New Rhenium-Doped SrCo1−xRexO3−δ Perovskites Performing as Cathodes in Solid Oxide Fuel Cells
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
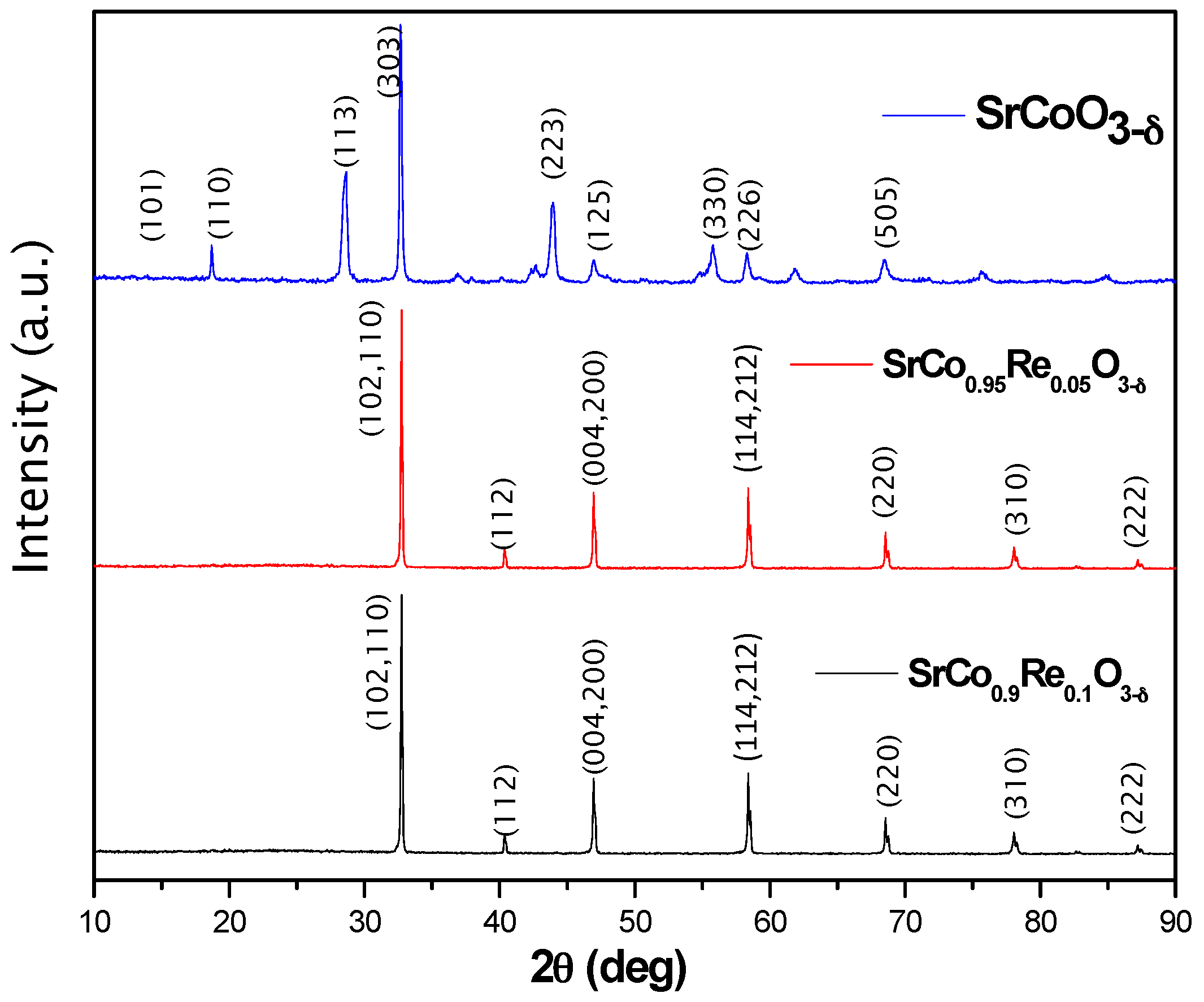
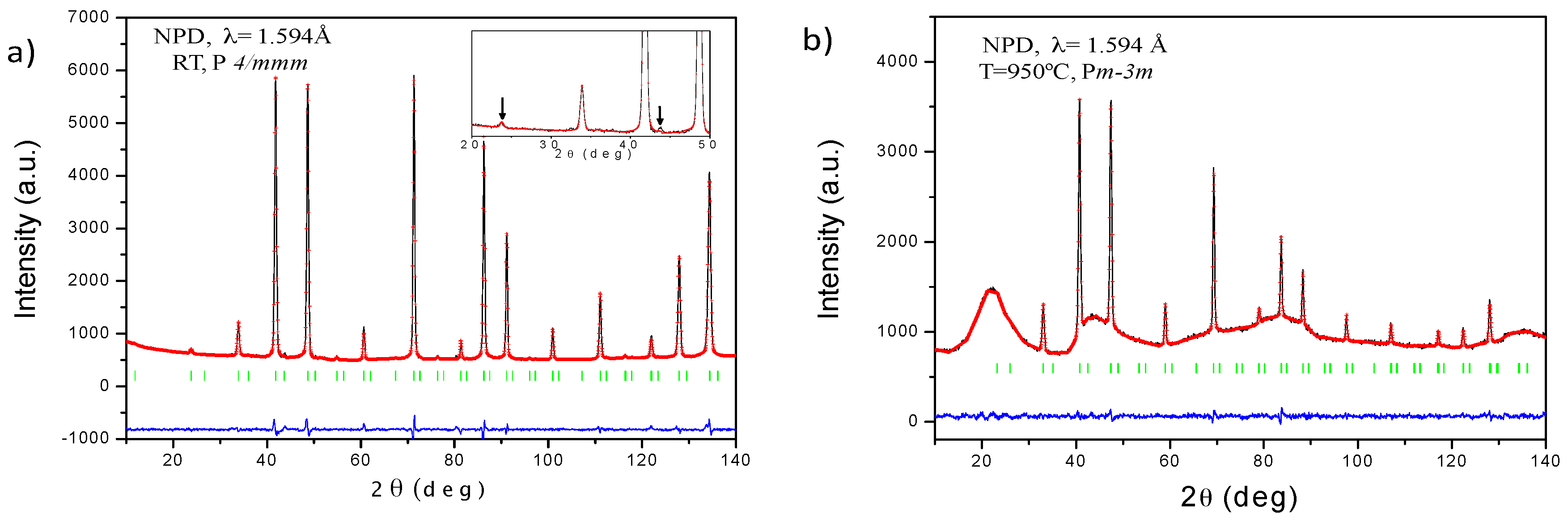
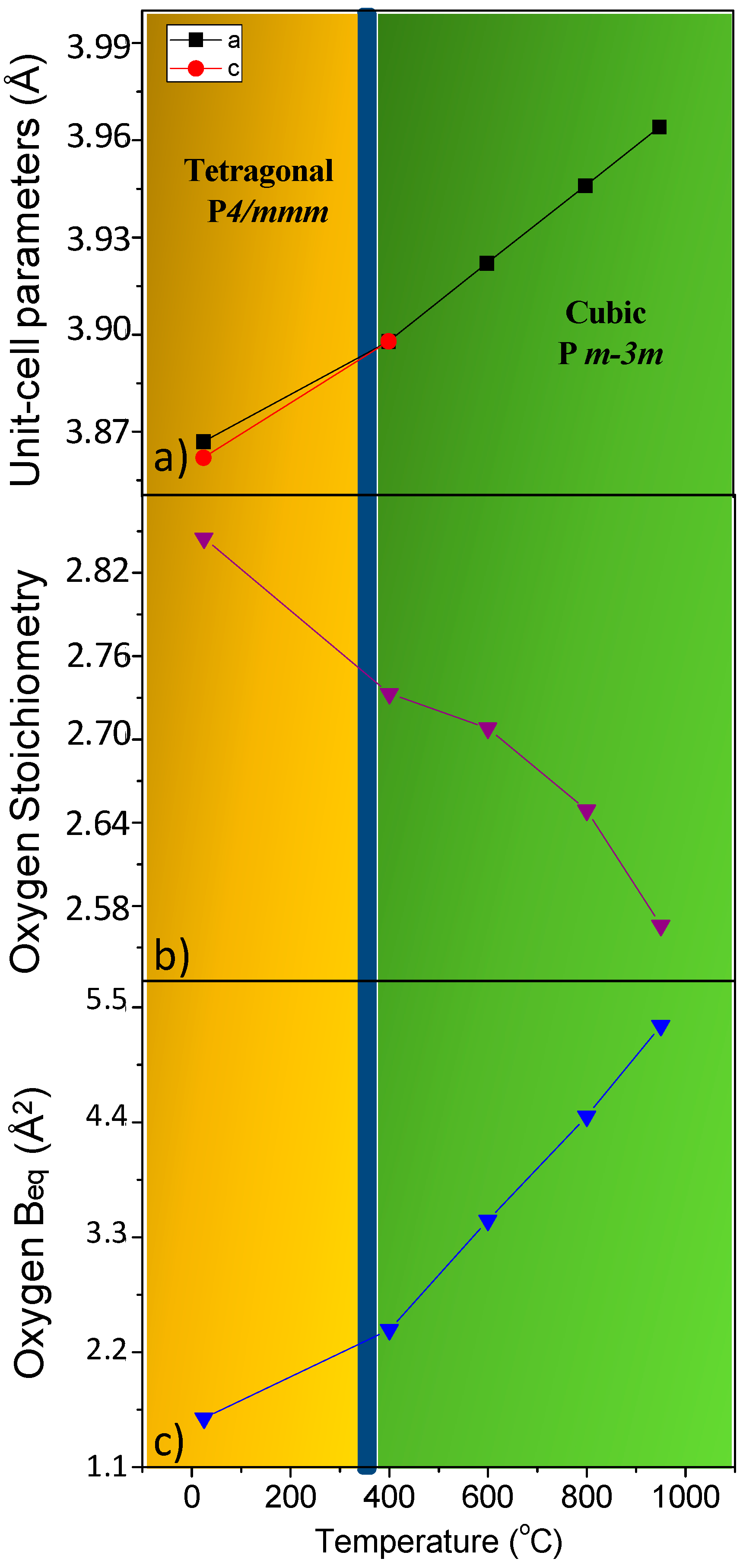
3.1. Crystallographic Characterization
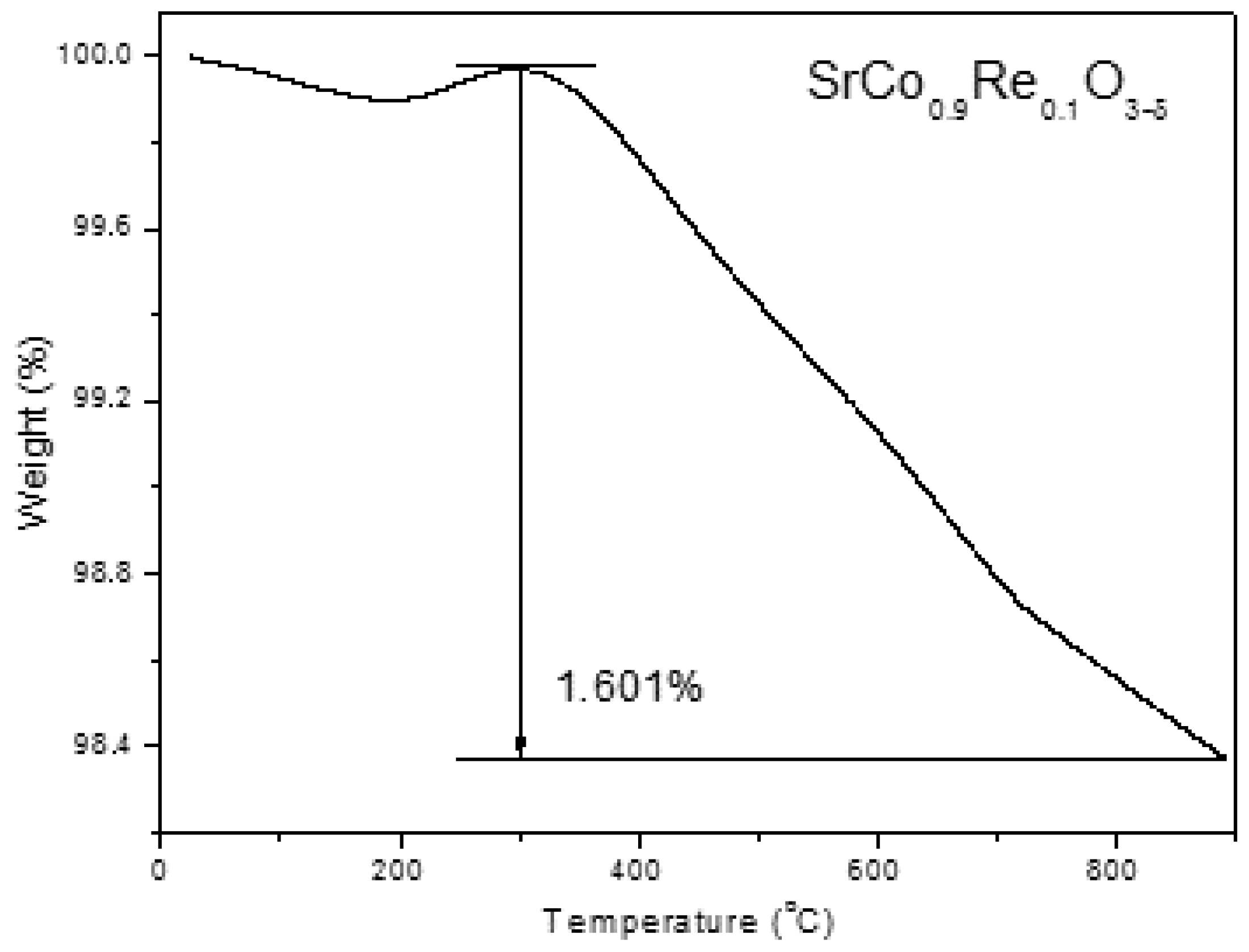
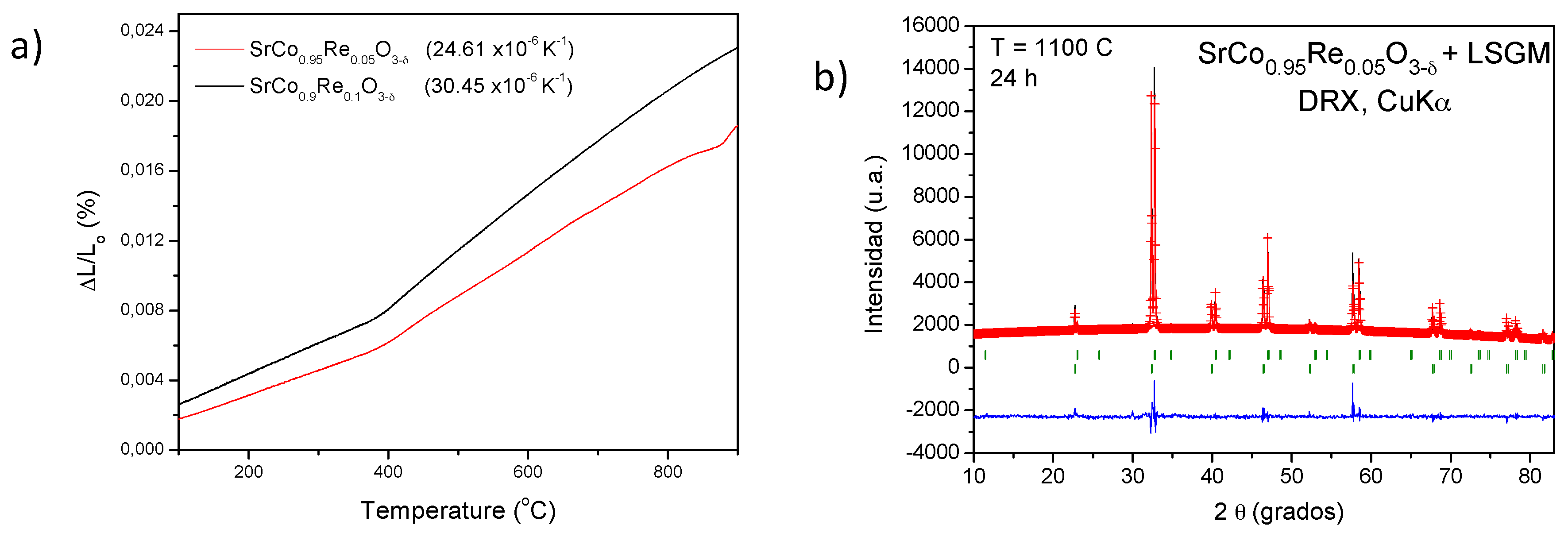
3.2. Thermal Analysis, Thermal Expansion Measurements and Chemical Compatibility
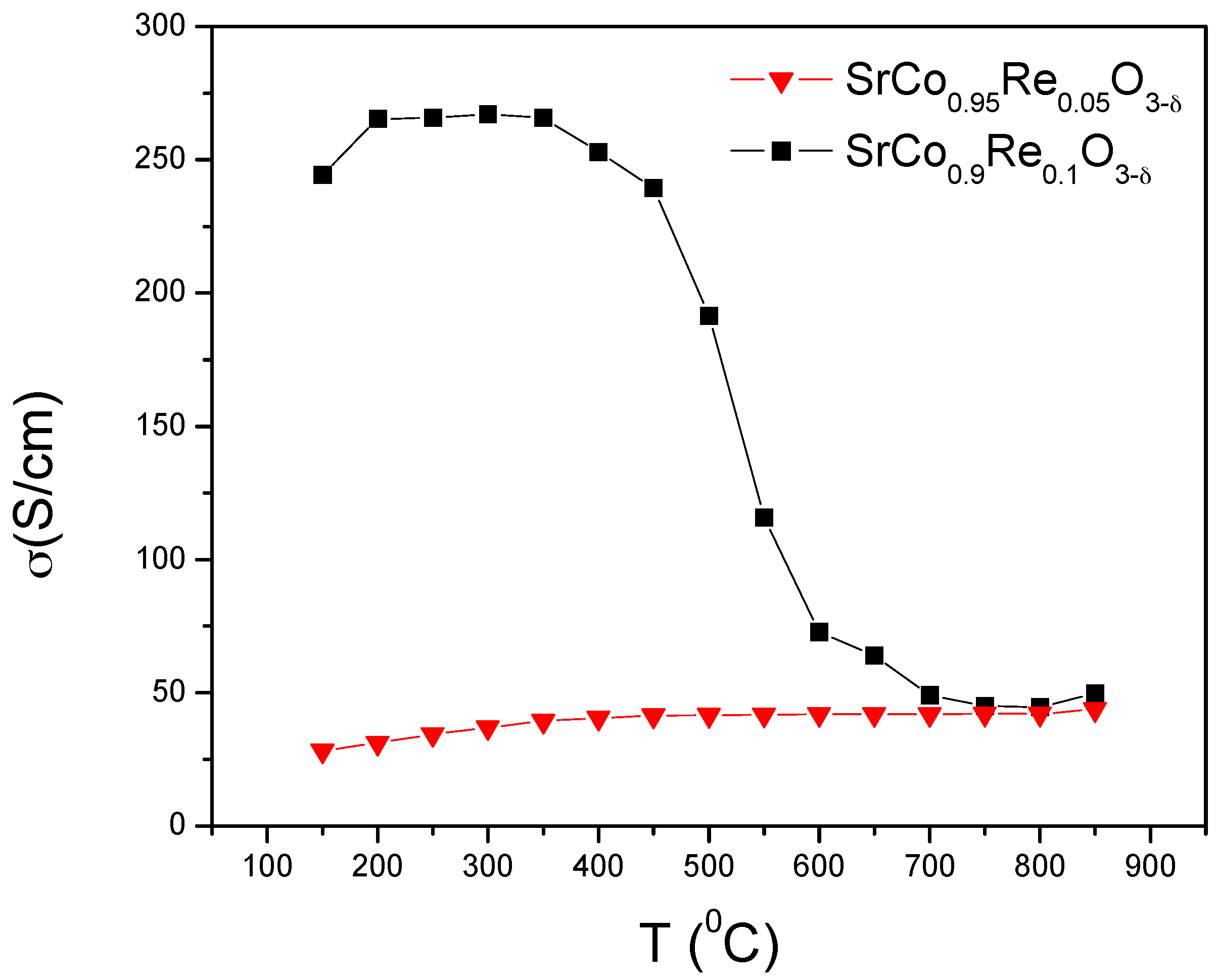
3.3. Electrical Conductivity Measurements
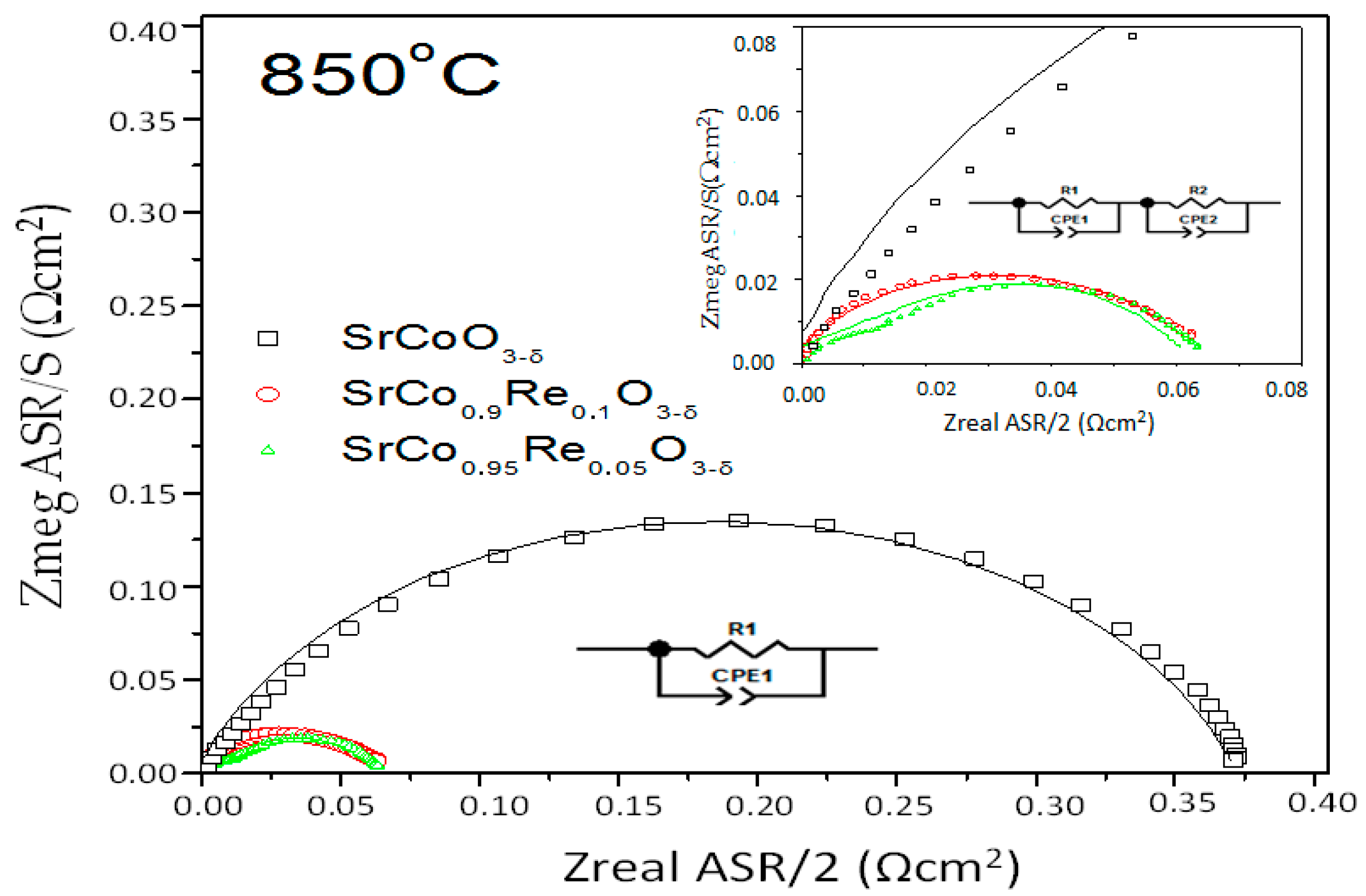
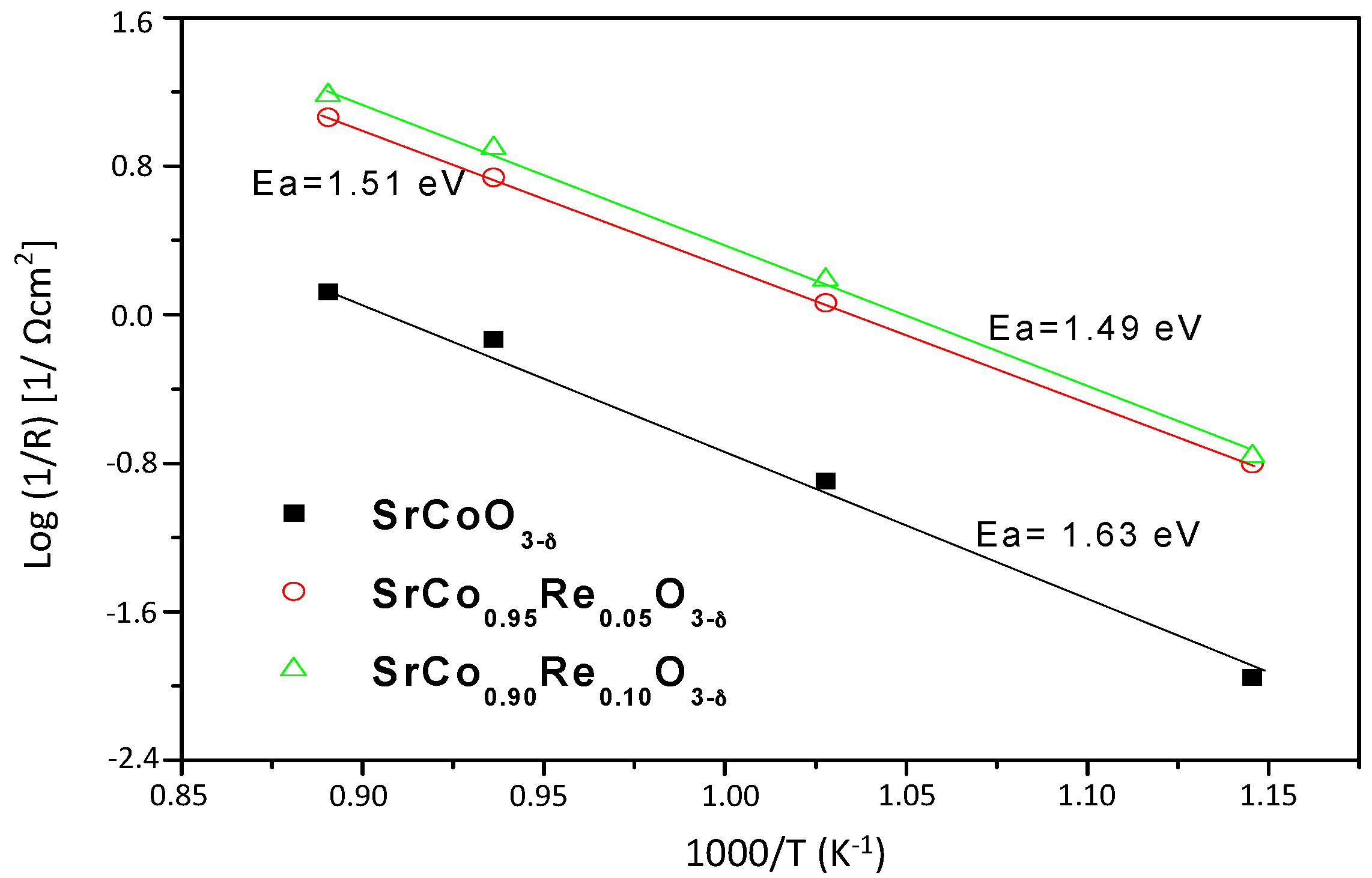
3.4. Impedance Spectroscopy Results
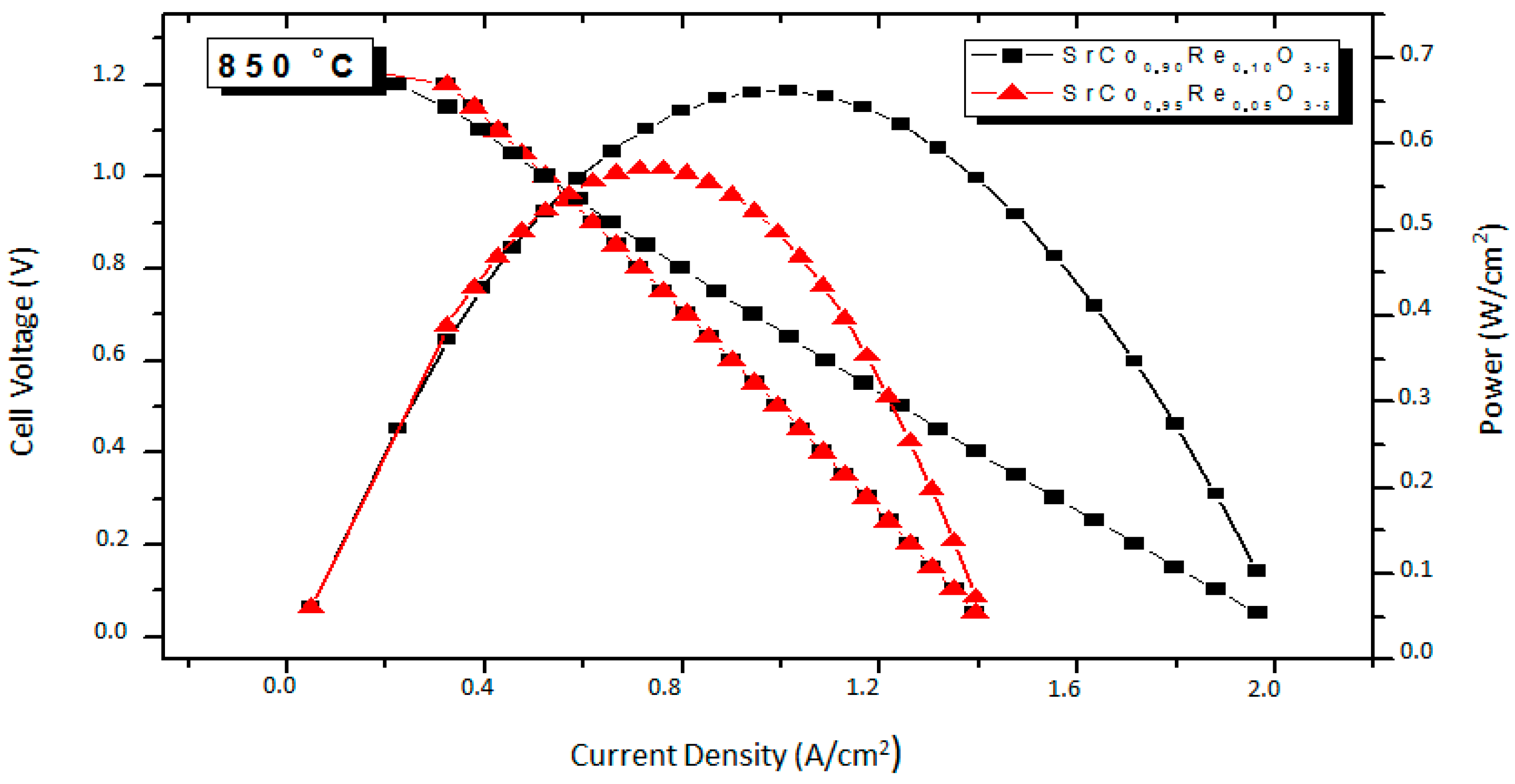
3.5. Fuel-Cell Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. J. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Coronado, R.; Alonso, J.A.; Cascos, V.; Fernández-Díaz, M.T. Crystal and magnetic structure of the Bi2RuMnO7 pyrochlore: A potential new cathode for solid oxide fuel cells. J. Power Sources 2014, 247, 876–882. [Google Scholar] [CrossRef]
- Cascos, V.; Martínez-Coronado, R.; Alonso, J.A.; Fernández-Díaz, M.T. Structural and electrical characterization of the Co-doped Ca2Fe2O5 brownmillerite: Evaluation as SOFC -cathode materials. Int. J. Hydrogen Energy 2015, 40, 5456–5468. [Google Scholar] [CrossRef]
- Sun, C.; Stimming, U. Recent anode advances in solid oxide fuel cells. J. Power Sources 2007, 171, 247–260. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Aguadero, A.; Fawcett, L.; Taub, S.; Woolley, R.; Wu, K.-T.; Xu, N.; Kilner, J.A.; Skinner, S.J. Materials development for intermediate-temperature solid oxide electrochemical devices. J. Mater. Sci. 2012, 47, 3925–3948. [Google Scholar] [CrossRef]
- De la Calle, C.C.; Aguadero, A.; Alonso, J.A.; Fernández-Díaz, M.T. Correlation between reconstructive phase transitions and transport properties from SrCoO2.5 brownmillerite: A neutron diffraction study. Solid State Sci. 2008, 10, 1924–1935. [Google Scholar] [CrossRef]
- Deng, Z.Q.; Yang, W.S.; Liu, W.; Chen, C.S. Relationship between transport properties and phase transformations in mixedconducting oxides. J. Solid State Chem. 2006, 179, 362–369. [Google Scholar] [CrossRef]
- Balamurugan, S.; Takayama-Muromachi, E. Structural and magnetic properties of high-pressure/high-temperature synthesized (Sr1−xRx)CoO3 (R = Y and Ho) perovskites. J. Solid State Chem. 2006, 179, 2231–2236. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, S.; Kim, J.W.; Woo, S.K. Enhancement of oxygen permeation by La0.6Sr0.4CoO3−δ coating in La0.7Sr0.3Ga0.6Fe0.4O3−δ membrane. Desalination 2002, 147, 439–444. [Google Scholar] [CrossRef]
- Ghadimi, A.; Alaee, M.A.; Behrouzifar, A.; Asadi, A.A.; Mohammadi, T. Oxygen permeation of BaxSr1−xCo0.8Fe0.2O3−δ perovskite-type membrane: Experimental and modeling. Desalination 2011, 270, 64–75. [Google Scholar] [CrossRef]
- Behrouzifar, A.; Asadi, A.A.; Mohammadi, T.; Pak, A. Experimental investigation and mathematical modeling of oxygen permeation through dense Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) perovskite-type ceramic membranes. Ceram. Int. 2012, 38, 4797–4811. [Google Scholar] [CrossRef]
- Cascos, V.; Martinez-Coronado, R.; Alonso, J.A.; Fernández-Díaz, M.T. Visualization by Neutron Diffraction of 2D Oxygen Diffusion in the Sr0.7Ho0.3CoO3−δ Cathode for Solid-Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 9194–9200. [Google Scholar] [CrossRef] [PubMed]
- Aguadero, A.; Perez-Coll, D.; Alonso, J.A.; Skinner, S.J.; Kilner, J.A. New family of Mo-doped SrCoO3−δ perovskites for application in reversible solid state electrochemical cells. Chem. Mater. 2012, 24, 2655–2663. [Google Scholar] [CrossRef]
- Cascos, V.; Martínez-Coronado, R.; Alonso, J.A. New Nb-doped SrCo1−xNbxO3−δ perovskites performing as cathodes in solid-oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 14349–14354. [Google Scholar] [CrossRef]
- Aguadero, A.; Retuerto, M.; Jiménez-Villacorta, F.; Fernández-Díaz, M.T.; Alonso, J.A. Evolution of cobalt spin states and magnetic coupling along the SrCo(1−x)Sb(x)O(3−δ) system: Correlation with the crystal structure. Phys. Chem. Chem. Phys. 2011, 13, 12835–12843. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, B.L.; Levasseur, G. Rhenium oxides having an ordered or related perovskite-type structure. Mat. Res. Bull. 1979, 14, 401–407. [Google Scholar] [CrossRef]
- Retuerto, M.; Li, M.-R.; Go, Y.B.; Ignatov, A.; Croft, M.; Ramanujachary, K.V.; Herber, R.H.; Nowik, I.; Hodges, J.P.; Dachraoui, W.; et al. High magnetic ordering temperature in the perovskites Sr4−xLaxFe3ReO12 (x = 0.0, 1.0, 2.0). J. Solid State Chem. 2012, 194, 48–58. [Google Scholar] [CrossRef]
- Yamamura, K.; Wakeshima, M.; Hinatsu, Y. Structural phase transition and magnetic properties of double perovskites Ba2CaMO6 (M = W, Re; Os). J. Solid State Chem. 2006, 179, 605–612. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by Neutron Powder Diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- ZPlot for Windows Version 2.1, Version 3.4c; Scribber Associates Inc.: Hampshire, UK, 1999–2014.
- Martínez-Coronado, R.; Alonso, J.A.; Aguadero, A.; Fernández-Díaz, M.T. Optimized energy conversion efficiency in solid-oxide fuel cells implementing SrMo1−xFexO3−δ perovskites as anodes. J. Power Sources 2012, 208, 153–158. [Google Scholar] [CrossRef]
- Aguadero, A.; de la Calle, C.; Alonso, J.A.; Escudero, M.J.; Fernández-Díaz, M.T.; Daza, L. Structural and Electrical Characterization of the Novel SrCo0.9Sb0.1O3−δ perovskite: Evaluation as a Solid Oxide Fuel Cell Cathode Material. Chem. Mater. 2007, 19, 6437–6444. [Google Scholar] [CrossRef]
- Aguadero, A.; Alonso, J.A.; Perez-Coll, D.; de la Calle, C.; Fernández-Díaz, M.T.; Goodenough, J.B. SrCo0.95Sb0.05O3−δ as Cathode Material for High Power Density Solid Oxide Fuel Cells. Chem. Mater. 2010, 22, 789–798. [Google Scholar] [CrossRef]
- Stevenson, J.W.; Hasinska, K.; Canfield, N.L.; Armstrong, T.R. Influence of cobalt and iron additions on the electrical and thermal properties of (La,Sr)(Ga,Mg)O3−δ. J. Electrochem. Soc. 2000, 147, 3213–3218. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, R.; Suzuki, A.; Hashimoto, T. Preparation, thermal expansion and electrical conductivity of La0.6Sr0.4Co1−xGaxO3−δ (x = 0.0–0.4) as a new cathode material of SOFC. Solid State Ion. 2004, 174, 157–162. [Google Scholar] [CrossRef]
- Shao, Z.P.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 2004, 431, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Grunbaum, N.; Mogni, L.; Prado, F.; Caneiro, A. Phase equilibrium and electrical conductivity of SrCo0.8Fe0.2O3−δ. J. Solid State Chem. 2004, 177, 2350–2357. [Google Scholar] [CrossRef]
- Cascos, V.; Troncoso, L.; Alonso, J.A. New families of Mn+-doped SrCo1−xMxO3−δ perovskites performing as cathodes in solid-oxide fuel cells. Int. J. Hydrogen Energy 2015, 40, 11333–11341. [Google Scholar] [CrossRef]

| Atoms | Site | x | y | z | Biso (Å2) | Beq (Å2) * | focc |
|---|---|---|---|---|---|---|---|
| Sr | 2h | 1/2 | 1/2 | 0.2513(9) | 1.14(2) | 1.00 | |
| Co1 | 1a | 0 | 0 | 0 | 0.09(4) | 1.00 | |
| (Co,Re)2 | 1b | 0 | 0 | 1/2 | 1.23(4) | 0.815(1)/0.185(1) | |
| O1 | 2e | 1/2 | 0 | 0 | 1.139 | 0.921(2) | |
| O2 | 2g | 0 | 0 | 0.755(1) | 2.227 | 1.00(2) | |
| O3 | 2f | 1/2 | 0 | 1/2 | 1.569 | 0.924(2) | |
| * Anisotropic Thermal Factors (× 10−4) (β12 = β13 = β23 = 0) | |||||||
| Atoms | β11 | β22 | β33 | ||||
| O1 | 100(56) | 151(59) | 80(18) | ||||
| O2 | 401(30) | β11 | 79(13) | ||||
| O3 | 141(60) | 207(67) | 109(20) | ||||
| T (°C) | 400 | 600 | 800 | 950 |
|---|---|---|---|---|
| a (Å) | 3.8979(4) | 3.9221(1) | 3.9460(1) | 3.9641(1) |
| V (Å3) | 59.227(1) | 60.333(1) | 61.442(2) | 62.292(2) |
| Atoms | ||||
| Sr | 1b (½,½,½) | |||
| Biso (Å2) | 2.06(5) | 2.59(6) | 3.19(8) | 3.78(9) |
| focc | 1.00 | 1.00 | 1.00 | 1.00 |
| (Co,Re) | 1a (0,0,0) | |||
| Biso (Å2) | 0.50(8) | 1.58(10) | 2.12(13) | 2.52(16) |
| focc | 0.9/0.1 | 0.9/0.1 | 0.9/0.1 | 0.9/0.1 |
| O | 3d (½, 0, 0) | |||
| β11 * | 317(13) | 407(15) | 534(19) | 655(22) |
| β22 = β33 * | 438(8) | 638(10) | 801(12) | 943(14) |
| Beq (Å2) | 2.415 | 3.461 | 4.456 | 5.324 |
| focc | 2.733(2) | 2.708(3) | 2.649(34) | 2.566(36) |
| Reliability factors | ||||
| χ2 | 2.49 | 1.56 | 1.40 | 1.37 |
| Rp (%) | 1.60 | 1.40 | 1.33 | 1.31 |
| Rwp (%) | 2.16 | 1.79 | 1.67 | 1.65 |
| RBragg (%) | 1.65 | 2.52 | 2.11 | 2.14 |
| Bond distances (Å) | ||||
| (Co,Re)–O (x6) | 1.94899(2) | 1.96105(2) | 1.97299(4) | 1.98205(5) |
| 700 °C | 800 °C | 850 °C | |
|---|---|---|---|
| SrCoO3−δ | |||
| R1/ASR (Ω·cm2) | 4.065 | 0.592 | 0.326 |
| SrCo0.95Re0.05O3−δ | |||
| R1 (Ω·cm2) | 0.072 | 0.018 | 0.047 |
| R2 (Ω·cm2) | 0.585 | 0.102 | 0.033 |
| ASR (Ω·cm2) | 0.658 | 0.120 | 0.080 |
| SrCo0.9Re0.1O3−δ | |||
| R1 (Ω·cm2) | 0.035 | 0.127 | 0.066 |
| R2 (Ω·cm2) | 0.565 | ||
| ASR (Ω·cm2) | 0.600 | 0.127 | 0.066 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troncoso, L.; Gardey, M.C.; Fernández-Díaz, M.T.; Alonso, J.A. New Rhenium-Doped SrCo1−xRexO3−δ Perovskites Performing as Cathodes in Solid Oxide Fuel Cells. Materials 2016, 9, 717. https://doi.org/10.3390/ma9090717
Troncoso L, Gardey MC, Fernández-Díaz MT, Alonso JA. New Rhenium-Doped SrCo1−xRexO3−δ Perovskites Performing as Cathodes in Solid Oxide Fuel Cells. Materials. 2016; 9(9):717. https://doi.org/10.3390/ma9090717
Chicago/Turabian StyleTroncoso, Loreto, María Celeste Gardey, María Teresa Fernández-Díaz, and José Antonio Alonso. 2016. "New Rhenium-Doped SrCo1−xRexO3−δ Perovskites Performing as Cathodes in Solid Oxide Fuel Cells" Materials 9, no. 9: 717. https://doi.org/10.3390/ma9090717
APA StyleTroncoso, L., Gardey, M. C., Fernández-Díaz, M. T., & Alonso, J. A. (2016). New Rhenium-Doped SrCo1−xRexO3−δ Perovskites Performing as Cathodes in Solid Oxide Fuel Cells. Materials, 9(9), 717. https://doi.org/10.3390/ma9090717








