Abstract
Contrast enhancement techniques serve the purpose of diminishing image noise and increasing the contrast of relevant structures. In the context of medical images, where the differentiation between normal and abnormal tissues can be quite subtle, precise interpretation might become challenging when noise levels are relatively elevated. The Fast Local Laplacian Filter (FLLF) is proposed to deliver a more precise interpretation and present a clearer image to the observer; this is achieved through the reduction of noise levels. In this study, the FLLF strengthened images through its unique contrast enhancement capabilities while preserving important image details. It achieved this by adapting to the image’s characteristics and selectively enhancing areas with low contrast, thereby improving the overall visual quality. Additionally, the FLLF excels in edge preservation, ensuring that fine details are retained and that edges remain sharp. Several performance metrics were employed to assess the effectiveness of the proposed technique. These metrics included Peak Signal-to-Noise Ratio (PSNR), Mean Squared Error (MSE), Root Mean Squared Error (RMSE), Normalization Coefficient (NC), and Correlation Coefficient. The results indicated that the proposed technique achieved a PSNR of 40.12, an MSE of 8.6982, an RMSE of 2.9492, an NC of 1.0893, and a Correlation Coefficient of 0.9999. The analysis highlights the superior performance of the proposed method when contrast enhancement is applied, especially when compared to existing techniques. This approach results in high-quality images with minimal information loss, ultimately aiding medical experts in making more accurate diagnoses.
1. Introduction
Angiography, sometimes referred to as arteriography or an angiogram, is a medical procedure used by physicians to examine the interior of blood vessels, typically arteries, and various organs within the body [1]. This procedure primarily focuses on investigating arteries, veins, and the chambers of the heart. Its applications are extensive, encompassing the diagnosis of a wide range of medical conditions and irregularities related to blood vessels. Angiography can be performed on various parts of the body, depending on the specific medical needs and diagnostic purposes, such as cerebral angiography, peripheral angiography, pulmonary angiography, renal angiography, aortic angiography, retinal angiography, and coronary angiography.
Cardiovascular diseases remain a leading cause of mortality worldwide. Coronary angiography plays a pivotal role in diagnosing and assessing coronary artery diseases and cardiovascular diseases, offering vital insights into the conditions of blood vessels and the overall cardiac health of patients. Additionally, coronary angiography is instrumental in facilitating medical interventions and treatments for issues such as vessel narrowing, blockages, and other conditions, particularly those impacting the heart. Despite its clinical significance, angiography images often exhibit variations in contrast, brightness, and overall quality due to diverse imaging conditions and patient characteristics [2]. Such variability can hinder accurate diagnosis and analysis, underscoring the need for advanced contrast enhancement techniques to improve the visibility of blood vessels and intricate vascular structures [3].
In recent years, digital image processing has emerged as a powerful tool for enhancing the quality of medical images, thereby facilitating more precise medical evaluations and diagnoses. Among the array of techniques available, contrast enhancement has garnered substantial attention due to its ability to improve image clarity and optimize visual information. This study delves into the application of the Fast Local Laplacian Filter (FLLF) technique for enhancing blood vessel contrast in coronary angiography images [4]. Modern medical imaging relies on digital processing methodologies to elevate image quality and extract crucial diagnostic information [5]. Contrast enhancement, in particular, has emerged as a promising avenue to amplify the visibility of relevant structures, thereby facilitating more accurate and confident clinical interpretations [6,7,8,9]. This study capitalizes on the Fast Local Laplacian Filter technique’s potential to enhance contrast while maintaining image details and minimizing undesirable artifacts.
The central objective of this study is to explore the effectiveness of Fast Local Laplacian Filter techniques in enhancing blood vessel contrast within coronary angiography images [10,11]. In the FLLF technique, a Laplacian pyramid is created to capture local image variations and details at different scales. After that, the modified Laplacian (ML) is introduced through a bilateral filter applied to each level of the Laplacian pyramid. This bilateral filter is controlled by two factors: the spatial distance (d) and the range similarity (r). It effectively preserves edges, reduces noise, and enhances image contrast. The FLLF technique offers a localized approach that adapts to the image’s intrinsic features, preserving image integrity while elevating its visual impact. The inherent adaptability of FLLF techniques to varying degrees of contrast enhancement makes it a compelling candidate for medical image enhancement applications.
This research paper encompasses an in-depth examination of the application and outcomes of Fast Local Laplacian Filter techniques in the context of coronary angiography [12]. The FLLF algorithm is dissected and tailored to the unique requirements of angiography images, with special attention to achieving an optimal balance between improved contrast and the preservation of diagnostic information.
This paper includes an overview of related works in contrast enhancement (Section 2); a detailed exposition of the FLLF methodology (Section 3); the experimental setup, dataset description, and the results and their implications (Section 4); and concluding remarks and avenues for future research (Section 5).
2. Literature Study
In recent times, medical image processing has emerged as a captivating and highly relevant research area for scholars. Within this field, two key aspects have garnered considerable attention: image enhancement, and the identification of low-contrast issues [13]. Both of these areas hold significant importance in the realm of medical image processing. Image enhancement aims to improve the quality and visual clarity of medical images, while the identification of low-contrast problems plays a crucial role in identifying and addressing issues related to image quality and visibility [14]. A summary of the literature review is shown in Table 1.
The current registration techniques fall short of preserving valuable information and image quality. To address this limitation and maintain the original image’s features, the proposed method combines contrast enhancement with the registration process. In this new approach, a scanned medical image is taken as the starting point. To enhance image quality, a dynamic histogram equalization method is used [15]. The proposed method focuses on making medical images look better, rather than just improving technical measures like PSNR or the amount of data that can be hidden in them. They split the images into two parts: one with important details (Region of Interest) and one without. When it comes to medical images, it is crucial that details are highlighted in the less vibrant areas to help doctors diagnose effectively [16].
It is important to improve image quality as specialists find it difficult to analyze poor-quality images. To address this issue, a method called “Dynamic Histogram Equalization with Modified Histogram Equalization on Fuzzy based Improved Particle Swarm Optimization (FIPSO)” was developed. This approach aims to enhance the image’s contrast, making it easier for specialists to examine. It achieves this by smoothing out the image details and distributing pixel intensity to nearby pixels using a Gaussian function [17].
The proposed approach aims to improve the contrast of medical images. The core concept revolves around creating an optimization challenge that takes into account both overall and specific enhancements in order to create a robust image enhancement method [18].
In [19], a new method called the “adaptive fuzzy gray-level difference histogram equalization algorithm” was proposed, in order to offer a more precise interpretation and cleaner images with less noise. Initially, the differences in gray levels in the input image were calculated using binary similar patterns. These gray-level differences were then made more flexible, in order to handle any uncertainties in the image. After this adjustment, a fuzzy gray-level difference clip limit was computed to control excessive contrast enhancement, ensuring that only relevant enhancements are made. In [20], an algorithm was introduced called “piecewise linear histogram equalization (PLHE).” This algorithm is a more advanced version of CHE, offering simplicity, a piecewise linear approach, and the convenience of a single parameter to manage the enhancement process. In order to demonstrate how well this new algorithm works, we have chosen two measures, information entropy and sharpness, that showcase the visual improvements it brings about. Another improved method for local contrast preservation is adaptive histogram equalization. This technique incorporates various image processing steps, including changing color spaces, inverting images, reducing haze, and boosting color saturation [21]. A chaos-based optimization algorithm has also been developed to enhance contrast in medical images. In this approach, a weighted combined framework was introduced as the method for improving contrast, serving as the cost function [22].
Several methods have been proposed to enhance the contrast and quality of medical images, addressing the limitations of current registration techniques. These approaches combine contrast enhancement with the registration process, aiming to preserve valuable image details. They often utilize techniques like dynamic histogram equalization, modified histogram equalization with fuzzy-based optimization, and piecewise linear histogram equalization, with a focus on improving visual quality rather than just technical measures like PSNR. Additionally, some methods emphasize local contrast preservation through adaptive histogram equalization and image processing techniques such as color space changes, image inversion, dehazing, and saturation adjustment. In these methods, an optimization challenge is presented, considering both global and specific enhancements, in order to create robust image enhancement techniques, ultimately benefiting medical image interpretation and diagnosis.

Table 1.
Summary of the Literature Review.
Table 1.
Summary of the Literature Review.
| Study | Data Type | Techniques | Descriptions | Performance Metrics | Results |
|---|---|---|---|---|---|
| [15] | CT images | Fuzzy employed dynamic histogram equalization | Contrast enhancement is considered a significant aspect of medical analysis because diagnosis error can be minimized only by utilizing a better-quality image. | Average Pixel Intensity, Standard Deviation, Average gradient, Entropy, Mutual Information | |
| [16] | MRI images | Contrast stretching | An effective data hiding technique that enhances the visual quality of watermarked images while ensuring efficient concealment of the embedded data. | PSNR, SSIM | 16.24 0.9932 |
| [17] | Digital images | Dynamic Histogram Equalization | Enhancing the quality of MRI-generated images is crucial, as the complexity of these images often poses challenges for specialists during their analysis and interpretation. | PSNR, SSIM, MSE | 32.00 0.9000 0.300 |
| [18] | X-ray Image | Modified shark smell optimization | The main idea here is to introduce an optimization problem by considering both global and local enhancement to achieve a strong image enhancement method | Contrast, CNR, EME, WPSNR, Homogeneity | 0.89, 78.12 32.41 17.13, 0.85 |
| [19] | X-ray image | Fuzzy gray-level difference histogram equalization algorithm | The gray-level difference of an input image is calculated using the binary similar patterns. | Entropy, PSNR | 7.01, 38.15 |
| [20] | MRI images | Piecewise linear histogram equalization | The primary function involves ensuring a uniform distribution of histogram components across the entire grayscale range | Entropy, edge enhancement index (EHI) | 5.2001 5040 |
| [23] | MRI image | Limited Dynamic Weighted Histogram Equalization | The proposed reversible data hiding-based limited dynamic weighted histogram equalization technique for abnormal tumor regions improves the contrast and transmits hidden secret information. | PSNR | 34.65 |
| [21] | Digital images | Adaptive Histogram Equalization | The algorithm’s efficacy extends beyond enhancing medical images; it also caters to the enhancement of ordinary images captured under both low-light and daylight conditions. | PSNR, NAE | 53.4047 1.0267 |
| [24] | MRI images | Histogram equalization, adaptive gamma correction | Extracting pertinent information from low-contrast and poor-quality MRI images proves to be a formidable task. | PSNR, MSE | 29.07 80.92 |
| [22] | CT and MRI images | Chaos-based optimization | Medical images often exhibit characteristics such as low contrast, significant noise, and compact dimensions. The accurate identification of anomalies within these images heavily relies on their quality and level of clarity. | Entropy, edge contents | 6.65 0.19 |
3. Proposed Methodology
In this study, the primary focus was the improvement of the quality of medical images, particularly for diagnosing coronary disease. The main goal was to enhance images that have low contrast and may contain noise, making it difficult for medical professionals to accurately diagnose heart-related conditions. The aim was to make these images clearer and cleaner, ultimately aiding in more effective and reliable diagnoses for coronary diseases.
3.1. Fast Local Laplacian Filter
The Fast Local Laplacian Filter (FLLF) technique is an advanced approach for enhancing the contrast of images while preserving their fine details and textures. Unlike traditional global contrast enhancement methods that can lead to oversaturation or loss of subtle details, the FLLF technique operates locally, adapting to the unique characteristics of different image regions [10], as shown in Figure 1.
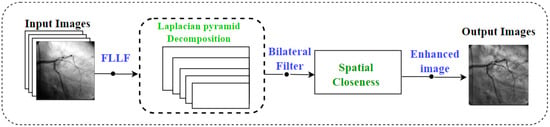
Figure 1.
The schematic framework of the proposed technique.
At the core of the FLLF technique lies the Laplacian pyramid. This pyramid is constructed by generating a series of Gaussian pyramids of varying scales and then computing the differences between consecutive levels. Mathematically, for a Gaussian pyramid level i denoted as Gi, the corresponding Laplacian pyramid level Li is calculated as:
where represents the Gaussian pyramid level Gi + 1 resized to the dimensions of Gi. This process generates a pyramid that captures local image variations and details at different scales.
The FLLF technique introduces the concept of the modified Laplacian (ML), obtained through a bilateral filter applied to each level of the Laplacian pyramid [4]. The bilateral filter is governed by two main factors: the spatial distance, d, and the range similarity, r. It preserves edges and reduces noise while enhancing the contrast shown in Figure 1. Mathematically, the modified Laplacian ML at level i can be expressed as:
where applies the bilateral filter to the Laplacian pyramid level Li with parameters d and r.
The final step involves reconstructing the enhanced image by combining the modified Laplacian ML with the base image . The reconstructed image is calculated as:
where is the number of pyramid levels. In this equation, is the base image, is the resized version of the modified Laplacian at the top level of the pyramid, and represents the sum of resized modified Laplacian levels across all pyramid levels. This reconstruction process adapts the contrast enhancement locally, allowing the technique to enhance different image regions individually based on their unique characteristics. Equation (3) can be broken down into more detail:
- E: This is the enhanced image to be created, which will have improved contrast and details.
- : This represents the base image, which is typically the original input image to be enhanced.
- : The modified Laplacian at the top level of the pyramid is resized to match the dimensions of the base image (). This means ensuring that the and have the same width and height.
- : The sum () is taken over all levels (i) of the Laplacian pyramid from 1 to ‘n’. For each level, the corresponding modified Laplacian is resized to match the dimensions of the base image () and then added to the result.
So, this equation shows that the enhanced image ‘E’ is formed by adding three components:
- The original base image .
- The modified Laplacian at the top level of the Laplacian pyramid (), ensuring that it matches the dimensions of .
- The sum of the modified Laplacian levels from all levels of the pyramid (), where each level has been resized to match .
This process combines the base image with information from the Laplacian pyramid, including the local image variations and details captured at different scales, to create an enhanced image that adapts contrast locally. The use of the Laplacian pyramid and modified Laplacian with resizing ensures that the enhancement process is applied on multiple scales, preserving fine details and textures while improving contrast. The final result, ‘E’, represents the enhanced image that retains local characteristics while having better contrast. Top of Form.
The Fast Local Laplacian Filter technique offers a sophisticated solution for contrast enhancement in images. By exploiting the Laplacian pyramid and the bilateral filter, it achieves localized enhancement while preserving essential details and textures. This adaptability makes the FLLF technique suitable for a wide range of images with varying contrast levels and textures, contributing to improved visual quality while retaining the natural appearance of the image. The FLLF algorithm (Algorithm 1) is presented below;
| Algorithm 1: Fast Local Laplacian Filter (FLLF) |
| Input: Image Mathematical Symbols: Gaussian Blur: Resizing: Laplacian: Bilateral Filter: Resizing: Enhanced Image: Parameters: Step 1. Generate Gaussian Pyramid: Create an empty Gaussian pyramid list Append to (base level) // Now this is a for-loop For Loop i in range Apply Gaussian blur to with kernel size proportional to Append the blurred image to End For Loop Step 2. Generate Laplacian Pyramid: Create an empty Laplacian pyramid list // Now this is a for-loop For Loop in range Resize to the dimensions of // Compute Laplacian pyramid using Equation (1) Append End For Loop Step 3. Enhance using Modified Laplacian: Create an empty modified Laplacian pyramid list // Now this is a for-loop For Loop each level Apply Bilateral filter to with spatial distance and range similarity Append the filtered result to End For Loop Step 4. Reconstruct Enhanced Image: Create an empty list // Now this is a for-loop For Loop in range ): //Compute modified Laplacian Resize to the dimensions of using Equation (2) Append the resized to using Equation (3) Sum all elements of and element-wise The reconstructed enhanced image is obtained End For Loop Output: Enhanced Image E |
3.2. Advantages of Fast Local Laplacian Filter
The Fast Local Laplacian Filter (FLLF) offers several advantages, and its development has been motivated by the need for effective contrast enhancement in various applications. Some of its key advantages and motivations include:
Preservation of Image Details: FLLF excels at enhancing image contrast while preserving important image details. It achieves this by selectively enhancing regions with low contrast, ensuring that fine details are retained and that edges remain sharp. This is crucial in applications where maintaining image fidelity is essential.
Adaptive Enhancement: The filter is adaptive and its enhancement strength can be adjusted based on the characteristics of the input image. This adaptability makes it suitable for a wide range of images with varying contrast levels.
Reduced Noise: FLLF effectively reduces image noise, resulting in cleaner and clearer images. This is particularly important in medical imaging, where noise can hinder the accurate interpretation of critical details.
Improved Visual Quality: By enhancing contrast and reducing noise, FLLF significantly improves the overall visual quality of images. This can enhance user experience and aid in more accurate image analysis.
Versatility: FLLF is a versatile technique that can be applied to various types of images, including medical images like coronary angiography. Its effectiveness in different domains makes it a valuable tool for researchers and professionals.
Enhanced Diagnostic Capabilities: In medical imaging, the ability to enhance contrast and preserve image details is crucial for accurate diagnosis. FLLF’s capabilities align with this need and can contribute to improved diagnostic accuracy.
Quantitative Evaluation: The performance of FLLF can be quantitatively assessed using metrics like PSNR, MSE, RMSE, NC, and CoC. This allows for rigorous evaluation and comparison with other methods, demonstrating its superiority in contrast enhancement.
Research Advancement: FLLF represents an advancement in the field of image processing and contrast enhancement. Its introduction opens up new possibilities for research and applications in image enhancement and analysis.
4. Experimentation
4.1. Performance Measures
Evaluation measures, such as Root Mean Squared Error (RMSE), Peak Signal-to-Noise Ratio (PSNR), Normalization Coefficient (NC), Mean Squared Error (MSE), and Correlation Coefficient (CoC), are commonly used to assess the performance of contrast enhancement methods in image processing. These measures provide a quantitative evaluation to compare the quality of the enhanced image with the original image. This section shall explore each measure and its corresponding equation:
Peak Signal-to-Noise Ratio: PSNR serves as a commonly employed metric for assessing the effectiveness of contrast-enhanced images [25]. It offers insight into the relationship between the highest attainable signal strength and the magnitude of noise present in the image. PSNR is calculated using the following equation:
where MAX is the maximum possible pixel value in the image (e.g., 255 for an 8-bit image) [26,27].
Mean Squared Error: MSE quantifies the average squared disparity between the pixel values of the initial image and the enhanced image. A diminished MSE value reflects more favorable outcomes, indicating that the enhancement has successfully minimized the differences between the two images [27,28]. MSE is calculated using the following equation:
where X and Y are the width and height of the image, I(r, s) represents the pixel value of the original image at coordinates (r, s), and D(r, s) represents the pixel value of the denoised image at the same coordinates.
Root Mean Squared Error: RMSE is the square root of the MSE and provides a measure of the average difference between the original and enhanced image pixel values [29]. It is commonly used as an error metric in image processing.
RMSE is calculated using the following equation:
RMSE = sqrt(MSE)
Correlation Coefficient: The CoC measures the linear relationship between the pixel values of the original and enhanced images. It provides a measure of how well the denoised image matches the original image. The Correlation Coefficient is calculated using the following equation:
where cov(I, D) represents the covariance between the pixel values of the original and denoised images, and and represent the standard deviations of the original and denoised images, respectively.
Normalization Coefficient: The NC is a measure used to assess the similarity of contrast enhancement results between original and enhanced images. A higher value of NC indicates that contrast enhancements were achieved.
The equation for calculating the Normalization Coefficient (NC) can vary depending on the general approach for calculating the NC based on the concept of normalization.
Here is a general equation for calculating the Normalization Coefficient (NC):
where NC is the Normalization Coefficient and represents the contrast enhancement image, while the is the original image.
These evaluation measures provide objective metrics to assess the performance of enhanced images. Lower RMSE and higher PSNR values indicate better quality, while a higher Correlation Coefficient signifies a stronger correlation between the original and enhanced images.
4.2. Results and Discussion
This article primarily focuses on the contrast enhancement techniques applied to coronary angiography images, with an emphasis on improving their quality by addressing issues such as low contrast. This study explores various techniques such as the Retinex Algorithm [30,31], Contrast Stretching (CS) [32], Gamma Correction (GC) [33], Histogram equalization (HE) [34], Local Bright Contrast (LBC) [35], Local Transformation Histogram Equalization (LTHE), Optimized maximum contrast (OMC) [36], Piecewise Linear Transformation (PLT), Sigmoid, Adaptive Histogram Equalization (AHE) [37], Bi-Histogram Equalization (BHE), Brightness Bi-Histogram Equalization (BBHE) [38], Contrast Limited Adaptive Histogram Equalization (CLAHE) [37], Dualistic Sub Image Histogram Equalization (DSIHE) [39], Logarithmic Transform (LT), Multi Histogram Equalization (MHE), Multi-Scale Retinex with Color Restoration (MSRCR) [40], Global Transformation Histogram Equalization (GTHE) [41], and Fast Local Laplacian Filter (FLLF) [10], and assesses their efficacy in enhancing the images before further analysis or diagnosis. This section of the article discusses the experiments conducted to evaluate the performance of noise removal and low contrast identification. It is divided into three subsections: simulation setup, visual analysis, and statistical analysis.
4.3. Simulation Setup
The simulation of image processing was conducted on a computer system running Windows 10 Pro. The computation time was measured using a computer equipped with an Intel(R) Core (TM) i5-6300U CPU @ 2.40 GHz 2.50 GHz and 8 GB of RAM. The experiment utilized the Jupiter Lab development environment, which is a web-based interactive IDE for scientific computing that is open-source. To access Jupiter Lab, an Anaconda emulator was used. Anaconda is a distribution of Python and R specifically designed for fields like data science, machine learning, and scientific computing. It provides a user-friendly environment for managing packages, handling dependencies, and executing code. The use of the Anaconda emulator ensures that Jupiter Lab is launched with all the necessary libraries and packages pre-installed, simplifying and streamlining the setup and execution process of the experiment.
Dataset Description: The dataset used in this study consisted of X-ray coronary angiography (invasive coronary angiography) images obtained from two renowned hospitals in Peshawar, Pakistan: The Cardiology Centre of the Hayatabad Hospital Complex and the Teaching Hospital of Khyber Peshawar. These images depict coronary arteries that exhibit varying thicknesses and complex vascular structures against a background. The dataset primarily revealed information about the intensity of blood vessels. It distinguished between various types of blood vessels, including healthy ones, constricted (narrow) vessels, and obstructed (blocked) vessels. Additionally, the dataset contained numerous instances of noise. Each coronary artery image in the dataset had a size of 512 × 512 pixels. The dataset contained information from a large number of patients, but in our study, we only utilized data from a couple of specific patients. The collection of angiographic images was performed under the supervision and guidance of a cardiologist.
4.4. Visual Analysis
The effectiveness of the proposed algorithm was evaluated by assessing the image quality through human observation and perception. To evaluate the efficacy of the proposed technique in comparison with existing methods, a set of low-contrast coronary angiography medical images from a widely recognized benchmark database was employed. The versatility and effectiveness of the proposed method were thoroughly tested in contrast with other established approaches [37]. The comparison of the proposed method’s performance against other existing methods is depicted in Figure 2 and Figure 3.
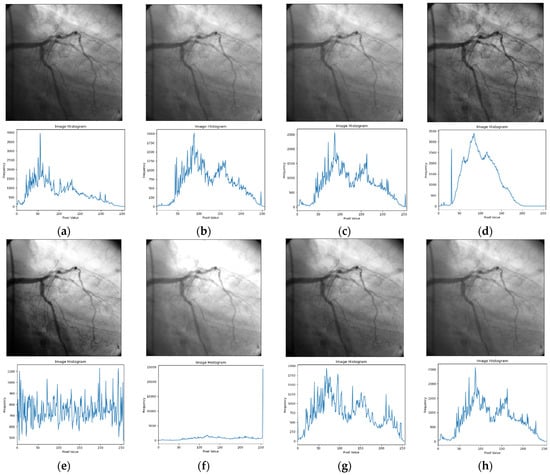
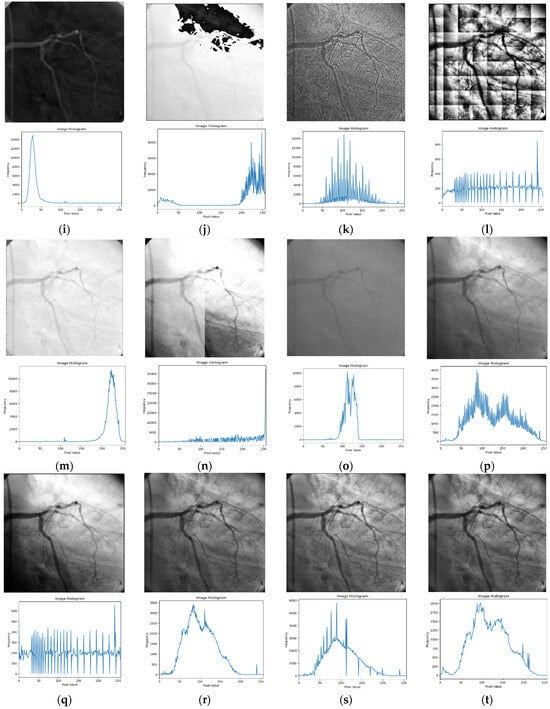
Figure 2.
Input image and its corresponding contrast enhancement results of Test Image 1: (a) input image, (b) Retinax, (c) CS, (d) GC, (e) HE, (f) LBC, (g) LTHE, (h) OMC, (i) PLT, (j) Sigmoid, (k) AHE, (l) BHE, (m) BBHE, (n) CLAHE, (o) DSIHE, (p) LT, (q) GTHE, (r) MHE, (s) MSRCR, and (t) FLLF.
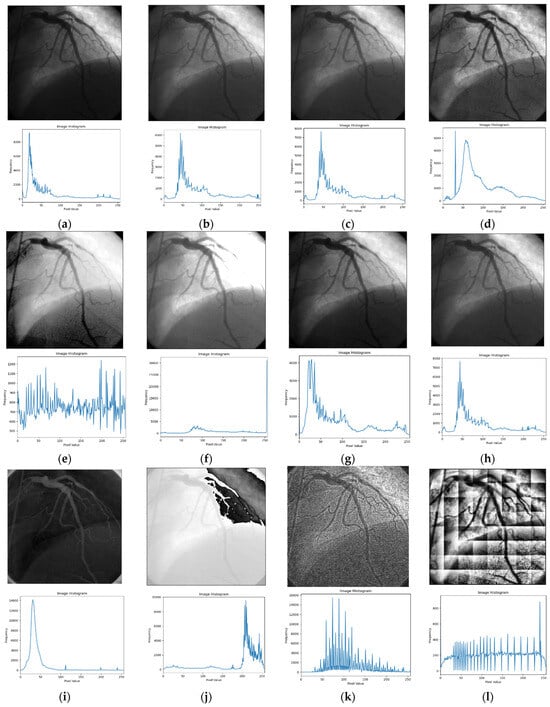
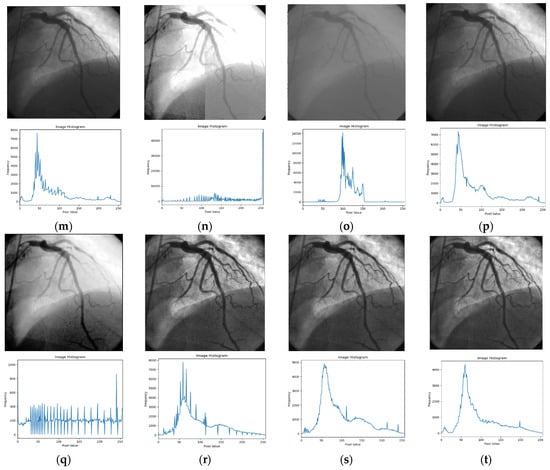
Figure 3.
Input image and its corresponding contrast enhancement results of Test Image 1: (a) input image, (b) Retinax, (c) CS, (d) GC, (e) HE, (f) LBC, (g) LTHE, (h) OMC, (i) PLT, (j) Sigmoid, (k) AHE, (l) BHE, (m) BBHE, (n) CLAHE, (o) DSIHE, (p) LT, (q) GTHE, (r) MHE, (s) MSRCR, and (t) FLLF.
The outcomes depicted in Figure 2 demonstrate the simulation findings of the ‘angiography’ testing image alongside its corresponding histogram. The Retinax technique, illustrated in Figure 2b, demonstrates a notable concern with excessive noise and over-enhancement. The angiography images generated by the CS and GC techniques in Figure 2c,d suffer from irregular intensity distribution, resulting in the distortion of subtle edges within the testing image.
As depicted in Figure 2e–h, the simulated angiography images derived from the HE, LBC, LTHE, and OMC methods exhibit multiple artifacts and struggle to preserve intricate edge features. Similarly, the results obtained from the PLT, Sigmoid, AHE, and BHE techniques (shown in Figure 2i–l) lack meaningful visual information due to their limited contrast adjustments. Furthermore, the experimental outcomes derived from the BBHE, CLAHE, and DSIHE methods (depicted in Figure 2m,o,p) produce contrast-enhanced images that are excessively bright.
Among the displayed results, Figure 2p–s showcase visually appealing outcomes, albeit with reduced significant details. Notably, the proposed FLLF method, illustrated in Figure 2t, demonstrates that the enhanced angiography image possesses a visually pleasing appearance, characterized by well-defined edge details and preserved mean brightness.
Through an in-depth analysis of the histogram plot, it becomes evident that the proposed FLLF technique adeptly mitigates insignificant contrast enhancement issues by curtailing undesirable segments within the histogram plot. Furthermore, the technique enhances image details by evenly distributing intensity occurrence levels across the entire dynamic range. This process collectively contributes to the overall improvement of the angiography image, aligning well with visual expectations.
Figure 3 presents the simulation results of the ‘angiography’ testing image, showcasing its histogram in parallel. Additionally, within Figure 3, each technique resultant image is visually illustrated alongside its accompanying histogram. This presentation allows for a comprehensive analysis and evaluation of the effectiveness and impact of each contrast enhancement technique.
4.5. Statistical Analysis
An objective approach to performance evaluation was conducted on the existing and proposed methods using five different metrics, including PSNR, MSE, RMSE, NC, and CoC.
An analysis of Table 1 and Table 2 makes it evident that the proposed Fast Local Laplacian Filter (FLLF) method offers the most promising outcomes. For both test image 1 and test image 2, the FLLF method achieved the highest PSNR values, recording values of 36.61 and 40.12, respectively. In terms of MSE and RMSE, which measure the average squared difference between pixel values of the original and processed images, the FLLF method consistently attained the lowest values among all the techniques evaluated.

Table 2.
Illustrates a comprehensive comparison between the proposed method and advanced noise removal techniques applied to test image 1.
Moreover, the CoC, a crucial metric for gauging enhancement quality, shows that the FLLF method closely approaches a value of 1. This indicates favorable results, particularly evident in the case of test image 2, and ranks the FLLF method as the third best for test image 1. Lastly, the NC metric highlights that the proposed algorithm achieved the highest value, indicating notable results for the LT technique on test image 1 and the OMC technique on test image 2.
Table 2 and Table 3 provide a visual representation of the most notable outcomes, with a clear distinction through color coding. The top three best results are visually differentiated using distinct colors: blue is assigned to the topmost results, green indicates the second-best outcomes, and orange highlights the third-best results. Figure 4 and Figure 5 also include bar charts that demonstrate the top three best results and emphasize the superiority of our proposed method over all the other methods.

Table 3.
Illustrates a comprehensive comparison between the proposed method and advanced noise removal techniques applied to test image 2.
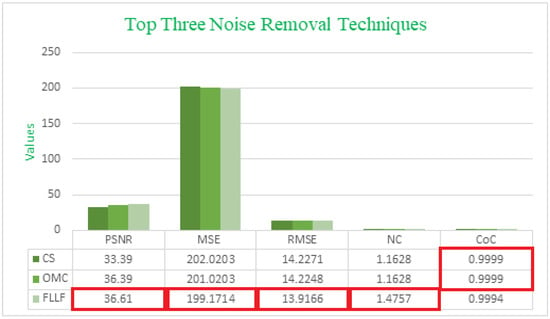
Figure 4.
Comparing the top three noise removal techniques using five different measures on test image 1.
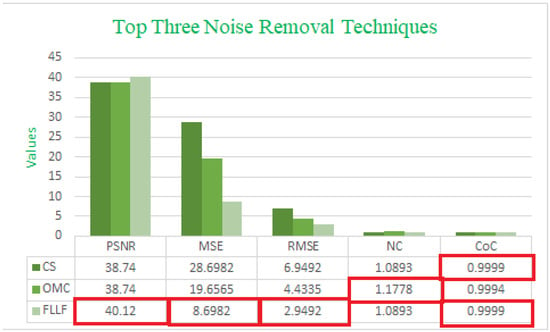
Figure 5.
Comparing the top three noise removal techniques using five different measures on test image 2.
Figure 6 and Figure 7 present a visual representation of the time comparison between the proposed Fast Local Laplacian Filter (FLLF) method and various state-of-the-art techniques. Notably, FLLF, MHE, and OME exhibit favorable time complexities, signifying efficient performance. Conversely, the PLT method demonstrates the poorest time efficiency among the evaluated techniques.
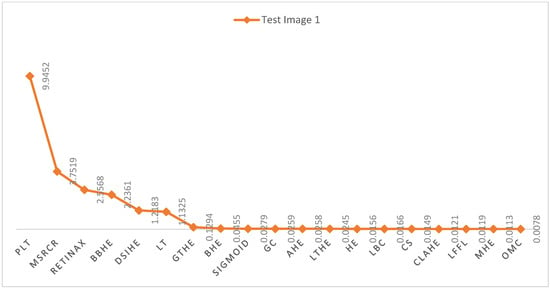
Figure 6.
Illustrates the time complexity (s) of the proposed technique in comparison with the existing state-of-the-art methods on test image 1.
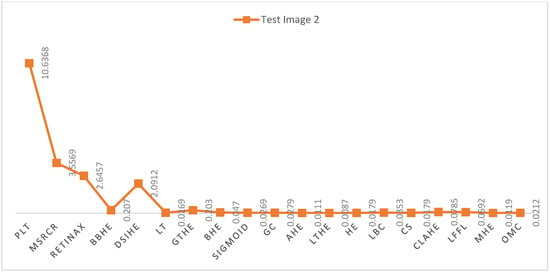
Figure 7.
Illustrates the time complexity (s) of the proposed technique in comparison with the existing state-of-the-art methods on test image 2.
5. Conclusions
This article introduces the Fast Local Laplacian Filter algorithm, a method designed to enhance the contrast and fine details in coronary angiography medical images while maintaining their natural appearance. This filter effectively reduces noise levels, resulting in more precise interpretations and sharper image presentations. To evaluate its performance, this article employs various metrics, such as PSNR, MSE, RMSE, NC, and CoC. The test results consistently demonstrate the superiority of the proposed approach compared to existing techniques in terms of enhancing both contrast and intricate details. This article underscores the significant potential of the proposed technique within the medical field. By improving the clarity and richness of information in coronary angiography images, it has the capacity to aid in accurate diagnoses and to promote a deeper understanding of the details present in these images. This conclusion is well-supported by the quantitative metrics used to evaluate the method’s performance, reinforcing the algorithm’s utility in advancing medical imaging and ultimately benefiting patient care and diagnosis.
Author Contributions
S.S.K.: conceptualization; methodology. S.S.K. and M.K.: formal analysis; investigation; roles/writing—original draft preparation. M.K. and Y.A.: supervision; validation; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no funding for this study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Mustapha, M.T.; Uzun, B.; Ozsahin, D.U.; Ozsahin, I. A comparative study of X-ray based medical imaging devices. In Applications of Multi-Criteria Decision-Making Theories in Healthcare and Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 163–180. [Google Scholar]
- Wood, M.L. Variability and standardization of quantitative imaging. Investig. Radiol. 2020, 55, 617–618. [Google Scholar] [CrossRef]
- Garland, L.M.; Holdsworth, D.W.; Cunningham, I.A. Enhancing bare metal stent visibility using multi-energy subtraction X-ray imaging. In Proceedings of the Medical Imaging 2023: Physics of Medical Imaging, SPIE, San Diego, CA, USA, 19–23 February 2023; Volume 12463, pp. 442–447. [Google Scholar]
- Aubry, M.; Paris, S.; Hasinoff, S.W.; Kautz, J.; Durand, F. Fast local Laplacian filters: Theory and applications. ACM Trans. Graph. 2014, 33, 167. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, L.; Soroushmehr, R.; Wood, A.; Gryak, J.; Nallamothu, B.; Najarian, K. Vessel segmentation for X-ray coronary angiography using ensemble methods with deep learning and filter-based features. BMC Med. Imaging 2022, 22, 10. [Google Scholar] [CrossRef]
- Jalili, M.H.; Yu, T.; Hassani, C.; Prosper, A.E.; Finn, J.P.; Bedayat, A. Contrast-enhanced MR Angiography without Gadolinium-based contrast material: Clinical applications using ferumoxytol. Radiol. Cardiothorac. Imaging 2022, 4, e210323. [Google Scholar] [CrossRef]
- Burger, W.; Burge, M.J. Digital Image Processing: An Algorithmic Introduction; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Liu, M.; Mei, S.; Liu, P.; Gasimov, Y.; Cattani, C. A New X-ray Medical-Image-Enhancement Method Based on Multiscale Shannon–Cosine Wavelet. Entropy 2022, 24, 1754. [Google Scholar] [CrossRef]
- Gao, F.; Wang, K.; Yang, Z.; Wang, Y.; Zhang, Q. Underwater image enhancement based on local contrast correction and multi-scale fusion. J. Mar. Sci. Eng. 2021, 9, 225. [Google Scholar] [CrossRef]
- Ullah, H.; Zhao, Y.; Abdalla, F.Y.O.; Wu, L. Fast local Laplacian filtering based enhanced medical image fusion using parameter-adaptive PCNN and local features-based fuzzy weighted matrices. Appl. Intell. 2022, 52, 7965–7984. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z. Medical imaging and image processing. Technologies 2023, 11, 54. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, H.; Nie, Y.; Zheng, W.-S. Pyramid Texture Filtering. arXiv 2023, arXiv:2305.06525. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, J.; Cao, E.; Pang, Y.; Sun, W. A medical endoscope image enhancement method based on improved weighted guided filtering. Mathematics 2022, 10, 1423. [Google Scholar] [CrossRef]
- Otgonbaatar, C.; Ryu, J.-K.; Shin, J.; Woo, J.Y.; Seo, J.W.; Shim, H.; Hwang, D.H. Improvement in image quality and visibility of coronary arteries, stents, and valve structures on CT angiography by deep learning reconstruction. Korean J. Radiol. 2022, 23, 1044. [Google Scholar] [CrossRef]
- Sunitha, T.O.; Rajalakshmi, R.; Sujatha, S.S. Fuzzy based dynamic histogram equalization for enhancing quality of registered medical image. J. Curr. Sci. Technol. 2022, 12, 243–264. [Google Scholar] [CrossRef]
- Showkat, S.; Parah, S.A.; Gull, S. Embedding in medical images with contrast enhancement and tamper detection capability. Multimed. Tools Appl. 2021, 80, 2009–2030. [Google Scholar] [CrossRef]
- Rao, B.S. Dynamic Histogram Equalization for contrast enhancement for digital images. Appl. Soft Comput. J. 2020, 89, 106114. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, J.; Du, Y.; Sheykhahmad, F.R. New Improved Optimized Method for Medical Image Enhancement Based on Modified Shark Smell Optimization Algorithm. Sens. Imaging 2020, 21, 20. [Google Scholar] [CrossRef]
- Subramani, B.; Veluchamy, M. Fuzzy Gray Level Difference Histogram Equalization for Medical Image Enhancement. J. Med. Syst. 2020, 44, 103. [Google Scholar] [CrossRef]
- Abdul-Adheem, W.R. Enhancement of magnetic resonance images through piecewise linear histogram equalization. J. Eng. Sci. Technol. 2020, 15, 2023–2039. [Google Scholar]
- Dar, K.A.; Mittal, S. An enhanced adaptive histogram equalization based local contrast preserving technique for HDR images. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1022, 012119. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Jimenez, G. Using chaos world cup optimization algorithm for medical images contrast enhancement. Concurr. Comput. Pract. Exp. 2020, 32, e5428. [Google Scholar] [CrossRef]
- Abbasi, R.; Chen, J.; Al-Otaibi, Y.; Rehman, A.; Abbas, A.; Cui, W. RDH-based dynamic weighted histogram equalization using for secure transmission and cancer prediction. Multimed. Syst. 2021, 27, 177–189. [Google Scholar] [CrossRef]
- Agarwal, M.; Rani, G.; Dhaka, V.S. Optimized contrast enhancement for tumor detection. Int. J. Imaging Syst. Technol. 2020, 30, 687–703. [Google Scholar] [CrossRef]
- Khan, S.S.; Khan, M.; Ran, Q. Multi-focus color image fusion using laplacian filter and discrete fourier transformation with qualitative error image metrics. In Proceedings of the 2nd International Conference on Control and Computer Vision, Jeju, Republic of Korea, 15–18 June 2019; pp. 41–45. [Google Scholar] [CrossRef]
- Khan, S.S.; Ran, Q.; Khan, M. Image pan-sharpening using enhancement based approaches in remote sensing. Multimed. Tools Appl. 2020, 79, 32791–32805. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, S. Comparison of Contrast Enhancement Techniques for Medical Image. In Proceedings of the 2016 Conference on Emerging Devices and Smart Systems (ICEDSS), Namakkal, India, 4–5 March 2016; pp. 155–159. [Google Scholar] [CrossRef]
- Shah, M.; Technology, I.; Khan, S.S.; Technology, I.; Khan, M.; Technology, S.; Ali, S. Multi-Focus Image Fusion using Unsharp Masking with Discrete Cosine Transform. In Proceedings of the 1st International Conference on Computing Technologies, Tools and Applications (ICTA PP-23), Peshawar, Pakistan, 9–11 May 2023; pp. 1–5. [Google Scholar]
- Khan, S.S.; Khan, M.; Alharbi, Y. Multi focus image fusion using image enhancement techniques with wavelet transformation. Int. J. Adv. Comput. Sci. Appl. 2020, 11, 414–420. [Google Scholar] [CrossRef]
- Daway, E.G.; Abdulameer, F.S.; Daway, H.G. X-ray Image Enhancement Using Retinex Algorithm Based On Color Restoration. J. Eng. Sci. Technol. 2022, 17, 1276–1286. [Google Scholar]
- Almalki, Y.E.; Jandan, N.A.; Soomro, T.A.; Ali, A.; Kumar, P.; Irfan, M.; Keerio, M.U.; Rahman, S.; Alqahtani, A.; Alqhtani, S.M. Enhancement of Medical Images through an Iterative McCann Retinex Algorithm: A Case of Detecting Brain Tumor and Retinal Vessel Segmentation. Appl. Sci. 2022, 12, 8243. [Google Scholar] [CrossRef]
- Lei, X.; Wang, H.; Shen, J.I.E.; Chen, Z.H.E.; Zhang, W. A novel intelligent underwater image enhancement method via color correction and contrast stretching. Microprocess. Microsyst. 2021, 10, 4040. [Google Scholar] [CrossRef]
- Acharya, A.; Giri, A.V. Contrast improvement using local gamma correction. In Proceedings of the 2020 6th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 6–7 March 2020; pp. 110–114. [Google Scholar]
- Agrawal, S.; Panda, R.; Mishro, P.K.; Abraham, A. A novel joint histogram equalization based image contrast enhancement. J. King Saud Univ. Inf. Sci. 2022, 34, 1172–1182. [Google Scholar] [CrossRef]
- Tung, T.-C.; Fuh, C.-S. ICEBIN: Image contrast enhancement based on induced norm and local patch approaches. IEEE Access 2021, 9, 23737–23750. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, S.; Zhuang, P.; Liang, Z.; Li, C. Underwater image enhancement via piecewise color correction and dual prior optimized contrast enhancement. IEEE Signal Process. Lett. 2023, 30, 229–233. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, S.; Yang, S. Contrast enhancement of low-contrast medical images using modified contrast limited adaptive histogram equalization. J. Med. Imaging Health Inform. 2020, 10, 1795–1803. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Zhang, J.; Li, Q.; Shi, Y. Image enhancement with the preservation of brightness and structures by employing contrast limited dynamic quadri-histogram equalization. Optik (Stuttg) 2021, 226, 165877. [Google Scholar] [CrossRef]
- Ezhilraja, K.; Shanmugavadivu, P. Contrast Enhancement of Lung CT Scan Images using Multi-Level Modified Dualistic Sub-Image Histogram Equalization. In Proceedings of the 2022 International Conference on Automation, Computing and Renewable Systems (ICACRS), Pudukkottai, India, 13–15 December 2022; pp. 1009–1014. [Google Scholar]
- Zhou, J.; Yao, J.; Zhang, W.; Zhang, D. Multi-scale retinex-based adaptive gray-scale transformation method for underwater image enhancement. Multimed. Tools Appl. 2022, 81, 1811–1831. [Google Scholar] [CrossRef]
- Khan, S.S.; Khan, M.; Khan, R.S. Automatic Segmentation and Classification to Diagnose Coronary Artery Disease (AuSC-CAD) Using Angiographic Images: A Novel Framework. In Proceedings of the IEEE International Conference on Emerging Technologies (ICET), Peshawar, Pakistan, 6–7 November 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).