Distribution, Habitat Preference, and Management of the Invasive Ambrosia Beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European Forests with an Emphasis on the West Carpathians
Abstract
:1. Introduction
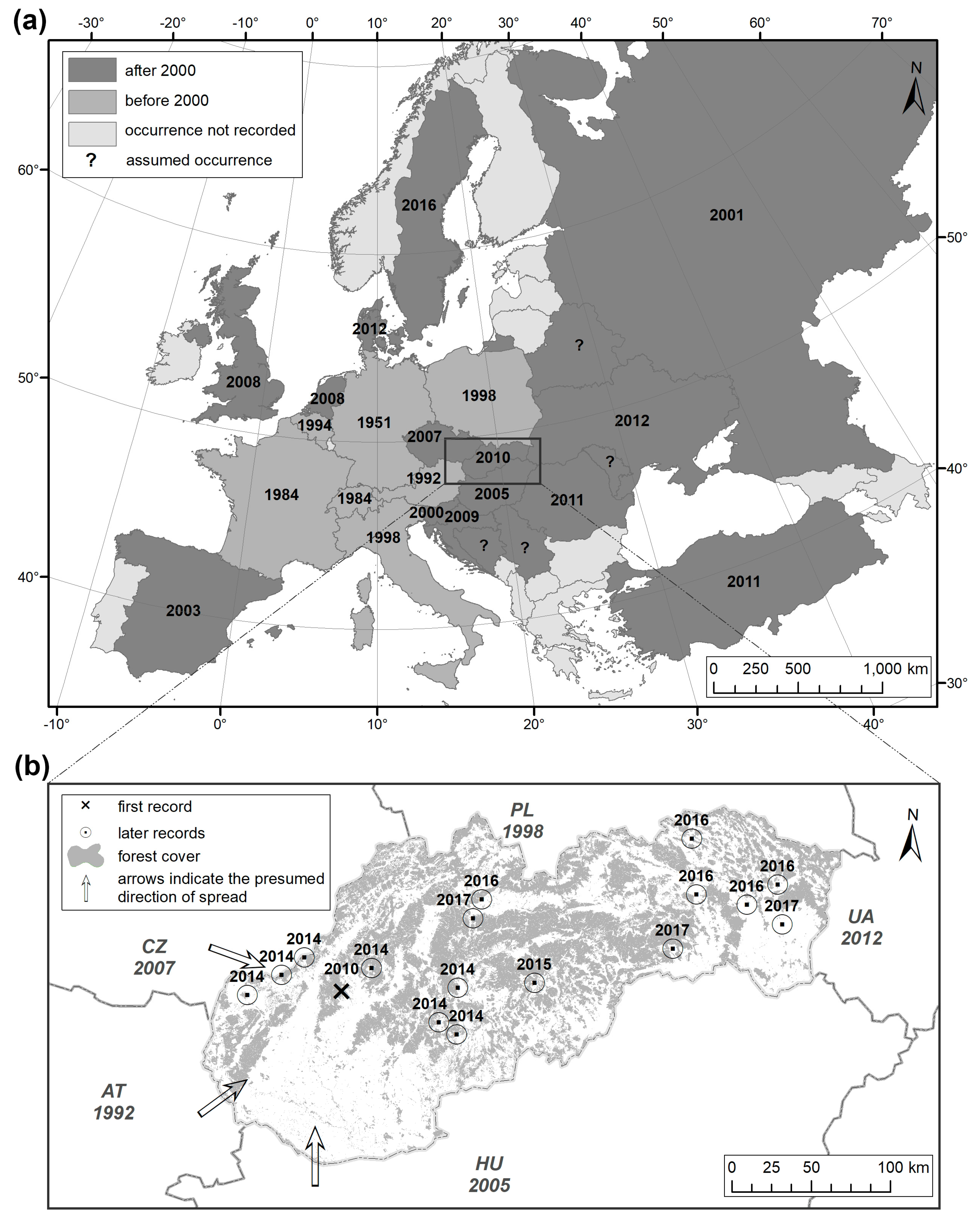
2. Spread of Xylosandrus germanus across Europe
2.1. Before 2000
2.1.1. Germany
2.1.2. Switzerland
2.1.3. France
2.1.4. Austria
2.1.5. Belgium
2.1.6. Poland
2.1.7. Italy
2.2. After 2000
2.2.1. Slovenia
2.2.2. Russia
2.2.3. Spain
2.2.4. Hungary
2.2.5. Czech Republic
2.2.6. Britain
2.2.7. The Netherlands
2.2.8. Croatia
2.2.9. Slovakia
2.2.10. Romania
2.2.11. Turkey
2.2.12. Ukraine
2.2.13. Denmark
2.2.14. Sweden
3. Spread and Occurrence of Xylosandrus germanus in Slovakia
4. Host Selection and Preference
5. Forest Management and Recommendations
- Logging, transport, storage, and processing of lumber should be carried out at periods without an increased abundance of technical pests. For instance, Franjević et al. [53] recommended the main felling period should be from October through March, harvesting should be prohibited during April and May, and thinning should take place from June through September.
- We recommend that valuable, top quality lumber, resulting from spring and winter logging stored at vulnerable sites from March to August, is preventively treated with an authorized insecticide (chemical treatment) [25].
- An alternative to chemical treatment is covering valuable lumber with protective nets infused with insecticides (Storanet®/Woodnet®, BASF®) [53,54]. According to Franjević et al. [53] and our personal observations, the netting system provided excellent control against bark and wood-boring insects attacking fresh cut logs. The Forest Stewardship Council (FSC) and World Health Organization (WHO) have approved the use of these chemically treated reusable fabrics [53].
- Auctions of high quality products should not take place at sites during periods when wood-boring insects occur there.
- Inspection of attacked wood material should be carried out visually (entrance holes, white piles of sawdust) (Figure 3). White sawdust is a typical sign of infestation.
- It is essential that personnel working with wood at vulnerable sites are aware of this species’ symptoms, since chemical treatments are only effective during the initial stages of attack.
- For monitoring the presence of X. germanus it is possible to use different types of traps [16,113] baited with ethanol. Traps provide information on the place, time, and abundance at which the monitored pest occurs [113]. However, mass trapping of X. germanus using ethanol-baited traps is not currently an effective management tactic [17,53].
- Heavily infested material should also be chipped or burned to avoid population build up [2].
- Wood products being imported into countries or regions where X. germanus has not yet reached should also be closely inspected and monitored.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rassati, D.; Faccoli, M.; Battisti, A.; Marini, L. Habitat and climatic preferences drive invasions of non-native ambrosia beetles in deciduous temperate forests. Biol. Invasions 2016, 18, 2809–2821. [Google Scholar] [CrossRef]
- Hulcr, J.; Stelinski, L.L. The ambrosia symbiosis: From evolutionary ecology to practical management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef]
- Haack, R.A. Non-native bark- and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Faccoli, M. Bark beetles and pinhole borers (Curculionidae, Scolytinae, Platypodinae) alien to Europe. ZooKeys 2010, 56, 227–251. [Google Scholar] [CrossRef] [Green Version]
- Haack, R.A.; Rabaglia, R.J.; Peña, J.E. Exotic bark and ambrosia beetles in the USA: Potential and current invaders. In Potential Invasive Pests of Agricultural Crops; Peña, J., Ed.; CAB International: Wallingford, UK, 2013; pp. 48–74. ISBN 9781845938291. [Google Scholar]
- Gomez, D.F.; Rabaglia, R.J.; Fairbanks, K.E.O.; Hulcr, J. North American Xyleborini north of Mexico: A review and key to genera and species (Coleoptera, Curculionidae, Scolytinae). ZooKeys 2018, 768, 19–68. [Google Scholar] [CrossRef]
- Smith, S.M.; Hulcr, J. Scolytus and other economically important bark and ambrosia beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press, Elsevier: Atlanta, GA, USA, 2015; pp. 495–531. ISBN 978-0-12-417156-5. [Google Scholar]
- Batra, L.R. Ambrosia beetles and their associated fungi: Research trends and techniques. Proc. Plant Sci. 1985, 94, 137–148. [Google Scholar]
- Weber, B.C.; McPherson, J.E. A Life history of the ambrosia beetle Xylosandrus germanus (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1983, 76, 455–462. [Google Scholar] [CrossRef]
- Křístek, J.; Urban, J. Lesnická Entomologie; Academia: Praha, Czech Republic, 2004; p. 446. ISBN 80-200-1052-1. [Google Scholar]
- Peer, K.; Taborsky, M. Outbreeding depression, but no inbreeding depression in haplodiploid ambrosia beetles with regular sibling mating. Evolution 2005, 59, 317–323. [Google Scholar] [CrossRef]
- CABI. Xylosandrus germanus (Black Timber Bark Beetle). Available online: https://www.cabi.org/isc/datasheet/57237#01273E6F-6FB1-49FC-B651-1D988B1C1ED6 (accessed on 26 November 2018).
- Mayers, C.G.; McNew, D.L.; Harrington, T.C.; Roeper, R.A.; Fraedrich, S.W.; Biedermann, P.H.W.; Castrillo, L.A.; Reed, S.E. Three genera in the Ceratocystidaceae are the respective symbionts of three independent lineages of ambrosia beetles with large, complex mycangia. Fungal Biol. 2015, 119, 1075–1092. [Google Scholar] [CrossRef]
- Dute, R.R.; Miller, M.E.; Davis, M.A.; Woods, F.M.; McLean, K.S. Effects of ambrosia beetle attack on Cercis canadensis. IAWA J. 2002, 23, 143–160. [Google Scholar] [CrossRef]
- Hulcr, J.; Rountree, N.R.; Diamond, S.E.; Stelinski, L.L.; Fierer, N.; Dunn, R.R. Mycangia of ambrosia beetles host communities of bacteria. Microb. Ecol. 2012, 64, 784–793. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Schultz, P.B.; Oliver, J.B.; Frank, S.D.; Addesso, K.M.; Chong, J.H.; Sampson, B.; Werle, C.; Gill, S.; et al. Biology, ecology, and management of nonnative ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in ornamental plant nurseries. J. Integr. Pest Manag. 2016, 7, 1–23. [Google Scholar] [CrossRef]
- Grégoire, J.-C.; Piel, F.; de Proft, M.; Gilbert, M. Spatial distribution of ambrosia beetle catches: A possibly useful knowledge to improve mass-trapping. Integr. Pest Manag. Rev. 2001, 6, 237–242. [Google Scholar] [CrossRef]
- Felt, E.P. A new pest in greenhouse grown grape stems. J. Econ. Entomol. 1932, 25, 418. [Google Scholar] [CrossRef]
- Groschke, F. Der «schwarze Nutzholzborkenkäfer», Xylosandrus germanus Blandf., ein neuer Schädling in Deutschland). Z. Angew. Entomol. 1953, 34, 297–302. [Google Scholar] [CrossRef]
- Kamp, H.J. Der “Schwarze Nutzholzborkenkäfer” Xylosandrus germanus Blandf., ein Neuling der heimischen Insektenfauna. Entomologische Blätter 1968, 64, 31–39. [Google Scholar]
- Björklund, N.; Boberg, J. Rapid Pest Risk Analysis Xylosandrus germanus. Tech. Rep. 2017, 22. [Google Scholar] [CrossRef]
- Inward, D.J.G. Rapid Pest Risk Analysis for Xylosandrus Germanus (Coleoptera: Scolytinae); Report; Forest Research, Alice Holt Lodge: Farnham, UK, 2015.
- Wichmann, H.E. Zur derzeitigen Verbreitung des Japanisches Nutzholzborkenkäfers Xylosandrus germanus Blandf. im Bundesgebiete. Z. Angew. Entomol. 1955, 37, 250–258. [Google Scholar] [CrossRef]
- Wichmann, H.E. Einschleppungsgeschichte und Verbreitung des Xylosandrus germanus Blandf. in Westdeutschland. Z. Angew. Entomol. 1957, 40, 82–99. [Google Scholar] [CrossRef]
- Maksymov, J.K. Erstmaliger massenbefall des schwarzen nutzholzborkenkäfers, Xylosandrus germanus Blandf., in der Schweiz. Schweiz. Z. Forstwes. 1987, 138, 215–227. [Google Scholar] [CrossRef]
- Schott, C. Catalogue et Atlas des Coleopteres d’Alsace, Tome 6: Scolytidae; Sociéte’ Alsacienne d’Entomologie. Musée zoologique de l’universite´ et de la ville de Strasbourg: Strasbourg, France, 1994; p. 85. ISBN 2908980053. [Google Scholar]
- Bouget, C.; Noblecourt, T. Short-term development of ambrosia and bark beetle assemblages following a windstorm in French broadleaved temperate forests. J. Appl. Entomol. 2005, 129, 300–310. [Google Scholar] [CrossRef]
- Wood, S.L.; Bright, D.E. A Catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic Index Volume A; Brigham Young University: Salt Lake City, UT, USA, 1992; 833p, ISBN 10-0842523103. [Google Scholar]
- Holzschuh, C. Erster Nachweis des Schwarzen Nutzholzborkenkäfers (Xylosandrus germanus) in Österreich. Forstschutz Aktuell 1993, 12, 10. [Google Scholar]
- Geiser, E.; Geiser, R. Erstnachweise und Wiederfunde von Alt-und Totholzkäfern in der Stadt Salzburg. Koleopterol. Rundsch. 2000, 70, 209–222. [Google Scholar]
- Holzinger, W.E.; Frieß, T.; Holzer, E.; Mehlmauer, P. Xylobionte Käfer (Insecta: Coleoptera part.) in Wäldern des Biosphärenparks Wienerwald (Österreich: Niederösterreich, Wien). Wissenschaftliche Mitteilungen des Niederösterreichischen Landesmuseums 2014, 25, 331–362. [Google Scholar]
- Bruge, H. Xylosandrus germanus (Blandford, 1894) (Belg. sp. nov.) (Coleoptera Scolytidae). Bulletin et Annales de la Société Royale Belge d’Entomologie 1995, 131, 249–264. [Google Scholar]
- Henin, J.M.; Versteirt, V. Abundance and distribution of Xylosandrus germanus (Blandford 1894) (Coleoptera Scolytidae) in Belgium: New observations and an attempt to outline its range. J. Pest Sci. 2004, 77, 57–63. [Google Scholar] [CrossRef]
- Mokrzycki, T.; Hilszczański, J.; Borowski, J.; Cieślak, R.; Mazur, A.; Miłkowski, M.; Szołtys, H. Faunistic review of Polish Platypodinae and Scolytinae (Coleoptera: Curculionidae). Pol. J. Entomol. 2011, 80, 343–364. [Google Scholar] [CrossRef] [Green Version]
- Mokrzycki, T.; Grodzki, W. Drzewotocz japoński Xylosandrus germanus (Bldf.) (Coleoptera: Curculionidae, Scolytinae) w Polsce. Sylwan 2014, 158, 590–594. [Google Scholar]
- Frigimelica, G.; Stergulc, F.; Zandigiacomo, P.; Faccoli, M.; Battisti, A. Xylosandrus germanus and Walnut Disease: An Association New to Europe. In Methodology of Forest Insect and Disease Survey in Central Europe; Forster, B., Knížek, M., Grodzki, W., Eds.; Swiss Federal Institute for Forest, Snow and Landscape Research: Sion, Switzerland, 1999; pp. 98–101. [Google Scholar]
- Stergulc, F.; Frigimelica, G.; Zandigiacomo, P.; Battisti, A. Gravi deperimenti del nocecommune in giovani impianti da legno in Friuli-Venezia Giulia. Sherwood 1999, 44, 27–30. [Google Scholar]
- Faccoli, M. Xylosandrus germanus (Blandford, 1894) (= Xylosandrus germanus)—Black stem borer (Coleoptera: Curculionidae, Scolytinae). In Alien Terrestrial Arthropods of Europe; Roques, A., Kenis, M., Lees, D., Lopez-Vaamonde, C., Rabitsch, W., Rasplus, J.Y., Roy, D.B., Eds.; Pensoft Publishers: Sofia, Bulgaria, 2010; pp. 902–903. ISBN 978-954-642-554-6. [Google Scholar]
- Jurc, M.; Borkovič, D.; Pavlin, R.; Meterc, G. Xylosandrus germanus (Blandfort, 1894) (Curculionidae: Scolytinae) in Slovenia (conference paper). In Proceedings of the Symposium Internationale Entomofaunisticum Europae Centralis XXII, Varaždin, Croatia, 29 June–3 July 2011; pp. 33–34. [Google Scholar]
- Jurc, M. Some harmful native and non-native insects in the forests of the Ljubljana area. Gozdar. Vest. 2010, 68, 321–329. (In Slovenian) [Google Scholar]
- Jurc, M.; Repe, A. Some new immigrant phytophagous insects on woody plants in Slovenia. Forstschutz Aktuell 2012, 55, 32–33. [Google Scholar]
- Mandelshtam, M.Y. New synonymy and new records in Palaearctic Scolytidae (Coleoptera). Zoosytematica Rossica 2001, 9, 203–204. [Google Scholar]
- Sweeney, J.D.; Silk, P.; Grebennikov, V.; Mandelshtam, M. Efficacy of semiochemical-baited traps for detection of Scolytinae species (Coleoptera: Curculionidae) in the Russian Far East. Eur. J. Entomol. 2016, 113, 84–97. [Google Scholar] [CrossRef] [Green Version]
- López, S.; Iturrondobeitia, J.C.; Goldarazena, A. Primera cita en la Península Ibérica de Gnathotrichus materiarius (Fitch, 1858) y Xylosandrus germanus (Blandford, 1894) (Coleoptera: Scolytinae). Boletín de la Sociedad Entomológica Aragonesa 2007, 40, 527–532. [Google Scholar]
- Goldarazena, A.; Bright, D.E.; Hishinuma, S.M.; López, S.; Seybold, S.J. First record of Pityophthorus solus (Blackman, 1928) in Europe. EPPO Bull. 2014, 44, 65–69. [Google Scholar] [CrossRef]
- Lakatos, F.; Kajimura, H. Occurrence of the introduced Xylosandrus germanus (Blandford, 1894) in Hungary—A genetic evidence (Coleoptera: Scolytidae). Folia Entomol. Hung. 2007, 68, 97–104. [Google Scholar]
- Knížek, M. Faunistic records from the Czech Republic—272. Coleoptera: Curculionidae: Scolytinae. Klapalekiana 2009, 45, 22. [Google Scholar]
- Knížek, M.; (Forestry and Game Management Research Institute, Jíloviště, Czech Republic). Personal communication, 2018.
- Kula, E.; (Mendel University, Brno, Czech Republic). Personal communication, 2018.
- Allen, A.J.; Hammond, P.M.; Telfer, M.G. Xylosandrus germanus (Blandford, 1894) (Curculionidae: Scolytinae) in Britain. Coleopterist 2015, 24, 72–75. [Google Scholar]
- Inward, D.; (Forest Research—Forestry Commission UK, Burnham, UK). Personal communication, 2018.
- Vorst, O.; Heijerman, T.; van Nunen, F.; van Wielink, P. Several bark beetles new to the Dutch fauna (Coleoptera: Curculionidae: Scolytinae). Nederl. Faun. Med. 2008, 29, 61–74. (In Dutch) [Google Scholar]
- Franjević, M.; Poršinsky, T.; Đuka, A. Integrated oak timber protection from ambrosia bark beetles: Economic and ecological importance in harvesting operations. Croat. J. For. Eng. 2016, 37, 353–364. [Google Scholar]
- Franjević, M. Novel Biotechnical Methods within the Integrated Protection of Oak Timber against Ambrosia Beetles. Ph.D. Thesis, Faculty of Forestry University of Zagreb, Zagreb, Croatia, 2012; pp. 1–224. (In Croatian). [Google Scholar]
- Galko, J. First record of the ambrosia beetle, Xylosandrus germanus (Blandford, 1894) (Coleoptera: Curculionidae, Scolytinae) in Slovakia. For. J. 2013, 58, 279. [Google Scholar]
- Galko, J.; Nikolov, C.; Kimoto, T.; Kunca, A.; Gubka, A.; Vakula, J.; Zúbrik, M.; Ostrihoň, M. Attraction of ambrosia beetles to ethanol baited traps in a Slovakian oak forest. Biologia 2014, 69, 1376–1383. [Google Scholar] [CrossRef] [Green Version]
- Olenici, N.; Knížek, M.; Olenici, V.; Duduman, M.-L.; Biriş, I.-A. First report of three scolytid species (Coleoptera: Curculionidae, Scolytinae) in Romania. Ann. For. Res. 2014, 57, 89–97. [Google Scholar] [CrossRef]
- Olenici, N.; Duduman, L.M.; Tomescu, R. Xylosandrus germanus (Coleoptera, Curculionidae, Scolytinae)—A potential pest of forests, orchards and vineyards in Romania. Bucov. For. 2015, 15, 207–216. [Google Scholar]
- Ak, K.; Saruhan, İ.; Tuncer, C.; Akyol, H.; Kılıç, A. Ordu İli Kivi Bahçelerinde Yazıcı böcek (Coleoptera: Scolytidae) türlerinin tespiti ve zarar oranları. Türkiye Entomoloji Bülteni 2011, 1, 229–234. [Google Scholar]
- Knížek, M. Scolytinae. Catalogue of Palaearctic Coleoptera 2011, 7, 204–251. [Google Scholar]
- Tuncer, C.; Knížek, M.; Hulcr, J. Scolytinae in hazelnut orchards of Turkey: Clarification of species and identification key (Coleoptera, Curculionidae). ZooKeys 2017, 710, 65. [Google Scholar] [CrossRef]
- Nazarenko, V.Y.; Gontarenko, A.V. The First Record of Xylosandrus germanus (Coleoptera, Curculionidae) in Ukraine. Vestn. Zool. 2014, 48, 570. [Google Scholar]
- Hansen, M.; Jørum, P. Records of beetles from Denmark, 2012 and 2013 (Coleoptera). Entomol. Med. 2014, 82, 113–168. [Google Scholar]
- Lundberg, S. Nytillkomna och strukna skalbaggsarter sedan 1995 års Catalogus Coleopterorum Sueciae. Ent. Tidskr. 2006, 127, 101–111. [Google Scholar]
- Björklund, N.; (Swedish University of Agricultural Sciences, Uppsala, Sweden). Personal communication, 2018.
- Galko, J.; (National Forest Centre, Zvolen, Slovakia). Personal communication, 2018.
- Zach, P.; Topp, W.; Kulfan, J.; Simon, M. Colonization of two alien ambrosia beetles (Coleoptera, Scolytidae) on debarked spruce logs. Biologia 2001, 56, 175–181. [Google Scholar]
- Haase, V.; Topp, W.; Zach, P. Eichen-Totholz im Wirtschaftswald als Lebensraum für xylobionte Insekten. Zeitschrift für Ökologie und Naturschutz 1998, 7, 137–153. [Google Scholar]
- Bussler, H.; Blaschke, M.; Walentowski, H. Bemerkenswerte xylobionte Käferarten in Naturwaldreservaten des Bayerischen Waldes (Coleoptera). Entomol. Z. 2010, 120, 263–268. [Google Scholar]
- Blaschke, M.; Bussler, H. Borkenkäfer und baumschädigende Holzpilze in einem Höhengradienten des Bayerischen Waldes. Forstschutz Aktuell 2012, 54, 10–15. [Google Scholar]
- Zach, P.; Patočka, J.; Kulfan, J.; Krištín, A.; Šušlík, V. Forest zonation and faunal assemblages of the Poľana Biosphere Reserve UNESCO. Ekológia 1995, 14, 353–365. [Google Scholar]
- Ranger, C.; (USDA-Agricultural Research Service, Wooster, OH, USA). Personal communication, 2018.
- Galko, J.; (National Forest Centre, Zvolen, Slovakia). Personal communication, 2015.
- Zach, P.; Galko, J.; Dzurenko, M.; Kulfan, J. Ekológia a Výskyt Drvinárika Cierneho Xylosandrus germanus na Slovensku in Aktuálne Problémy v Ochrane Lesa; Nový Smokovec, S., Kunca, A., Eds.; Národné Lesnícke Centrum: Zvolen, Slovakia, 2017; pp. 87–91. [Google Scholar]
- Dzurenko, M.; (Institute of Forest Ecology SAS, Zvolen, Slovakia). Personal communication, 2018.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Ito, M.; Kajimura, H.; Hamaguchi, K.; Araya, K.; Lakatos, F. Genetic structure of Japanese populations of an ambrosia beetle, Xylosandrus germanus (Curculionidae: Scolytinae). Entomol. Sci. 2008, 11, 375–383. [Google Scholar] [CrossRef]
- Weber, B.C.; McPherson, J.E. World list of host plants of Xylosandrus germanus (Blandford) (Coleoptera: Scolytidae). Coleop. Bull. 1983, 37, 114–134. [Google Scholar]
- Weber, B.C.; McPherson, J.E. Attack on black walnut trees by the ambrosia beetle Xylosandrus germanus (Coleoptera: Scolytidae). For. Sci. 1984, 30, 864–870. [Google Scholar] [CrossRef]
- Oliver, J.B.; Mannion, C.M. Ambrosia beetle (Coleoptera: Scolytidae) species attacking chestnut and captured in ethanol-baited traps in middle Tennessee. Environ. Entomol. 2001, 30, 909–918. [Google Scholar] [CrossRef]
- Reding, M.E.; Oliver, J.B.; Schultz, P.B.; Ranger, C.M. Monitoring flight activity of ambrosia beetles in ornamental nurseries with ethanol-baited traps: Influence of trap height on captures. J. Environ. Hort. 2010, 28, 85–90. [Google Scholar]
- Reding, M.E.; Schultz, P.B.; Ranger, C.M.; Oliver, J.B. Optimizing ethanol-baited traps for monitoring damaging ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in ornamental nurseries. J. Econ. Entomol. 2011, 104, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Reding, M.E.; Ranger, C.M.; Sampson, B.J.; Werle, C.T.; Oliver, J.B.; Schultz, P.B. Movement of Xylosandrus germanus (Coleoptera: Curculionidae) in ornamental nurseries and surrounding habitats. J. Econ. Entomol. 2015, 108, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Galko, J.; Kunca, A.; Rell, S.; Zúbrik, M.; Nikolov, C.; Gubka, A.; Vakula, J. Drvinárik čierny (Xylosandrus germanus), ako nový technický škodca dreva na Slovensku. In Aktuálne Problémy v Ochrane Lesa; Nový Smokovec, S., Kunca, A., Eds.; Národné Lesnícke Centrum: Zvolen, Slovakia, 2015; pp. 35–40. [Google Scholar]
- Ranger, C.M.; Tobin, P.C.; Reding, M.E. Ubiquitous volatile compound facilitates efficient host location by a non-native ambrosia beetle. Biol. Invasions 2015, 17, 675–686. [Google Scholar] [CrossRef]
- Agnello, A.; Breth, D.; Tee, E.; Cox, K.; Warren, H.R. Ambrosia beetle—An emergent apple pest. N. Y. Fruit Q. 2015, 23, 25–28. [Google Scholar]
- Agnello, A.M.; Breth, D.I.; Tee, E.M.; Cox, K.D.; Villani, S.M.; Ayer, K.M.; Wallis, A.E.; Donahue, D.J.; Combs, D.B.; Davis, A.E.; et al. Xylosandrus germanus (Coleoptera: Curculionidae: Scolytinae) occurrence, fungal associations, and management trials in New York apple orchards. J. Econ. Entomol. 2017, 110, 2149–2164. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Manser, P. Beitrag zum eingeschleppten Schwarzen Nutzholzborkenkäfer Xylosandrus germanus. Biologie und Schadenpotential an im Wald gelagertem Rundholz im Vergleich zu Xyloterus lineatus und Hylecoetus dermestoides. Schweiz. Z. Forstwes. 2000, 151, 271–281. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.; Persad, A.; Herms, D. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) and other ambrosia beetles. Agric. For. Entomol. 2010, 12, 177–185. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Schultz, P.B.; Oliver, J.B. Ambrosia beetle (Coleoptera: Curculionidae) responses to volatile emissions associated with ethanol-injected Magnolia virginiana L. Environ. Entomol. 2012, 41, 636–647. [Google Scholar] [CrossRef]
- Ranger, C.M.; Biedermann, P.H.W.; Phuntumart, V.; Beligala, G.U.; Ghosh, S.; Palmquist, D.E.; Mueller, R.; Barnett, J.; Schultz, P.B.; Reding, M.E.; et al. Symbiont selection via alcohol benefits fungus farming by ambrosia beetles. Proc. Natl. Acad. Sci. USA 2018, 115, 4447–4452. [Google Scholar] [CrossRef] [Green Version]
- Kimmerer, T.W.; Kozlowski, T.T. Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiol. 1982, 69, 840–847. [Google Scholar] [CrossRef]
- Kimmerer, T.W.; MacDonald, R.C. Acetaldehyde and ethanol biosynthesis in leaves of plants. Plant Physiol. 1987, 84, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, R.G.; Joseph, G. Attraction of Scolytus unispinosus bark beetles to ethanol in water-stressed Douglas-fir branches. For. Ecol. Manag. 2001, 144, 229–238. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Beh, M.M.; Shaw, D.C.; Manter, D.K. Ethanol attracts scolytid beetles to Phytophthora ramorum cankers on coast live oak. J. Chem. Ecol. 2013, 39, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Ranger, C.M.; Schultz, P.B.; Frank, S.D.; Chong, J.H.; Reding, M.E. Non-native ambrosia beetles as opportunistic exploiters of living but weakened trees. PLoS ONE 2015, 10, e0131496. [Google Scholar] [CrossRef]
- La Spina, S.; De Cannière, C.; Dekri, A.; Grégoire, J.C. Frost increases beech susceptibility to scolytine ambrosia beetles. Agric. For. Entomol. 2013, 15, 157–167. [Google Scholar] [CrossRef]
- Ranger, C.M.; Schultz, P.B.; Frank, S.D.; Reding, M.E. Freeze stress of deciduous trees induces attacks by opportunistic ambrosia beetles. Agric. For. Entomol. 2018. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Schultz, P.B.; Oliver, J.B. Influence of flood-stress on ambrosia beetle host selection and implications for their management in a changing climate. Agric. For. Entomol. 2013, 15, 56–64. [Google Scholar] [CrossRef]
- Kelsey, R.G. Ethanol and ambrosia beetles in Douglas fir logs with and without branches. J. Chem. Ecol. 1994, 20, 3307–3319. [Google Scholar] [CrossRef]
- Kelsey, R.G. Ethanol synthesis in Douglas-fir logs felled in November, January, and March and its relationship to ambrosia beetle attack. Can. J. For. Res. 1994, 24, 2096–2104. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Joseph, G. Ambrosia beetle host selection among logs of Douglas fir, western hemlock, and western red cedar with different ethanol and α-pinene concentrations. J. Chem. Ecol. 1997, 23, 1035–1051. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Joseph, G. Ethanol and ambrosia beetles in Douglas fir logs exposed or protected from rain. J. Chem. Ecol. 1999, 25, 2793–2809. [Google Scholar] [CrossRef]
- Hale, F.A. Entomological research priorities for nursery and landscape ornamentals. In Proceeding 52nd Annual Southern Nursery Association Research Confernce, Atlanta, GA; James, B.L., Ed.; Southern Nursery Association, Inc.: Acworth, GA, USA, 2007; pp. 17–21. [Google Scholar]
- Solomon, J.D. Guide to insect borers in North American broadleaf trees and shrubs. In Agriculture Handbook (Washington) (AH-706); U.S. Department of Agriculture-Forest Service: Washington, DC, USA, 1995; 735p. [Google Scholar]
- Haack, R.A.; Petrice, T.R. Bark-and wood-borer colonization of logs and lumber after heat treatment to ISPM 15 specifications: The role of residual bark. J. Econ. Entomol. 2009, 102, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Labonte, J.R.; Mudge, A.D.; Johnson, K.J.R. Nonindigenous woodboring coleoptera (Cerambycidae, Curculionidae: Scolytinae) new to Oregon and Washington, 1999–2002: Consequences of the intracontinental movement of raw wood products and solid wood packing materials. Proc. Entomol. Soc. Wash. 2005, 107, 554–564. [Google Scholar]
- Faccoli, M. Bioecologia di coleotteri scolitidi Ips typographus (Linnaeus) e species di recente interesse per la selvicoltura italiana. III contributo. Reperti su specie di scolitidi nuove per il territorio italiano. Bollettino dell’ Istituto di Entomologia ‘Guido Grandi’ della Universita degli Studi di Bologna 2000, 54, 77–90. [Google Scholar]
- Graf, E.; Manser, P. Der Schwarze Nutzholzborkenkäfer, Xylosandrus germanus, in der Schweiz. Holz-Zentralblatt 1996, 122, 454–456. [Google Scholar]
- Galko, J.; (National Forest Centre, Zvolen, Slovakia). Personal communication, 2016.
- Lesy, S.R.; (Forests of the Slovak Republic, Banská Bystrica, Slovakia). Personal communication, 2018.
- Galko, J.; Kunca, A.; Rell, S.; Zúbrik, M.; Nikolov, C.H.; Vakula, J.; Gubka, A. Charakteristika najzávažnejších drevokazných druhov hmyzích škodcov a opatrenia ochrany lesa proti nim. In Aktuálne problémy v ochrane lesa; Nový Smokovec, S., Kunca, A., Eds.; Národné Lesnícke Centrum: Zvolen, Slovakia, 2016; pp. 22–29. [Google Scholar]
- Galko, J.; Nikolov, C.; Kunca, A.; Vakula, J.; Gubka, A.; Zúbrik, M.; Rell, S.; Konôpka, B. Effectiveness of pheromone traps for the European spruce bark beetle: A comparative study of four commercial products and two new models. For. J. 2016, 62, 207–215. [Google Scholar] [CrossRef]
- Suh, S.J. Lethal temperature for the black timber bark beetle, Xylosandrus germanus (Coleoptera: Scolytidae), in infested wood using microwave energy. Curr. Res. Agric. Life Sci. 2014, 32, 131–134. [Google Scholar] [CrossRef]
- Galko, J.; Vakula, J.; Rell, S.; Gubka, A.; Kunca, A. Ochrana dreva na lesných skladoch a charakteristika najvýznamnejších drevokazných druhov fuzáčov a píloviek. In Aktuálne Problémy v Ochrane Lesa; Nový Smokovec, S., Kunca, A., Eds.; Národné Lesnícke Centrum: Zvolen, Slovakia, 2017; pp. 92–98. [Google Scholar]
- Castrillo, L.A.; Griggs, M.H.; Ranger, C.M.; Reding, M.E.; Vandenberg, J.D. Virulence of commercial strains of Beauveria bassiana and Metarhizium brunneum (Ascomycota: Hypocreales) against adult Xylosandrus germanus (Coleoptera: Curculionidae) and impact on brood. Biol. Control 2011, 58, 121–126. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Griggs, M.H.; Vandenberg, J.D. Granulate ambrosia beetle, Xylosandrus crassiusculus (Coleoptera: Curculionidae), survival and brood production following exposure to entomopathogenic and mycoparasitic fungi. Biol. Control 2013, 67, 220–226. [Google Scholar] [CrossRef]
- Carrillo, D.; Dunlap, C.A.; Avery, P.B.; Navarrete, J.; Duncan, R.E.; Jackson, M.A.; Behle, R.W.; Cave, R.D.; Crane, J.; Rooney, A.P.; et al. Entomopathogenic fungi as biological control agents for the vector of the laurel wilt disease, the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae). Biol. Control 2015, 81, 44–50. [Google Scholar] [CrossRef]
- Murphy, J. Predictions of climate change over Europe using statistical and dynamical downscaling techniques. Int. J. Climatol. 2000, 20, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Chang. 2014, 14, 563–578. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galko, J.; Dzurenko, M.; Ranger, C.M.; Kulfan, J.; Kula, E.; Nikolov, C.; Zúbrik, M.; Zach, P. Distribution, Habitat Preference, and Management of the Invasive Ambrosia Beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European Forests with an Emphasis on the West Carpathians. Forests 2019, 10, 10. https://doi.org/10.3390/f10010010
Galko J, Dzurenko M, Ranger CM, Kulfan J, Kula E, Nikolov C, Zúbrik M, Zach P. Distribution, Habitat Preference, and Management of the Invasive Ambrosia Beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European Forests with an Emphasis on the West Carpathians. Forests. 2019; 10(1):10. https://doi.org/10.3390/f10010010
Chicago/Turabian StyleGalko, Juraj, Marek Dzurenko, Christopher M. Ranger, Ján Kulfan, Emanuel Kula, Christo Nikolov, Milan Zúbrik, and Peter Zach. 2019. "Distribution, Habitat Preference, and Management of the Invasive Ambrosia Beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European Forests with an Emphasis on the West Carpathians" Forests 10, no. 1: 10. https://doi.org/10.3390/f10010010
APA StyleGalko, J., Dzurenko, M., Ranger, C. M., Kulfan, J., Kula, E., Nikolov, C., Zúbrik, M., & Zach, P. (2019). Distribution, Habitat Preference, and Management of the Invasive Ambrosia Beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European Forests with an Emphasis on the West Carpathians. Forests, 10(1), 10. https://doi.org/10.3390/f10010010




