversity measurements were performed using the alpha_rarefaction.py script in QIIME. Weighted and unweighted UniFrac distances were calculated from a rarefied OTU table using the beta_diversity_through_plots.py script in QIIME [28].
2.7. Statistical Analysis
Excel 2013 software was used to pre-arrange all data, and SPSS 21.0 (IBMCorp, Armonk, NY, USA) was used for statistical analysis. One-way analysis of variance (ANOVA), followed by Duncan’s test, was used to compare the differences of soil properties among different land uses. Linear regression analysis was conducted after two-tailed Pearson product-moment correlation analysis. Based on the obtained abundance table of OTU and abundance table of different classification levels, community alpha diversity and beta diversity were analyzed by QIIME. The environmental variables and phylum-level pyrosequencing data were redundantly analyzed by CANOCO 4.5 (Microcomputer Power, Ithaca, NY, USA), and the significance of their differences was tested by the Monte Carlo method.
First-order kinetic model fitting C = C0 (1 − e−kt) was performed using the “Non-linear curve fit” program (OriginLab Corp, and used to evaluate the decomposition ability of organic C during incubation, where C is the amount of mineralized carbon (mg·kg−1), C0 is a potentially mineralizable C pool, and k is a mineralization constant.
3. Results
3.1. Physical and Chemical Properties of Soil
Physicochemical characteristics differed significantly among the four studied soils (Table 1 and Figure 1b). The values of TOC, TN (total nitrogen), DOC, DON, and TP in the topsoil layer of undisturbed bamboo soils (M0 and M1) were 50%–178%, 67%–153%, 1.6%–90.8%, 37%–212%, and 48%–68% higher, respectively, than those in CK and, in the subsoil layer, were 82%–217%, 95%–189%, 26.1%–62.1%, 62%–237%, and 20%–89% higher, respectively. The corresponding contents were significantly lower in M2 soil layers. The soil bulk density was significantly higher in M2 sites than in other studied soils (p < 0.05).

Table 1.
Physiochemical properties of soils in the studied forests.
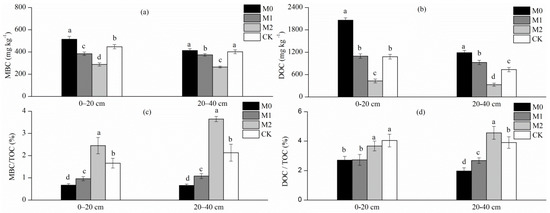
Figure 1.
MBC (microbial biomass C) and DOC (dissolved organic C) contents (a,b), and the ratio of MBC to TOC (total organic C) and that of DOC to TOC (c,d) in the soil of the different studied forests.
3.2. Soil Organic Carbon Structure
C–OH stretching of alcoholic groups (peak 1158 cm−1), OH deformation, and C–O stretching of phenolic groups (peak 1375 cm−1), aromatic C=C (peak 1644 cm−1), aliphatic C–H stretching (peaks 2920 and 2851 cm−1) were found in all studied soils (Figure 2). The intensity of the peaks at 1158 cm−1, 1375 cm−1, 1644 cm−1, 2851 cm−1, and 2920 cm−1 were significantly stronger in M0 and M1 in both soil layers analyzed, than in CK and M2 (Table 2). The ratio of the peaks at aliphatic C–H (2851 cm−1 + 2920 cm−1)/aromatic C=C (1644 cm−1) was calculated as the parameter to describe soil hydrophobicity (repellency) or C chemical recalcitrance. The higher this value, the more stable the SOC present. In the present study, M0 sites exhibited a higher hydrophobicity index than CK did, followed by M1, while M2 had the lowest hydrophobicity index.
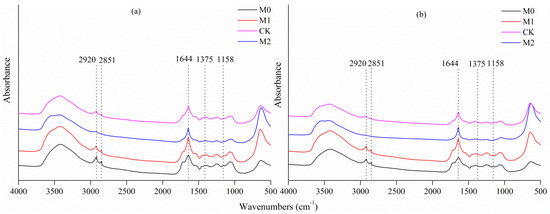
Figure 2.
FTIR (fourier transform infrared spectra) of bulk soil organic carbon (SOC) in topsoil (a) and subsoil (b) soil layer under different management strategies. Spectra were stacked to facilitate visualization of the differences between different management strategies.

Table 2.
The intensity (area) of each peak determined by FTIR spectroscopy in SOM (mean ± SD, n = 3).
3.3. SOC Mineralization
The first-order exponential equation C = C0 (1 − e−kt) of mineralization dynamics, for four land use types, is shown in Table 3. M0 and M1 showed significantly higher potentially labile organic C (C0) in the soil than that of CK and M2. The final rate of organic carbon decomposition was calculated as the carbon mineralization rate on a per-gram soil C basis. M0 and M1 significantly (p < 0.05) decreased cumulative C release per gram of soil, compared with CK and M2. M2 showed the highest rate of carbon mineralization; consequently, it showed the lowest organic carbon content.

Table 3.
Parameter values for the first-order kinetic model C = C0 (1 − e−kt) of carbon mineralization as well as decomposition rate (mean ± SD, n = 3).
3.4. Bacterial Community Differences
Figure 3 shows the relative abundance of different bacterial phyla under the four land use types. Acidobacteria and Proteobacteria were the predominant phyla, constituting 21.9%–45.1% and 15.8%–28.7% of the bacterial sequences obtained from the studied soils respectively, followed by Verrucomicrobia (4.6%–11.1%), Actinobacteria (4.2%–10.7%), Chloroflexi (1.6%–6.9%), and Planctomycetes (1.1%–5.3%). Compared with the CK forest, the relative abundances of Acidobacteria were 26.5%–40.6% and 40.8%–61.2% lower in the topsoil and subsoil, respectively, than of those observed in the bamboo plantations with different management strategies, whereas the abundances of Proteobacteria, Planctomycetes, and Bacteroidetes were significantly higher (p < 0.05). The relative abundances of Chloroflexi and Bacteroidetes, in M0 and M1 soils, were significantly higher in both the soil layers than those in M2 soil (p < 0.05), in which the relative abundance of Verrucomicrobia was significantly higher (p < 0.05).
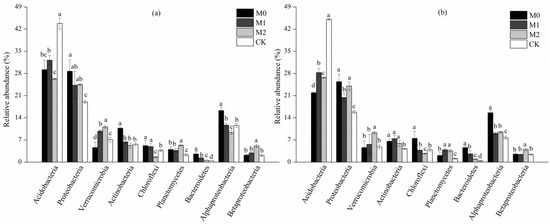
Figure 3.
Relative abundances of major bacterial phyla and class in topsoil (a) and subsoil (b) soil layer under different studied soils.
Alphaproteobacteria was the most abundant (9.1%–16.3%) class in all studied soils. The relative abundance of Alphaproteobacteria in M0 soil was significantly higher than those in M2 and CK soils (p < 0.05). By contrast, in the M2 sites, the relative abundance of Betaproteobacteria was significantly higher in both soil layers, compared to those in other soils (p < 0.05).
The alpha-diversity of the soil bacterial communities differed in the different soils studied (Table 4). Both the OTUs and Chao 1 index values were lower for the M0 bamboo forest, than those for CK. By contrast, M2 had significantly higher OTUs, Chao 1, and Shannon index values compared with the other land use types.

Table 4.
Sequence statistics and diversity indexes of soil bacteria under different land use types (mean ± S.E., n = 3).
Principal coordinates analysis (PCoA), based on weighted UniFrac distance, which is the weighted sum of the branch lengths in a phylogenetic tree of the sequences from the communities, was used to determine whether the two communities differed. The first two principal coordinates for the topsoil and subsoil layers represented 85.3% and 79.8% of the variation in the bacterial communities, respectively (Figure 4). Clear separations were observed between the secondary forest and Moso bamboo plantations in both the soil layers along the first principal coordinate, which indicates a large shift in the bacterial community structure. A significant difference in bacterial community structure between M0 and M2 was also detected, indicating that the disturbance of forest management significantly altered the bacterial community structure.
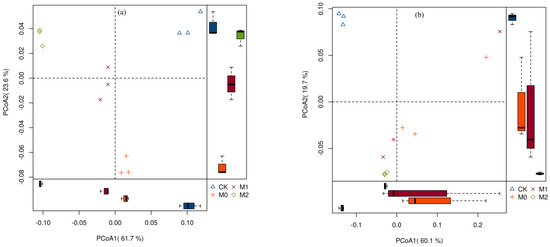
Figure 4.
The beta-diversity of the bacterial community in topsoil (a) and subsoil (b) soil layer under different land use types.
3.5. Relationships between Soil Carbon-Related Properties and Bacterial Communities
For both soil layers, the C mineralization rate was significantly positively correlated with the MBC/TOC ratio and the values of Chao 1 and Shannon’s index (p < 0.05), and negatively correlated with soil hydrophobicity and MBC content (p < 0.05) (Table 5). By contrast, the TOC content showed the opposite trend.

Table 5.
Pearson’s correlation for testing the association among bacterial diversity indices, soil hydrophobicity, and C mineralization rate.
RDA (redundancy analysis) was carried out to discern the relationships of bacterial community structure and TOC with DOC content, C/N ratio, and other soil variables (Figure 5). The first two axes of the RDA accounted for 75.45% and 87.41% of the total variance in bacterial community composition in the topsoil and subsoil, respectively. From the results of a Monte Carlo permutation test, we identified DON (F = 25.6, p = 0.001), soil pH (F = 24.1, p = 0.001), DOC (F = 15.2, p = 0.002), TN (F = 6.9, p = 0.017), TOC (F = 6.1, p = 0.033), and C/N ratio (F = 5.0, p = 0.047) as being the six most important contributors to variation in the topsoil bacterial communities, as they individually accounted for 27.7%, 26.1%, 16.5%, 7.4%, 6.6%, and 5.4% of the variation, respectively. Other factors, such as C mineralization rate, cumulative C mineralization, soil hydrophobicity, and TP content, explained 3.8%, 2.3%, 1.5%, and 1.5% of the total variance, respectively. Although for the subsoil, all the examined environmental variables together explained 96.9% of microbial community variation between samples, only DON content (F = 9.3, p = 0.047) appeared to have a significant influence.
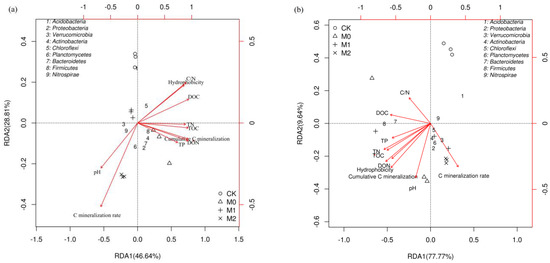
Figure 5.
Redundancy analysis (RDA) of soil bacterial community compositions (phylum level) and soil characteristics for individual samples in topsoil (a) and subsoil (b) soil layer. pH: soil acidity; TOC: total organic carbon; TN: total nitrogen; TP: total phosphorus; DOC: dissolved organic carbon; DON: dissolved organic nitrogen; C/N: TOC to TN ratio.
4. Discussion
4.1. Effects of Forest Management Strategies on Soil Properties
Bamboo plantations undisturbed by human activity (M0) or subjected only to recurrent thinning (M1) were found to have higher SOC content and lower soil bulk density than those subjected to more intensive management strategies (M2). This difference could be attributed to a reduction in physical disturbance, promoting the succession of vegetation with vigorous rhizome systems in those sites with lighter management strategies, which increases the input of organic C derived from root biomass, root exudates, leaves, and photosynthetic products [29]. By contrast, M2 soil from sites that are more heavily managed had a significantly lower TOC content and higher soil bulk density, presumably as a consequence of tillage and understory removal [30].
Previous studies have shown that variation in plant species contributes differently to soil C inputs, thus changing the SOC composition [31]. The increase in the content of aliphatic and aromatic groups, in the whole soil of M0 and M1 sites, indicated that the Moso bamboo forest with no, or only limited, human disturbance could accumulate more recalcitrant chemical components and enhance the stability of SOC, compared to the natural secondary forest. Aliphatic and aromatic compounds were mostly represented by compounds associated with microbial metabolism, and those resulting from incomplete degradation of more recalcitrant plant residues, such as tannins and lignins, which are relatively difficult for soil microorganisms to utilize [32]. Similar results were reported for the SOC content in a Pinus massoniana plantation, where it was likely to be more stable compared to the SOC content in broadleaf plantations, because of the higher content of aliphatic compounds in the coniferous needles [33]. As a result, the increased soil hydrophobicity in M0 and M1 sites showed that SOC was more recalcitrant to mineralization, which could affect microbial metabolic activity and the efficiency of organic carbon utilization by microorganisms, resulting in the marked decrease in DOC/TOC and MBC/TOC ratios, and lower C mineralization rate and, in turn, contribute to greater SOC accumulation in Moso bamboo forests.
4.2. Effects of Forest Management Strategy on the Diversity of Soil Bacterial Communities
Forest management practices, including harvesting, tillage, and weeding, can alter not only the physicochemical properties of soil but, also, the structure and metabolic activity of microbial communities, by altering the soil conditions and the composition of the associated vegetation [34]. In this regard, it has been demonstrated that, in frequently tilled soils, bacterial communities are more diverse than those found in soils with lower levels of disturbance [35]. M2 had a significantly higher number of OTUs and higher Chao 1 and Shannon index values compared with the other studied forests soils, which is consistent with the observations of Schneider et al. [36], who reported that managed land use systems (rubber plantations and oil palm plantations) had a higher diversity of soil prokaryotic communities (bacteria and archaea) than that in rainforests. The main reason for this difference might be that the heterogeneity associated with management practices (e.g., soil disturbance from tillage, fertilization, and weed control) can compensate for the loss of the biotically imposed heterogeneity of undisturbed woodlands. M2 forest soils are characterized by lower vegetation coverage and are, thus, exposed to more sunlight, consequently experiencing higher fluctuations in soil moisture, which might also increase the diversity of bacterial communities. Presumably, the disturbance caused by forest management plays a key role in influencing both the bacterial community diversity and soil structure under similar climatic conditions [17].
Our PCoA of bacterial communities showed that land use changes or disturbances in bamboo ecosystems led to differences in the structure of soil bacterial communities. Different management strategies could affect soil microbial community composition by changing the soil conditions. In the present study, this variation indicated that soil pH; TOC, TN, DOC, and DON contents; and C/N ratio could serve as important predictors for describing soil bacterial communities in differently managed bamboo forests. Following the conversion of broadleaf forests into bamboo plantations, the soil pH increased, resulting in a reduction in the relative abundance of Acidobacteria, which are known to prefer acidic environments [37]. A large amount of litter in undisturbed bamboo forests promoted the formation of thick humus in the soil, and increased the relative abundance of eutrophic and heterotrophic bacterial groups, particularly Bacteroidetes, which are usually stimulated by C-rich environments. An increase in the relative abundance of Alphaproteobacteria was, also, possibly due to higher levels of C and N in M0 and M1 soils. The phylum Chloroflexi was considered to decompose more recalcitrant compounds, such as aromatic and detrital proteins in plant residues [38] and, therefore, it appeared to be more abundant in M0 sites, contributing more to the transformation of fresh C and the sequestration of these transformed compounds in SOC. By contrast, the relative abundance of Verrucomicrobia was higher in the soils under M2 practices, which was mainly because Verrucomicrobia are known to be oligotrophs, and to prefer conditions of limited nutrient availability [39,40].
4.3. Effects of Changes in Soil Microbiological Properties on Carbon Sequestration Potential and Ecological Indications
Some previous studies have shown that the structure of microbial communities and enzyme activities in soils might be affected by the quality of the substrate and cause different degrees of mineralization and humification of organic matter [41]. The recalcitrant nature of organic carbon in the M0 soil may have selected for specific bacterial populations capable of using it, resulting in changes in the bacterial community structure. This, in turn, may potentially lead to weakened mineralization and accelerated humification, thereby increasing the SOC content. By contrast, the bacterial diversity increased from M0 to M2 when the disturbance intensity increased, which might explain the increase in the carbon mineralization rate in M2. This was consistent with the results of Tardy et al. [9], in that bacterial diversity was significantly positively correlated with the C mineralization rate. It is worth noting that the soil bacterial diversity did not change significantly after the secondary broadleaf forest was converted to an extensively managed bamboo forest, indicating that the long-term extensive management measures (M1) of no fertilizer application, tillage, and selective harvesting of bamboo trunks and shoots, will not cause a significant decline in soil bacterial diversity. The ecosystem diversity of Moso bamboo forest is superior, and such forests will continue to be managed for a long time. In summary, these results provide evidence to suggest that employing bamboo forest management strategies with low levels of disturbance can maintain soil biodiversity, promote soil carbon sequestration, and increase soil fertility of bamboo forest, thus supporting sustainable soil functioning.
5. Conclusions
The study results suggest that the conversion of secondary broadleaf forest into Moso bamboo plantation, and the subsequent management practices, significantly changed the SOC and bacterial community structure, but did not reduce Shannon index values of bacterial diversity. Differences in soil pH and nutrient contents (e.g., C and N) could serve as important factors determining bacterial community structure. The bamboo forest under no, or only limited, human disturbance (M0 and M1) was beneficial for SOC accumulation. Compared with the secondary natural broadleaf forest, M0 and M1 significantly enhanced the biochemical stability of the SOC pool by increasing recalcitrant chemical groups and soil hydrophobicity, while decreasing the MBC/TOC ratio. By contrast, the bamboo forest under intensive management (M2) increased DOC/TOC and MBC/TOC ratios, and accelerated the turnover of stable organic matter into the active DOC component, which resulted in a higher C mineralization rate and lower SOC. Our results indicate that reducing disturbance to soil and moderate harvesting can maintain better diversity of the bamboo forest ecosystem, and increase the soil carbon sequestration potential.
Author Contributions
C.Y. and Z.Z. conceived and designed the experiments; F.B. and X.D. collected and analyzed the samples; C.Y. and X.Z. analyzed the data; C.Y. wrote the original draft; Z.Z reviewed and edited the manuscript.
Funding
This study was supported by the Special Fund for Scientific Research of the Forestry Public Welfare Industry (Grant No.201504407).
Acknowledgments
The authors thank Huijing Ni for assistance in the conception and sample collection phase of this research. We are sincerely grateful to the anonymous reviewers for valuable suggestions to improve the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Global Forest Resources Assessment; FAO Forestry Paper: Rome, Italy, 2010. [Google Scholar]
- Qin, H.; Niu, L.; Wu, Q.; Chen, J.; Li, Y.; Liang, C.; Xu, Q.; Fuhrmann, J.J.; Shen, Y. Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil 2017, 420, 407–421. [Google Scholar] [CrossRef]
- Yen, T.M.; Lee, J.S. Comparing aboveground carbon sequestration between Moso bamboo (phyllostachys heterocycla) and china fir (cunninghamia lanceolata) forests based on the allometric model. For. Ecol. Manag. 2011, 261, 995–1002. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Zhang, Y.; Booth, T.; He, X. Changes of carbon stocks in bamboo stands in china during 100 years. For. Ecol. Manag. 2009, 258, 1489–1496. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Gu, H.; Qi, L. Management practices amplify the effects of n deposition on leaf litter decomposition of the Moso bamboo forest. Plant Soil 2015, 395, 391–400. [Google Scholar] [CrossRef]
- Lin, Y.T.; Jangid, K.; Whitman, W.B.; Coleman, D.C.; Chiu, C.Y. Change in bacterial community structure in response to disturbance of natural hardwood and secondary coniferous forest soils in central Taiwan. Microb. Ecol. 2011, 61, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, J.; Meng, M.; Guo, X.; Wu, Y.; Liu, X.; Zhao, K.; Ding, L.; Shao, Y.; Fu, W. Forest-type shift and subsequent intensive management affected soil organic carbon and microbial community in southeastern China. Eur. J. For. Res. 2017, 136, 689–697. [Google Scholar] [CrossRef]
- Tardy, V.; Spor, A.; Mathieu, O.; Lévèque, J.; Terrat, S.; Plassart, P.; Regnier, T.; Bardgett, R.D.; van der Putten, W.H.; Roggero, P.P.; et al. Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol. Biochem. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Chen, F.; Zheng, H.; Zhang, K.; Ouyang, Z.; Lan, J.; Li, H.; Shi, Q. Changes in soil microbial community structure and metabolic activity following conversion from native Pinus massoniana plantations to exotic eucalyptus plantations. For. Ecol. Manag. 2013, 291, 65–72. [Google Scholar] [CrossRef]
- Xiao, W.; Feng, S.; Liu, Z.; Su, Y.; Zhang, Y.; He, X. Interactions of soil particulate organic matter chemistry and microbial community composition mediating carbon mineralization in karst soils. Soil Biol. Biochem. 2017, 107, 85–93. [Google Scholar] [CrossRef]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Du, M.; Zhang, H. Soil respiration and net ecosystem production in relation to intensive management in Moso bamboo forests. Catena 2016, 137, 219–228. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, P.; Xu, Z. Soil microbial functional diversity under intensively managed bamboo plantations in southern China. J. Soils Sediments 2008, 8, 177–183. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Sun, D.D.; Xu, Q.F.; Tian, T.; Liu, B.R. Investigation on soil microbial biomass and structure in Phyllostachys edulis plantations with increasing cultivation time. Sci. Silvae Sin. 2011, 47, 181–186. [Google Scholar]
- Lin, Y.T.; Tang, S.L.; Pai, C.W.; Whitman, W.B.; Coleman, D.C.; Chiu, C.Y. Changes in the soil bacterial communities in a cedar plantation invaded by Moso bamboo. Microb. Ecol. 2014, 67, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, E.; Yan, C.; Tian, J.; Zhang, H.; Zhang, Y. Impact of no tillage vs. Conventional tillage on the soil bacterial community structure in a winter wheat cropping succession in northern china. Eur. J. Soil Biol. 2017, 80, 35–42. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Li, Q.; Yang, H.; Xu, K. Analysis of soil degradation causes in Phyllostachys edulis forests with different mulching years. Forests 2018, 9, 149. [Google Scholar] [CrossRef]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.; Miltner, A.; Schroth, G. Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Gross, C.D.; James, J.N.; Turnblom, E.C.; Harrison, R.B. Thinning treatments reduce deep soil carbon and nitrogen stocks in a coastal pacific northwest forest. Forests 2018, 9, 238. [Google Scholar] [CrossRef]
- Chang, E.-H.; Tian, G.; Chiu, C.-Y. Soil microbial communities in natural and managed cloud montane forests. Forests 2017, 8, 33. [Google Scholar] [CrossRef]
- He, D.; Ruan, H. Long term effect of land reclamation from lake on chemical composition of soil organic matter and its mineralization. PLoS ONE 2014, 9, e99251. [Google Scholar] [CrossRef] [PubMed]
- Ellerbrock, R.H.; Gerke, H.H. Characterizing organic matter of soil aggregate coatings and biopores by fourier transform infrared spectroscopy. Eur. J. Soil Sci. 2004, 55, 219–228. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.B.; Romanek, C.S.; Alvarez-Lloret, P.; Gaines, K.F. Effect of in ovo exposure to PCBs and Hg on clapper rail bone mineral chemistry from a contaminated salt marsh in coastal Georgia. Environ. Sci. Technol. 2006, 40, 4936–4942. [Google Scholar] [CrossRef] [PubMed]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- West, N.J.; Lepere, C.; Manes, C.L.; Catala, P.; Scanlan, D.J.; Lebaron, P. Distinct spatial patterns of SAR11, SAR86, and actinobacteria diversity along a transect in the ultra-oligotrophic South Pacific Ocean. Front. Microbiol. 2016, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.-J.; Su, J.-Q.; Li, H.; Zhu, Y.-G.; Cao, Z.-H. Bacterial succession along a long-term chronosequence of paddy soil in the yangtze river delta, china. Soil Biol. Biochem. 2017, 104, 59–67. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by chinese bamboo forests and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Balota, E.L.; Calegari, A.; Nakatani, A.S.; Coyne, M.S. Benefits of winter cover crops and no-tillage for microbial parameters in a brazilian oxisol: A long-term study. Agric. Ecosyst. Environ. 2014, 197, 31–40. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.-R.; Mo, J.-M.; Wang, J.-X.; Makeschin, F.; Wolff, M. Soil organic carbon stock and chemical composition in four plantations of indigenous tree species in subtropical China. Ecol. Res. 2010, 25, 1071–1079. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, A.; Yang, W.; Zhang, J. Accumulation of organic components and its association with macroaggregation in a sandy loam soil following conservation tillage. Plant Soil 2017, 416, 1–15. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Yu, X. Decline of soil fertility during forest conversion of secondary forest to chinese fir plantations in subtropical china. Land Degrad. Dev. 2011, 22, 444–452. [Google Scholar] [CrossRef]
- Guo, X.; Chen, H.Y.H.; Meng, M.; Biswas, S.R.; Ye, L.; Zhang, J. Effects of land use change on the composition of soil microbial communities in a managed subtropical forest. For. Ecol. Manag. 2016, 373, 93–99. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.H.; Jenkins, M.B.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Schneider, D.; Engelhaupt, M.; Allen, K.; Kurniawan, S.; Krashevska, V.; Heinemann, M.; Nacke, H.; Wijayanti, M.; Meryandini, A.; Corre, M.D.; et al. Impact of lowland rainforest transformation on diversity and composition of soil prokaryotic communities in Sumatra (Indonesia). Front. Microbiol. 2015, 6, 1339. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, M.; Flower, C.; Knight, K.; Gonzalez-Meler, M. Evidence of ash tree (Fraxinus spp.) specific associations with soil bacterial community structure and functional capacity. Forests 2018, 9, 187. [Google Scholar] [CrossRef]
- Oni, O.E.; Schmidt, F.; Miyatake, T.; Kasten, S.; Witt, M.; Hinrichs, K.U.; Friedrich, M.W. Microbial communities and organic matter composition in surface and subsurface sediments of the Helgoland mud area, North sea. Front. Microbiol. 2015, 6, 1290. [Google Scholar] [CrossRef] [PubMed]
- Nemergut, D.R.; Townsend, A.R.; Sattin, S.R.; Freeman, K.R.; Fierer, N.; Neff, J.C.; Bowman, W.D.; Schadt, C.W.; Weintraub, M.N.; Schmidt, S.K. The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: Implications for carbon and nitrogen cycling. Environ. Microbiol. 2008, 10, 3093–3105. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y.; Wu, J.; Fu, X.; Sun, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 319, 194–203. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
