Advanced Treatment of Direct Dye Wastewater Using Novel Composites Produced from Hoshanar and Sunny Grey Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
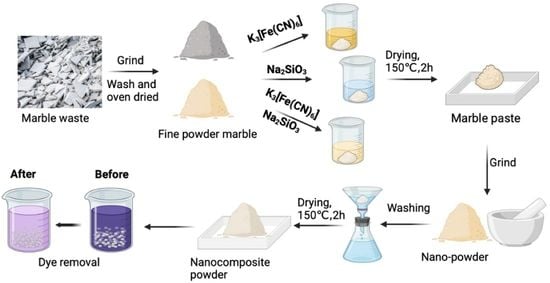
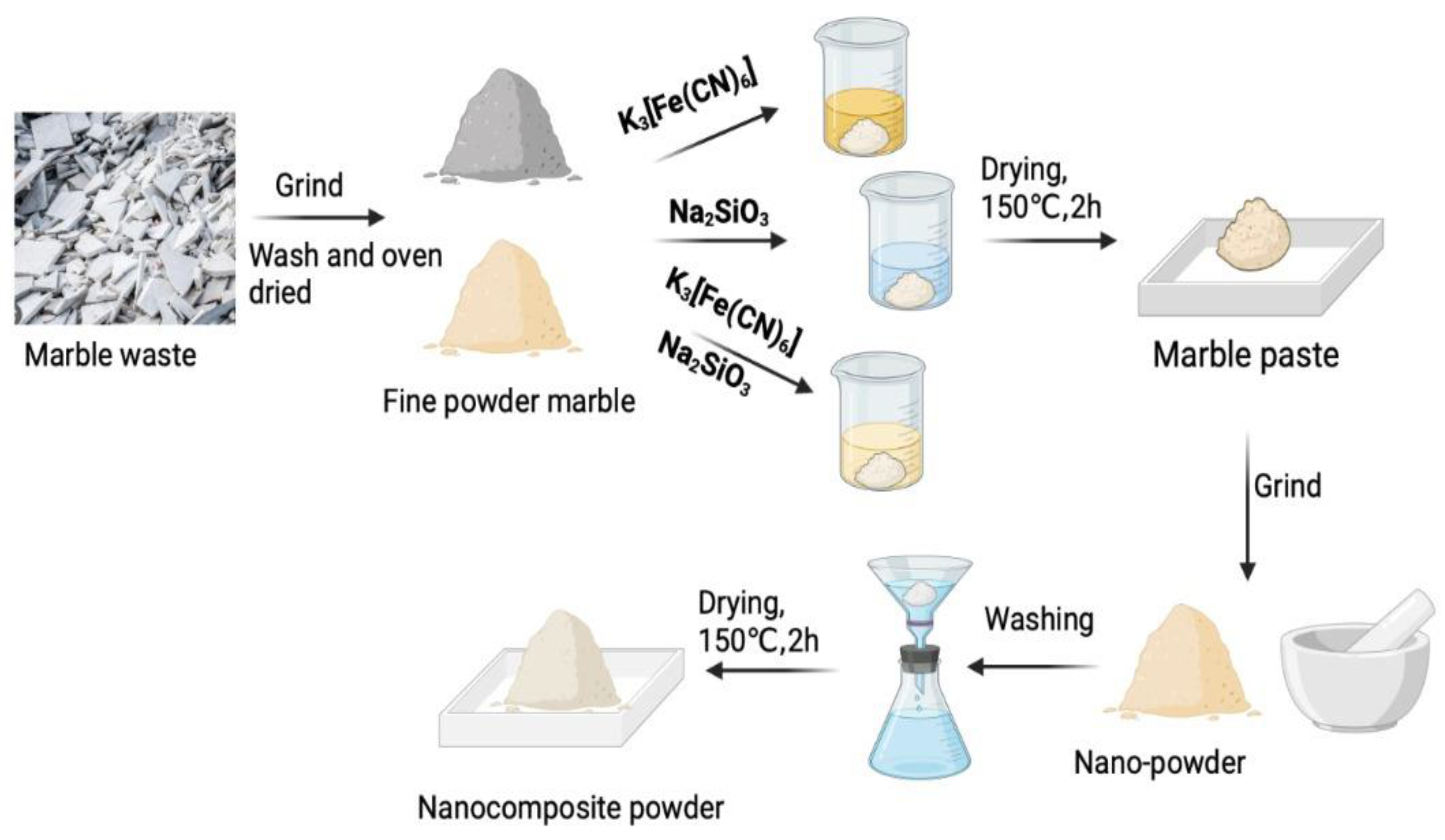
2.2. Preparation of Adsorbents
2.3. Optimization of Process Parameters
2.3.1. Optimization of Initial Dye Concentration
2.3.2. Optimization of Adsorbent Dosage
2.3.3. Optimization of Time and Temperature
2.3.4. Optimization of pH
2.4. Determination of λmax before Treatment
3. Results and Discussion
3.1. Spectrophotometric Analysis
3.2. Optimization of Parameters
3.2.1. Effect of Initial Dye Concentration
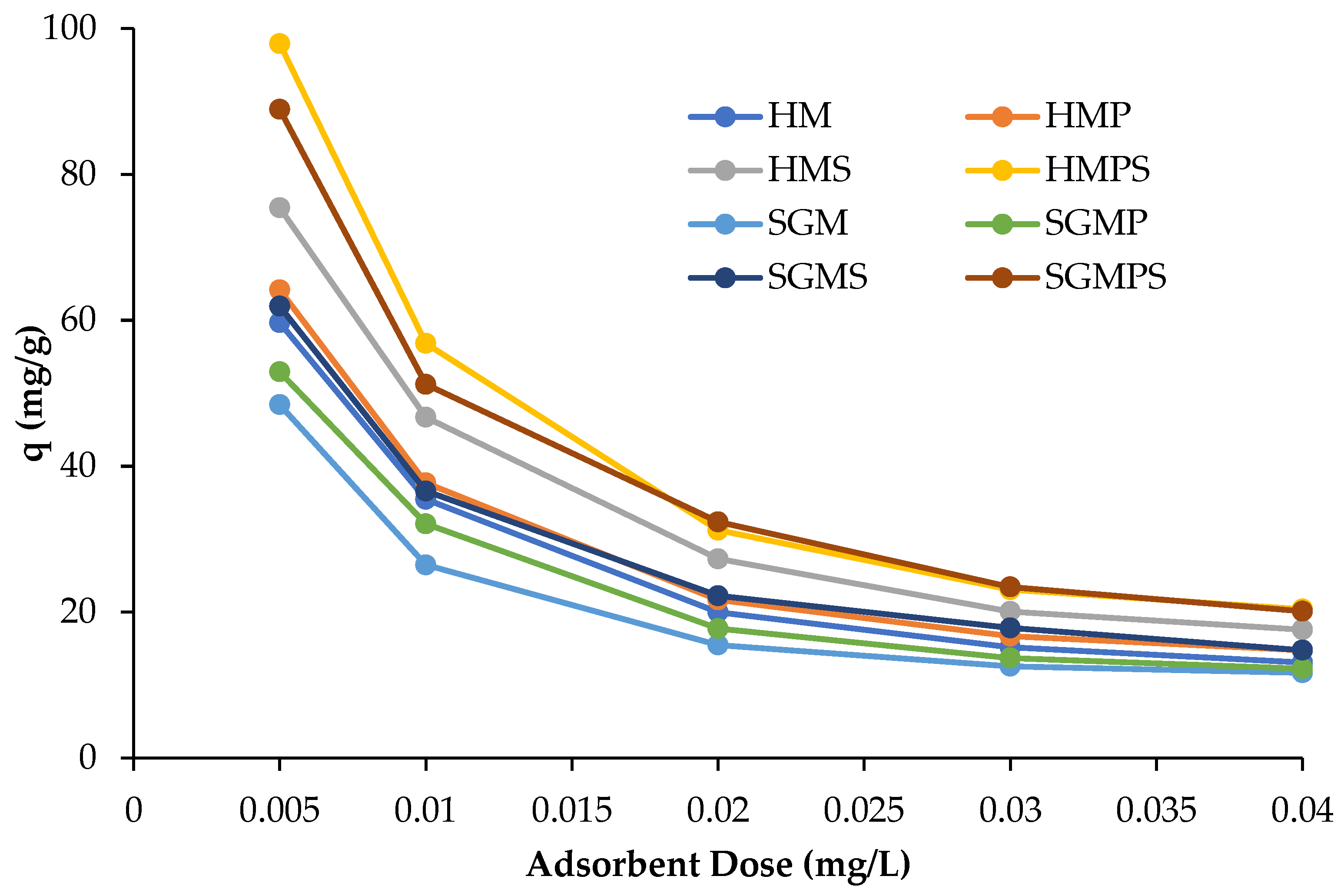
3.2.2. Effect of Adsorbent Dose on Efficiency of Dye Removal
3.2.3. Effect of Solution pH on Dye Adsorption
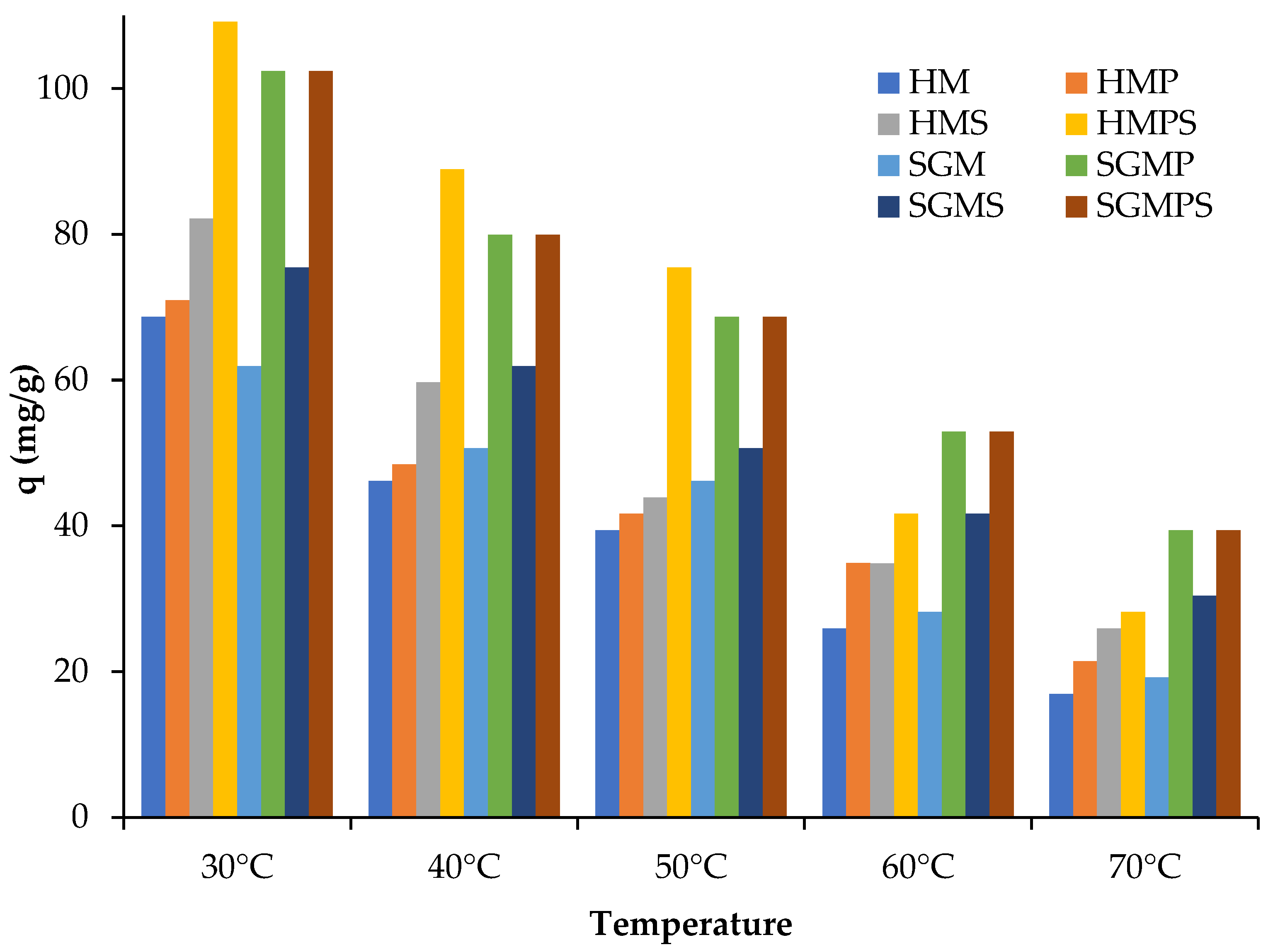
3.2.4. Effect of Contact Time and Solution Temperature
3.3. Optimization of Parameters by Theoretical Modelling
3.3.1. Adsorption Isothermal Modelling
3.3.2. Kinetic Studies
3.4. Possible Mechanism of Adsorption
3.5. Charaterization of Composites
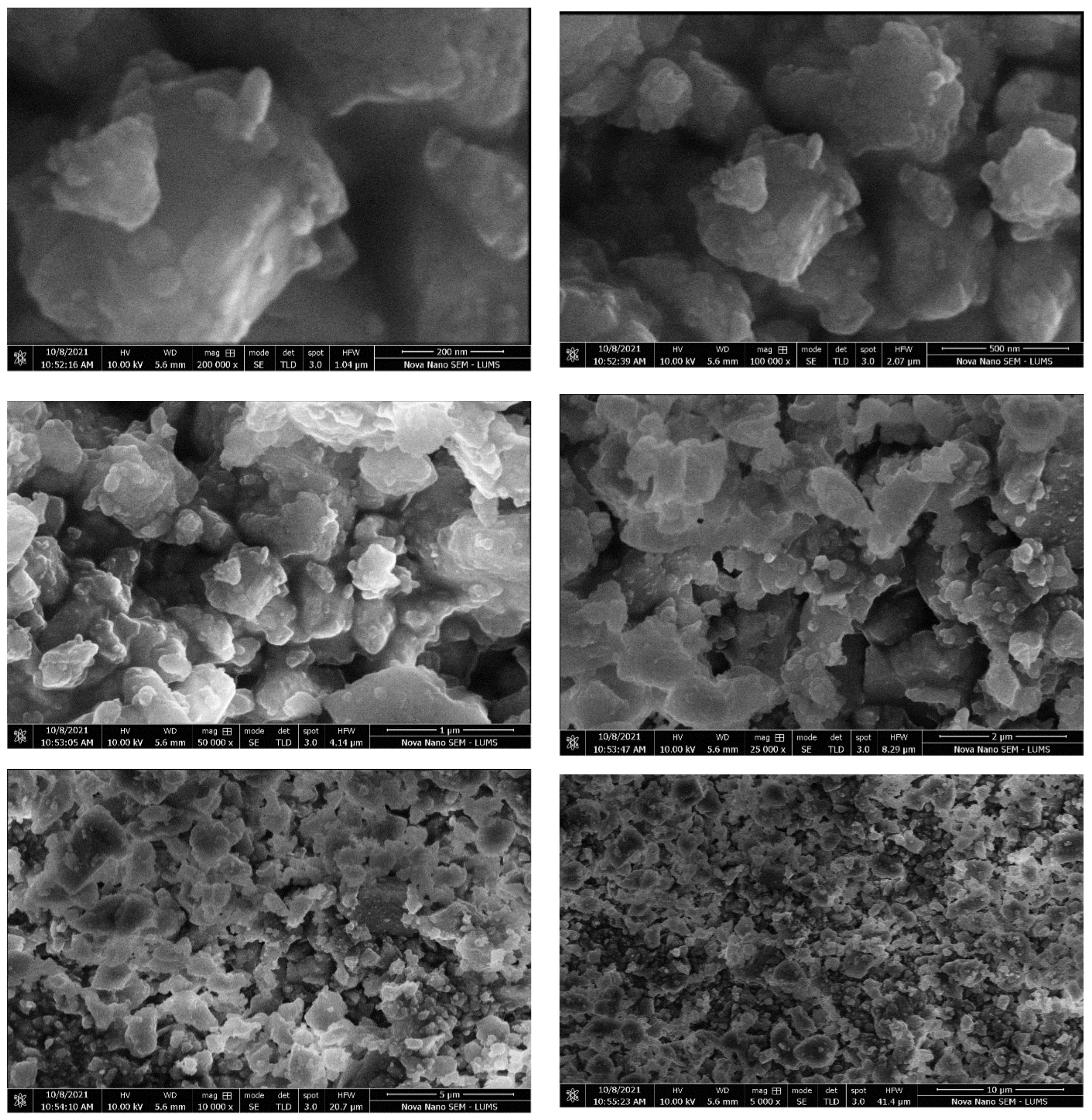
3.5.1. SEM Analysis
3.5.2. FTIR Analysis
3.6. Study of Desorption and Reusability of Adsorbents
3.7. Comparative Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halder, J.N.; Islam, M.N. Water pollution and its impact on the human health. J. Environ. Hum. 2015, 2, 36–46. [Google Scholar] [CrossRef]
- Khan, R.; Bhawana, P.; Fulekar, M. Microbial decolorization and degradation of synthetic dyes: A review. Rev. Environ. Sci. Bio. Technol. 2013, 12, 75–97. [Google Scholar] [CrossRef]
- Pawari, M.; Gawande, S. Ground water pollution and its consequence. Int. J. Eng. Res. Gen. Sci. 2015, 3, 773–776. [Google Scholar]
- Ullah, R.; Malik, R.N.; Qadir, A. Assessment of groundwater contamination in an industrial city, Sialkot, Pakistan. Afr. J. Environ. Sci. Technol. 2009, 3, 429–446. [Google Scholar]
- Azizullah, A.; Khattak, M.N.K.; Richter, P.; Häder, D.-P. Water pollution in Pakistan and its impact on public health—A review. Environ. Int. 2011, 37, 479–497. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Zwiener, C.; Richardson, S.D.; De Marini, D.M.; Grummt, T.; Glauner, T.; Frimmel, F.H. Drowning in disinfection byproducts? Assessing swimming pool water. Environ. Sci. Technol. 2007, 41, 363–372. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Wastewater treatment: An overview. In Green Adsorbents for Pollutant Removal. Environmental Chemistry for a Sustainable World; Crini, G., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 18. [Google Scholar]
- Sharma, S.; Kaur, A. Various methods for removal of dyes from industrial effluents-a review. Indian J. Sci. Technol. 2018, 11, 1–21. [Google Scholar] [CrossRef]
- Akpor, O. Wastewater effluent discharge: Effects and treatment processes. In Proceedings of the 2011 3rd International Conference on Chemical, Biological and Environmental Engineering, Singapore, 23–25 September 2011; Volume 20, pp. 85–91. [Google Scholar]
- Montaño-Medina, C.U.; Lopéz-Martínez, L.M.; Ochoa-Terán, A.A.; López-Maldonado, E.A.; Salazar-Gastelum, M.I.; Trujillo-Navarrete, B.; Pérez-Sicairos, S.; Cornejo-Bravoc, J.M. New pyridyl and aniline-functionalized carbamoylcarboxylic acids for removal of metal ions from water by coagulation-flocculation process. Chem. Eng. J. 2023, 451, 138396. [Google Scholar] [CrossRef]
- Duan, X.; Srinivasakannan, C.; Wang, X.; Wang, F.; Liu, X. Synthesis of activated carbon fibers from cotton by microwave induced H3PO4 activation. J. Taiwan Inst. Chem. Eng. 2017, 70, 374–381. [Google Scholar] [CrossRef]
- Glassmeyer, S.T.; Furlong, E.T.; Kolpin, D.W.; Cahill, J.D.; Zaugg, S.D.; Werner, S.L.; Meyer, M.T.; Kryak, D.D. Transport of chemical and microbial compounds from known wastewater discharges: Potential for use as indicators of human fecal contamination. Environ. Sci. Technol. 2005, 39, 5157–5169. [Google Scholar] [CrossRef]
- Mehta, D.; Mondal, P.; George, S. Utilization of marble waste powder as a novel adsorbent for removal of fluoride ions from aqueous solution. J. Environ. Chem. Eng. 2016, 3, 932–942. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Sekki, A.; Madani, K.; Vial, C.; Barkaoui, M. Studies on the decolorization of textile dye wastewater by continuous electrocoagulation process. Chem. Eng. J. 2009, 149, 207–214. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 1–856. [Google Scholar]
- Ahmad, R.; Kumar, R.; Haseeb, S. Adsorption of Cu2+ from aqueous solution onto iron oxide coated eggshell powder: Evaluation of equilibrium, isotherms, kinetics, and regeneration capacity. Arab. J. Chem. 2012, 5, 353–359. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Baqar, M.; Sarwar, A.; Shahwar, D.-e.; Nazir, R.; Shahid, S.; Shaikh, I.A.; Arslan, M.; Machado, S. Adsorption of acid yellow-73 and direct violet-51 dyes from textile wastewater by using iron doped corncob charcoal. Pak. J. Anal. Environ. Chem. 2015, 16, 31–38. [Google Scholar]
- Bouberka, Z.; Khenifi, A.; Benderdouche, N.; Derriche, Z. Removal of supranol yellow 4GL by adsorption onto Cr-intercalated montmorillonite. J. Hazard. Mater. 2006, 133, 154–161. [Google Scholar] [CrossRef]
- Senturk, H.B.; Ozdes, D.; Duran, C. Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunus dulcis) as a low cost biosorbent. Desalination 2010, 252, 81–87. [Google Scholar] [CrossRef]
- Sadaf, S.; Bhatti, H.N. Biosorption of Foron turquoise SBLN using mixed biomass of white rot fungi from synthetic effluents. Afr. J. Biotechnol. 2011, 10, 13548–13554. [Google Scholar] [CrossRef]
- Aqdam, S.R.; Otzen, D.E.; Mahmoodi, N.M.; Morshedi, D. Adsorption of azo dyes by a novel bio-nanocomposite based on whey protein nanofibrils and nano-clay: Equilibrium isotherm and kinetic modeling. J. Colloid Interface Sci. 2021, 602, 490–503. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Adsorptive removal of reactive black 5 from wastewater using bentonite clay: Isotherms, kinetics and thermodynamics. Sustainability 2015, 7, 15302–15318. [Google Scholar] [CrossRef]
- Bushra, R.; Mohamad, S.; Alias, Y.; Jin, Y.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review. Microp. Mesoporous Mater. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Ruan, W.; Hu, J.; Qi, J.; Hou, Y.; Zhou, C.; Wei, X. Removal of dyes from wastewater by nanomaterials: A review. Adv. Mater. Lett. 2019, 10, 9–20. [Google Scholar] [CrossRef]
- Hamed, M.M.; Ahmed, I.; Metwally, S. Adsorptive removal of methylene blue as organic pollutant by marble dust as eco-friendly sorbent. J. Ind. Eng. Chem. 2014, 20, 2370–2377. [Google Scholar] [CrossRef]
- Ricci, C.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A. Non-invasive identification of surface materials on marble artifacts with fiber optic mid-FTIR reflectance spectroscopy. Talanta 2006, 69, 1221–1226. [Google Scholar] [CrossRef]
- Utara, S.; Phataib, P. Adsorption Characteristics of Direct Violet Dye by Natural Rubber Chips. In Advanced Materials Research; Trans Tech Publications, Ltd.: Wollerau, Switzerland, 2013; Volume 844, pp. 391–394. [Google Scholar]
- Chrastil, J. Adsorption of direct dyes on cotton: Kinetics of dyeing from finite baths based on new information. Text. Res. J. 1990, 60, 413–416. [Google Scholar] [CrossRef]
- Sadaf, S.; Bhatti, H.N.; Nausheen, S.; Noreen, S. Potential use of low-cost lignocellulosic waste for the removal of direct violet 51 from aqueous solution: Equilibrium and breakthrough studies. Arch. Environ. Contam. Toxicol. 2014, 66, 557–571. [Google Scholar] [CrossRef]
- Ho, S. Removal of dyes from wastewater by adsorption onto activated carbon: Mini review. J. Geosci. Environ. Prot. 2020, 8, 120–131. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Bastani, D. Direct dyes removal using modified magnetic ferrite nanoparticle. J. Environ. Health Sci. Eng. 2014, 12, 1–10. [Google Scholar] [CrossRef]




| Isothermal Models | Adsorbents | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HM | HMP | HMS | HMPS | SGM | SGMP | SGMS | SGMPS | ||
| Langmuir | qmax (exp) | 67.19 | 69.11 | 80.95 | 105.31 | 60.06 | 60.70 | 71.2 | 99.02 |
| qcal (mg/g) | 37.45 | 98.03 | 87.72 | 117.65 | 69.45 | 93.45 | 80 | 116.26 | |
| KL (L/mg) | 0.473 | 0.056 | 0.281 | 0.209 | 0.156 | 0.043 | 0.151 | 0.116 | |
| R2 | 0.96 | 0.97 | 0.98 | 0.98 | 0.99 | 0.97 | 0.98 | 0.97 | |
| Freundlich | qmax (exp) | 67.19 | 69.11 | 80.95 | 105.31 | 60.06 | 60.70 | 71.2 | 99.02 |
| qcal (mg/g) | 51.94 | 73.93 | 87.31 | 114.99 | 68.14 | 67.31 | 71.80 | 99.08 | |
| Kf (L/mg) | 33.34 | 9.11 | 31.41 | 32.12 | 15.58 | 6.18 | 21 | 22.82 | |
| R2 | 0.20 | 0.97 | 0.90 | 0.93 | 0.90 | 0.97 | 0.98 | 0.99 | |
| N | 8.65 | 1.79 | 3.65 | 2.88 | 2.60 | 1.58 | 3.05 | 2.51 | |
| Dubinin-Radushkevich | qmax (exp) | 67.19 | 69.11 | 80.95 | 105.31 | 60.06 | 60.70 | 71.2 | 99.02 |
| qcal (mg/g) | 49.13 | 48.67 | 67.87 | 82.35 | 42.54 | 44.52 | 54.92 | 70.04 | |
| R2 | 0.49 | 0.70 | 0.74 | 0.73 | 0.78 | 0.82 | 0.77 | 0.72 | |
| E (KJ/mol) | −1118 | −500 | −1000 | −1000 | −500 | −408 | −745 | −790 | |
| Β (mol2/J2) | |||||||||
| Temkin | qmax (exp) | 67.19 | 69.11 | 80.95 | 105.31 | 60.06 | 60.70 | 71.2 | 99.02 |
| qcal (mg/g) | 52.67 | 61.89 | 83.43 | 107.43 | 63.23 | 62.71 | 68.60 | 93.24 | |
| R2 | 0.17 | 0.95 | 0.89 | 0.91 | 0.92 | 0.97 | 0.97 | 0.96 | |
| B | 4.91 | 21.02 | 15.16 | 22.65 | 14.85 | 19.89 | 14.83 | 22.74 | |
| At (L/g) | 976.16 | 0.579 | 5.86 | 2.90 | 1.62 | 0.461 | 2.373 | 1.504 | |
| Harkin jura | qmax (exp) | 67.19 | 69.11 | 80.95 | 105.31 | 60.06 | 60.70 | 71.2 | 99.02 |
| qcal (mg/g) | 24.49 | 10.83 | 24.91 | 25.01 | 14.33 | 8 | 18.35 | 20.7 | |
| R2 | 0.29 | 0.86 | 0.83 | 0.84 | 0.81 | 0.73 | 0.86 | 0.86 | |
| A | −2500 | −370 | −2000 | −2000 | −666.7 | −200 | −1111.1 | −125 | |
| B | −2.5 | −1.51 | −1.6 | −1.6 | −1.6 | −1.48 | −1.7 | −0.15 | |
| Adsorbents | Pseudo 1st Order Kinetics | Pseudo 2nd Order Kinetics | ||||||
|---|---|---|---|---|---|---|---|---|
| Qe | Q° | K1 | R2 | Qe | Q° | K2 | R2 | |
| 30 °C | ||||||||
| HM | 68.67 | 10.78 | 0.0126 | 0.97 | 68.67 | 69.45 | 0.99 | |
| HMP | 70.92 | 10.76 | −0.0133 | 0.99 | 70.92 | 71.94 | 0.99 | |
| HMS | 82.16 | 10.91 | −0.0123 | 0.99 | 82.16 | 83.33 | 0.99 | |
| HMPS | 109.16 | 10.54 | −0.0134 | 0.99 | 109.16 | 10.9.81 | 0.99 | |
| SGM | 61.92 | 10.50 | −0.0131 | 0.99 | 61.92 | 62.89 | 0.99 | |
| SGMP | 66.42 | 10.44 | −0.0131 | 0.99 | 66.42 | 67.14 | 0.99 | |
| SGMS | 75.41 | 6.68 | −0.0096 | 0.92 | 75.41 | 75.76 | 0.99 | |
| SGMPS | 102.41 | 9.91 | −0.0131 | 0.99 | 102.41 | 103.09 | 0.99 | |
| 40 °C | ||||||||
| HM | 46.17 | 10.21 | −0.0131 | 0.99 | 46.17 | 47.17 | 0.99 | |
| HMP | 48.42 | 10.61 | −0.0132 | 0.99 | 48.42 | 49.26 | 0.99 | |
| HMS | 59.67 | 10.43 | −0.0132 | 0.99 | 59.67 | 60.61 | 0.99 | |
| HMPS | 88.91 | 11.97 | −0.0145 | 0.96 | 88.91 | 90.09 | 0.99 | |
| SGM | 50.67 | 10.50 | −0.0129 | 0.99 | 50.67 | 51.54 | 0.99 | |
| SGMP | 57.42 | 10.79 | −0.0127 | 0.97 | 57.42 | 58.47 | 0.99 | |
| SGMS | 61.92 | 10.74 | −0.0131 | 0.99 | 61.92 | 62.89 | 0.99 | |
| SGMPS | 79.91 | 12.09 | −0.0085 | 0.97 | 79.91 | 80.64 | 0.99 | |
| 50 °C | ||||||||
| HM | 39.42 | 12.37 | −0.0087 | 0.98 | 39.42 | 40.65 | 0.99 | |
| HMP | 41.67 | 11.07 | −0.0135 | 0.98 | 41.67 | 42.73 | 0.99 | |
| HMS | 43.92 | 11.22 | −0.0013 | 0.98 | 43.92 | 45.04 | 0.99 | |
| HMPS | 75.41 | 25.23 | −0.0115 | 0.97 | 75.41 | 78.12 | 0.99 | |
| SGM | 46.17 | 11.37 | −0.0138 | 0.98 | 46.17 | 47.16 | 0.99 | |
| SGMP | 48.42 | 12.51 | −0.0147 | 0.97 | 48.42 | 49.50 | 0.99 | |
| SGMS | 50.67 | 10.76 | −0.0133 | 0.99 | 50.67 | 51.54 | 0.99 | |
| SGMPS | 68.67 | 12.09 | −0.0085 | 0.97 | 68.67 | 69.93 | 0.99 | |
| 60 °C | ||||||||
| HM | 25.92 | 11.37 | −0.0138 | 0.98 | 25.92 | 27.24 | 0.99 | |
| HMP | 34.92 | 12.73 | −0.0087 | 0.98 | 34.92 | 36.63 | 0.99 | |
| HMS | 34.82 | 10.60 | −0.0131 | 0.99 | 34.82 | 35.97 | 0.99 | |
| HMPS | 41.67 | 11.66 | −0.0140 | 0.97 | 41.67 | 42.73 | 0.99 | |
| SGM | 28.17 | 11.07 | −0.0135 | 0.98 | 28.17 | 29.41 | 0.99 | |
| SGMP | 39.42 | 10.47 | −0.0131 | 0.99 | 39.42 | 40.04 | 0.99 | |
| SGMS | 41.67 | 11.37 | −0.0138 | 0.98 | 41.67 | 42.73 | 0.99 | |
| SGMPS | 52.92 | 10.76 | −0.0133 | 0.99 | 52.92 | 53.76 | 0.99 | |
| 70 °C | ||||||||
| HM | 16.93 | 10.60 | −0.0131 | 0.99 | 16.93 | 18.55 | 0.99 | |
| HMP | 21.42 | 11.37 | −0.0138 | 0.98 | 21.42 | 22.93 | 0.99 | |
| HMS | 25.92 | 11.07 | −0.0135 | 0.98 | 25.92 | 27.17 | 0.99 | |
| HMPS | 28.17 | 10.65 | −0.0131 | 0.99 | 28.17 | 29.32 | 0.99 | |
| SGM | 19.18 | 11.37 | −0.0138 | 0.98 | 19.18 | 20.83 | 0.99 | |
| SGMP | 25.92 | 11.22 | −0.0135 | 0.98 | 25.92 | 27.24 | 0.99 | |
| SGMS | 30.42 | 10.91 | −0.0133 | 0.99 | 30.42 | 31.54 | 0.99 | |
| SGMPS | 39.42 | 10.45 | −0.0131 | 0.99 | 39.42 | 40.48 | 0.99 | |
| Adsorbents | Removal Capacity (mg/g) | Ref. |
|---|---|---|
| HM | 67.19 | This study |
| HMPS | 105.31 | This study |
| Natural rubber | 0.0245 | [30] |
| Cotton fabric | 2.26 | [31] |
| Sugarcane bagasse | 36.17 | [32] |
| Activated carbon | 26 | [33] |
| Modified magnetic ferrite nanoparticles | 55.5 | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, A.; Hanif, M.A.; Rashid, U.; Bhatti, I.A.; Alharthi, F.A.; Kazerooni, E.A. Advanced Treatment of Direct Dye Wastewater Using Novel Composites Produced from Hoshanar and Sunny Grey Waste. Separations 2022, 9, 425. https://doi.org/10.3390/separations9120425
Akhtar A, Hanif MA, Rashid U, Bhatti IA, Alharthi FA, Kazerooni EA. Advanced Treatment of Direct Dye Wastewater Using Novel Composites Produced from Hoshanar and Sunny Grey Waste. Separations. 2022; 9(12):425. https://doi.org/10.3390/separations9120425
Chicago/Turabian StyleAkhtar, Amna, Muhammad Asif Hanif, Umer Rashid, Ijaz Ahmad Bhatti, Fahad A. Alharthi, and Elham Ahmed Kazerooni. 2022. "Advanced Treatment of Direct Dye Wastewater Using Novel Composites Produced from Hoshanar and Sunny Grey Waste" Separations 9, no. 12: 425. https://doi.org/10.3390/separations9120425
APA StyleAkhtar, A., Hanif, M. A., Rashid, U., Bhatti, I. A., Alharthi, F. A., & Kazerooni, E. A. (2022). Advanced Treatment of Direct Dye Wastewater Using Novel Composites Produced from Hoshanar and Sunny Grey Waste. Separations, 9(12), 425. https://doi.org/10.3390/separations9120425