Fabrication and Physical Evaluation of Gelatin-Coated Carbonate Apatite Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Gelatin-Free CO3Ap Foam
2.2. Reinforcement of CO3Ap Foam with Gelatin
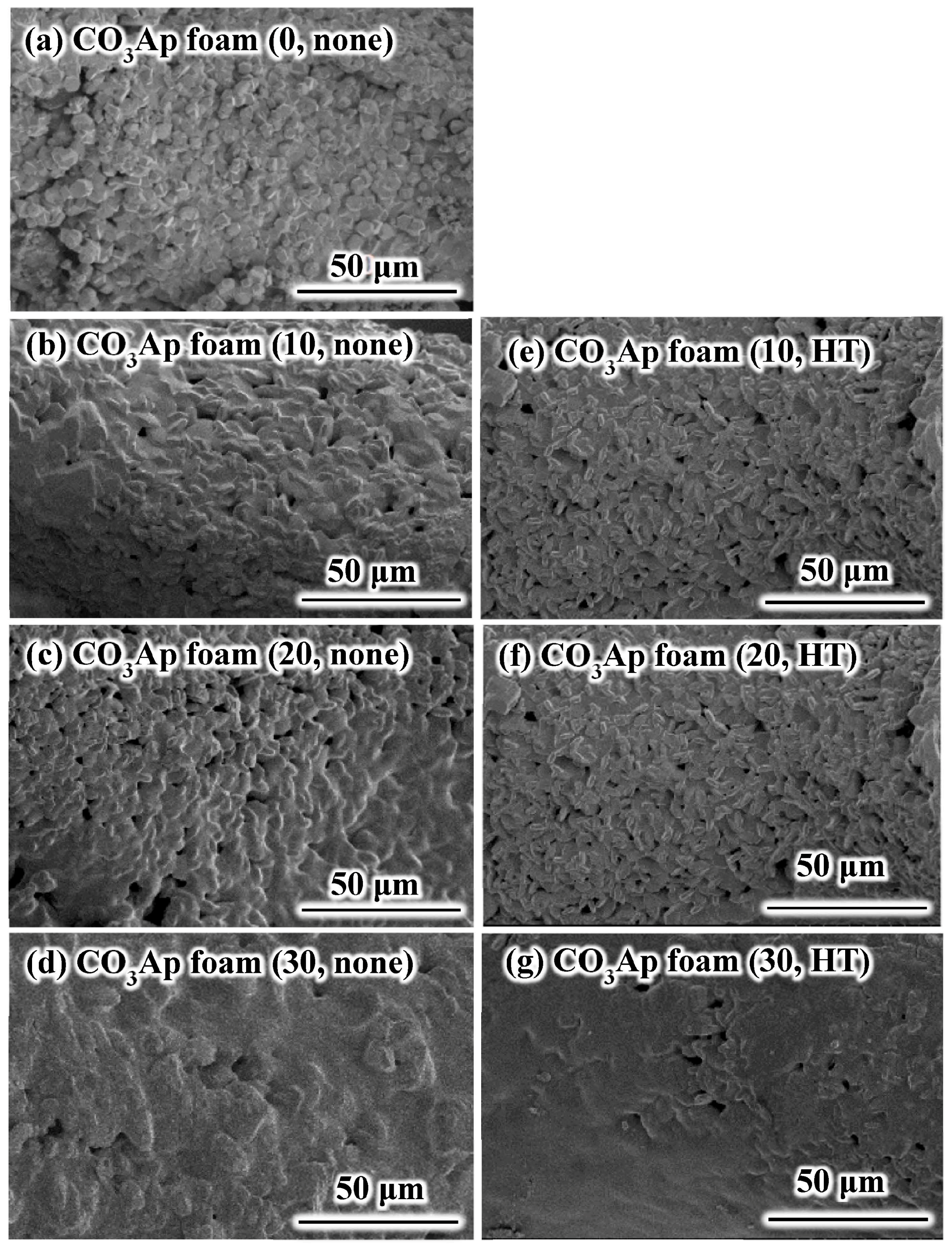
2.3. Scanning Electron Microscopy Observation
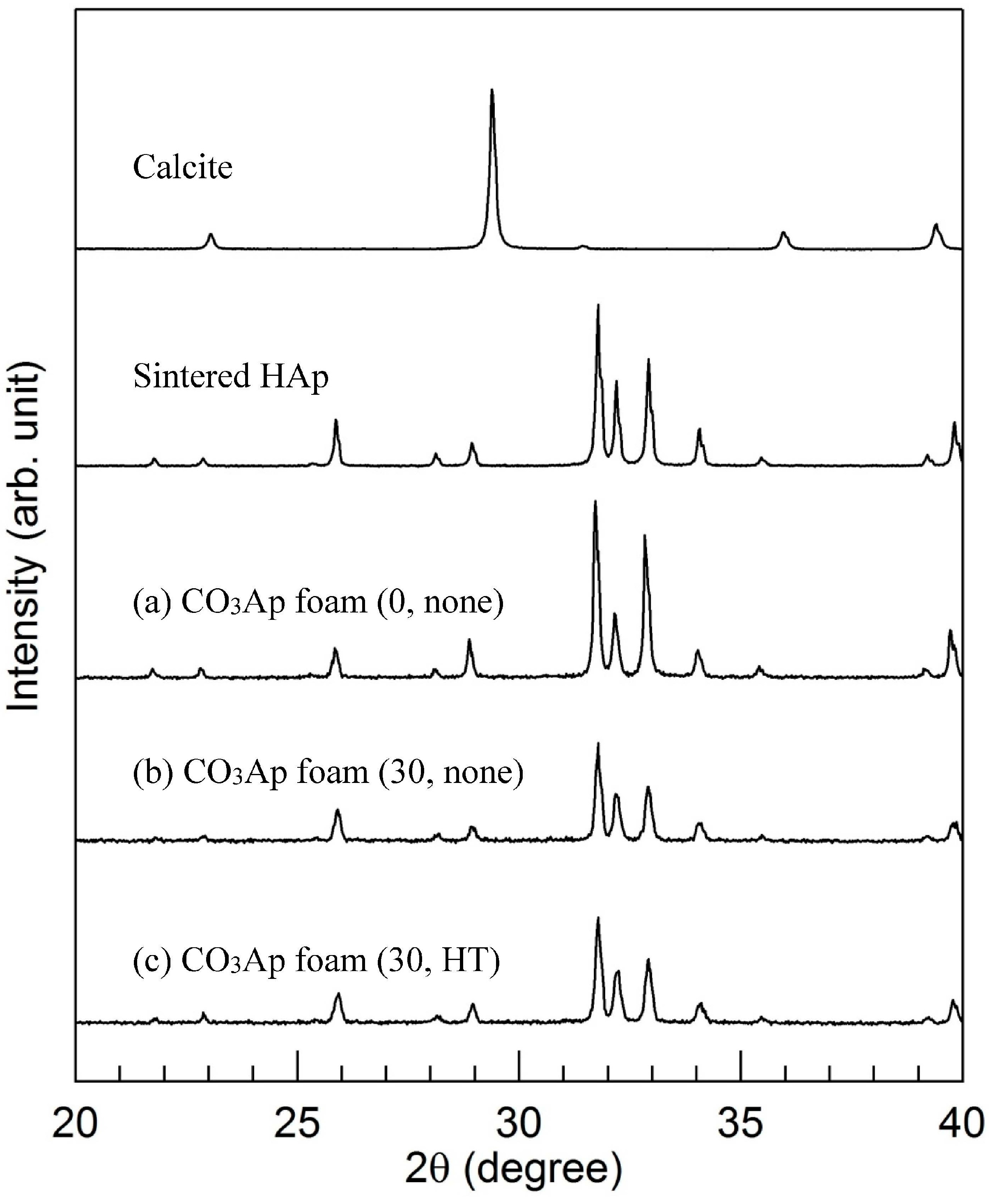
2.4. X-ray Diffraction Analysis
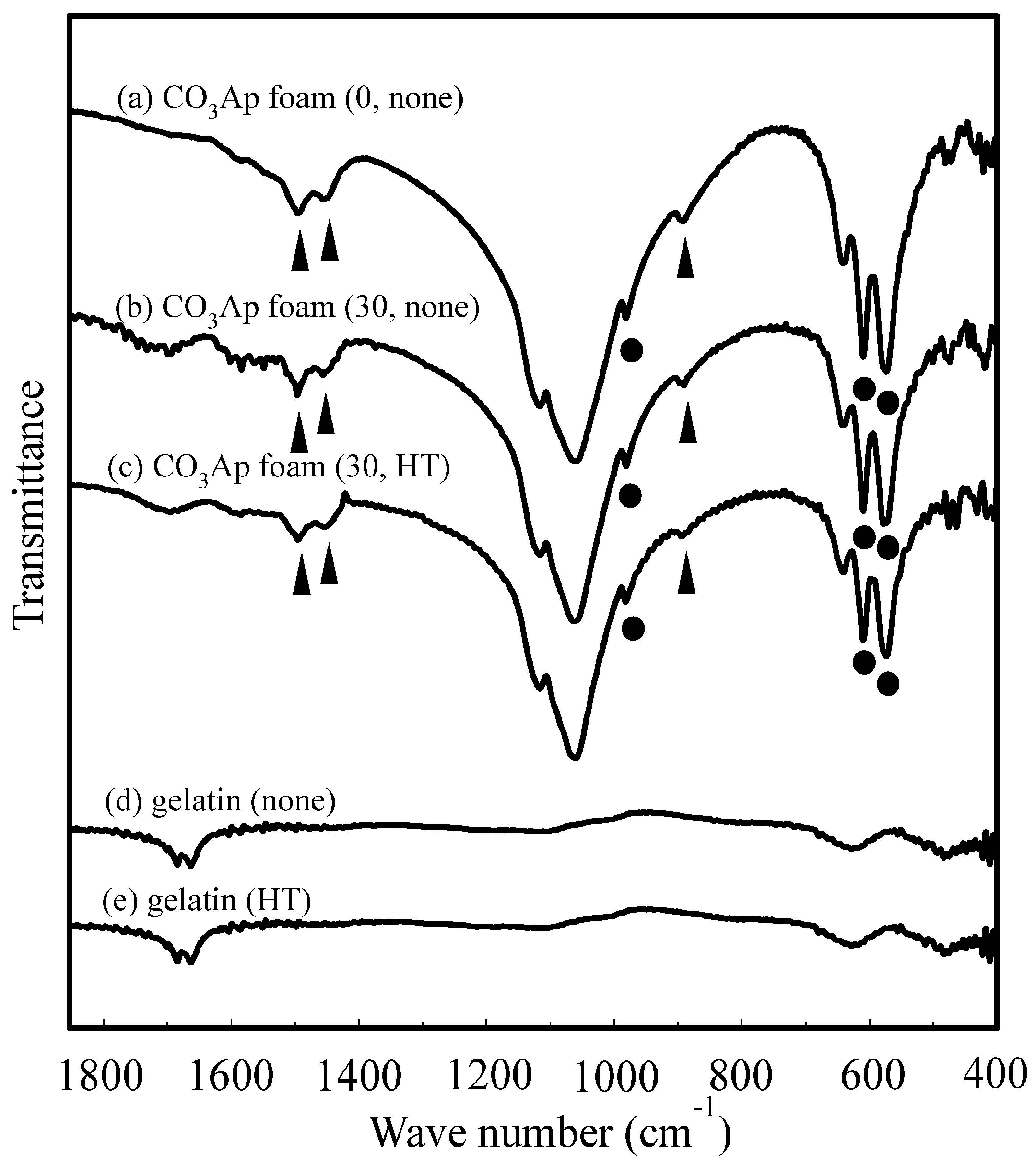
2.5. Fourier-Transformed Infrared Spectroscopy
2.6. Mechanical Strength Evaluation
2.7. Stability of CO3Ap Foam in the Saline Solution
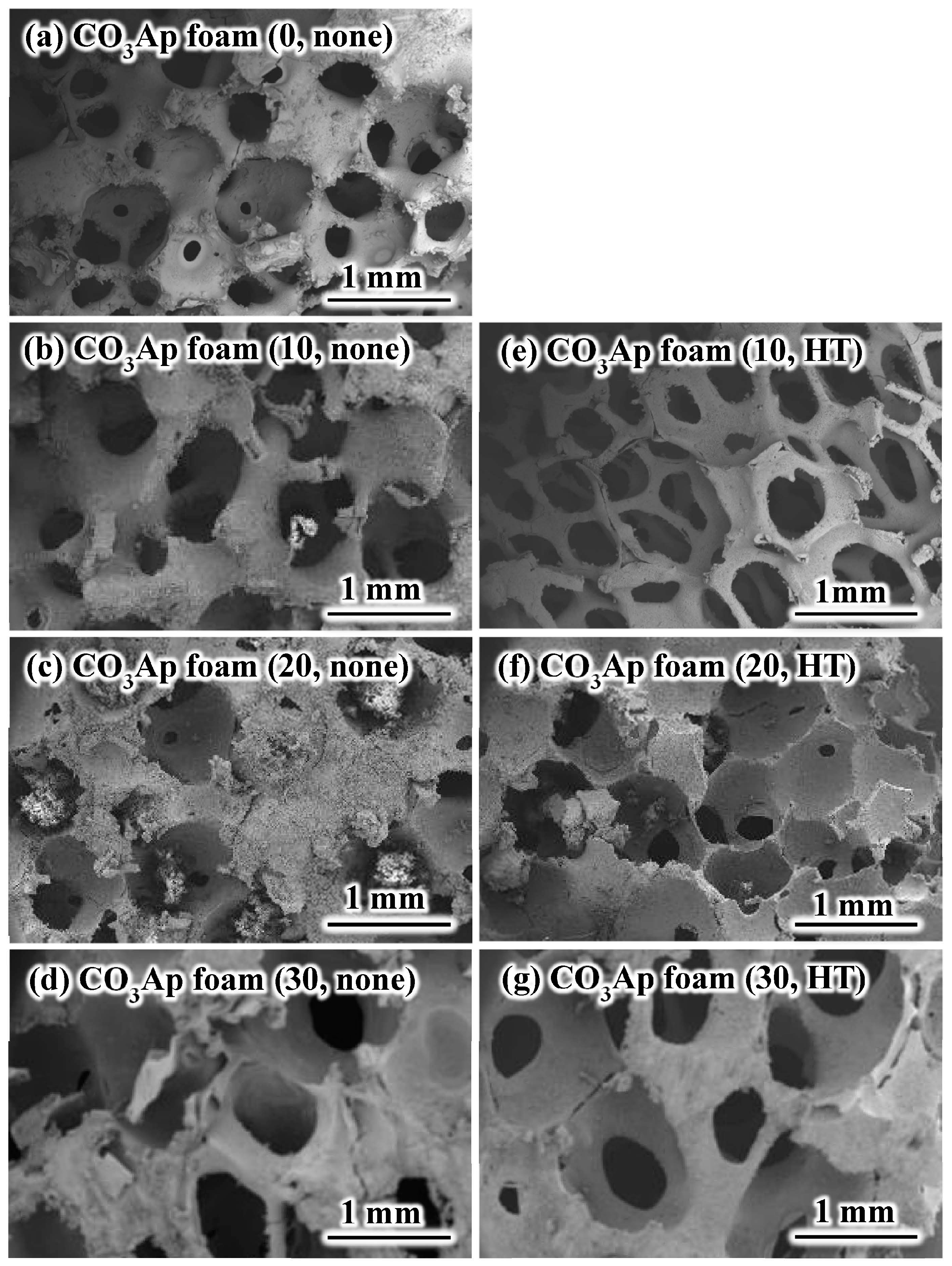
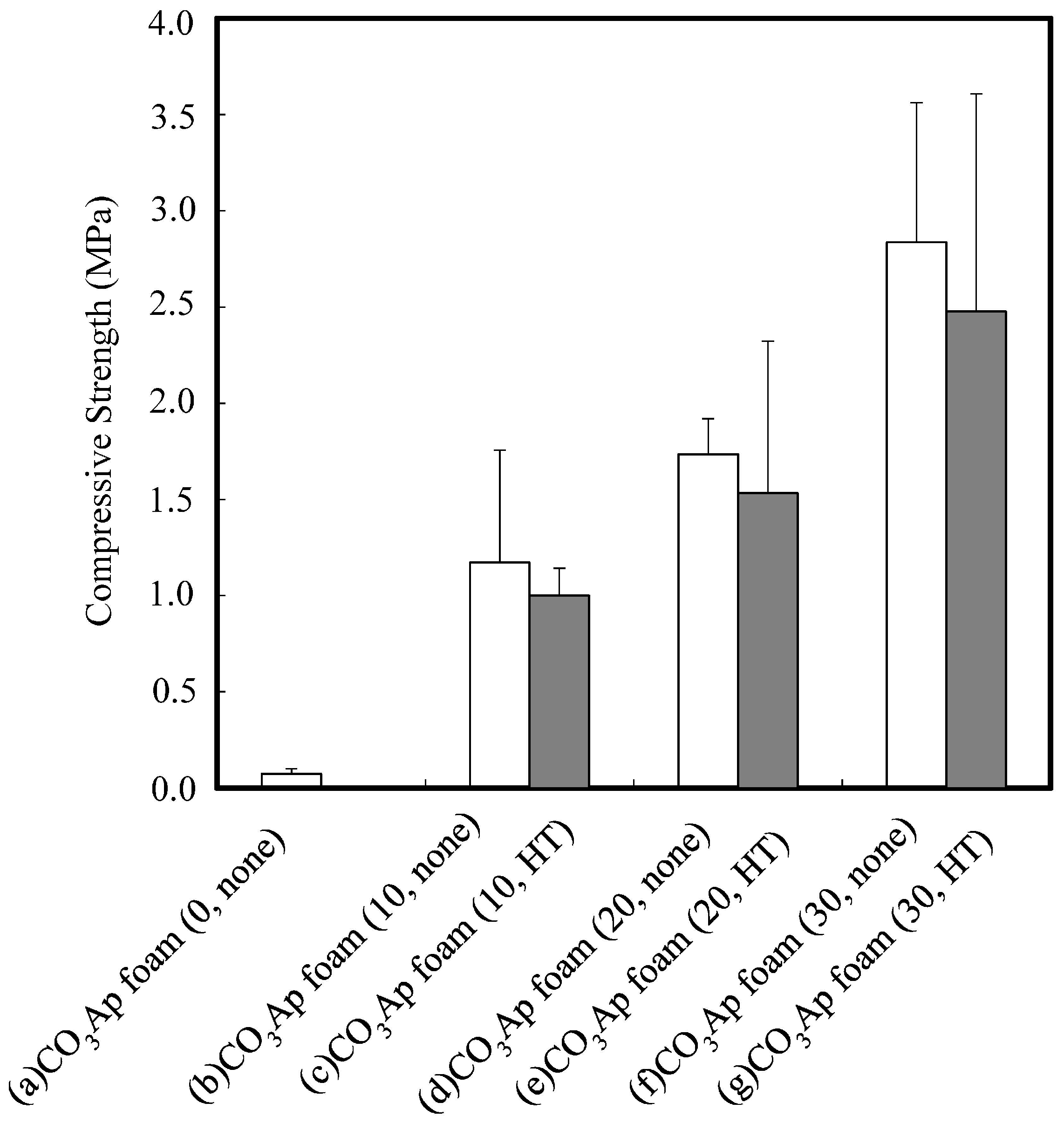
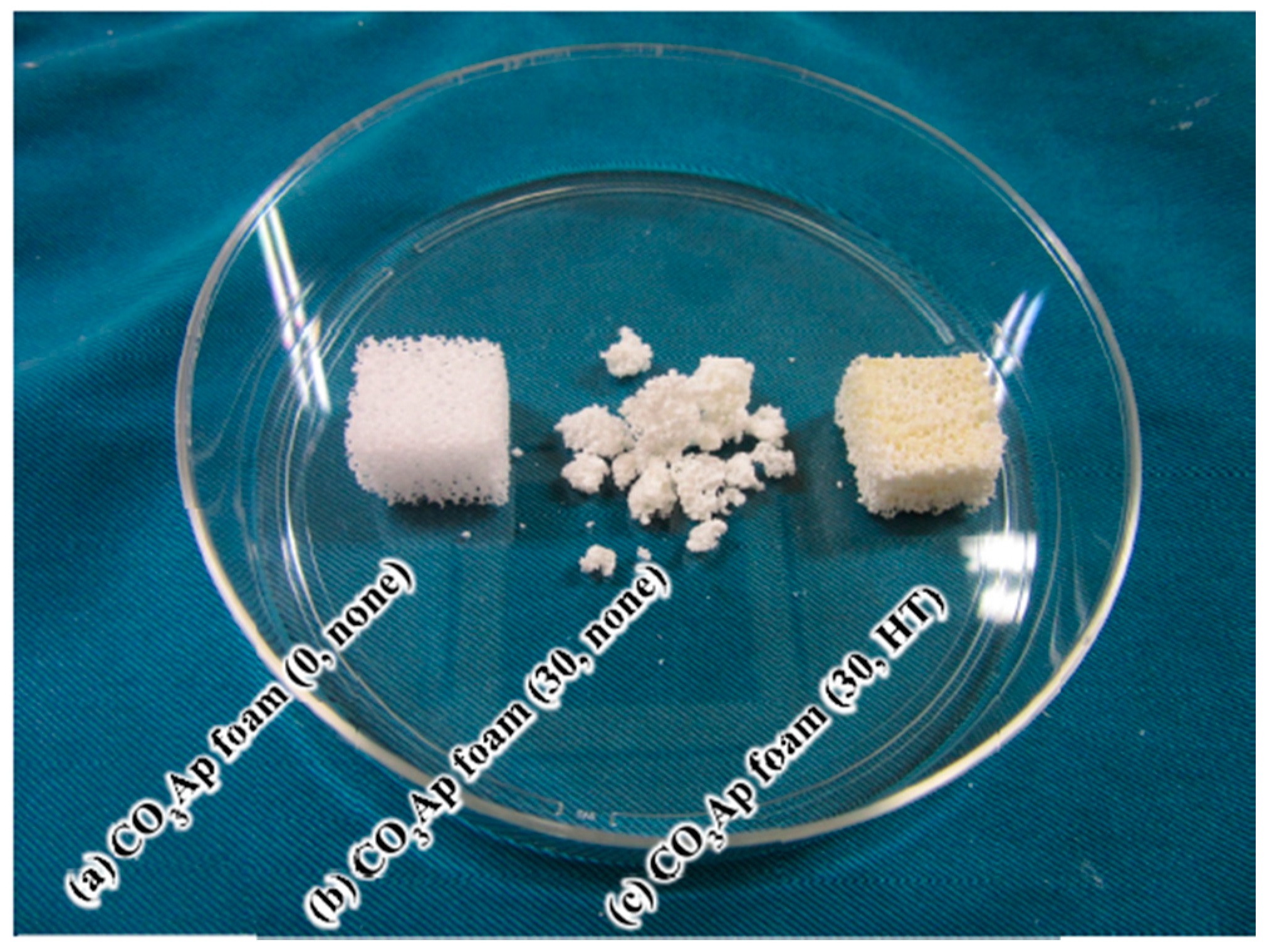
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wakae, H.; Takeuchi, A.; Udoh, K.; Matsuya, S.; Munar, M.L.; LeGeros, R.Z.; Nakamura, A.; Ishikawa, K. Fabrication of macroporous carbonate apatite foam by hydrothermal conversion of α-tricalcium phosphate in carbonate solutions. J. Biomed. Mater. Res. A 2008, 87, 957–963. [Google Scholar] [PubMed]
- Maruta, M.; Matsuya, S.; Nakamura, S.; Ishikawa, K. Fabrication of low-crystalline carbonate apatite foam bone replacement based on phase transformation of calcite foam. Dent. Mater. J. 2011, 30, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Karashima, S.; Takeuchi, A.; Matsuya, S.; Udoh, K.; Koyano, K.; Ishikawa, K. Fabrication of low-crystallinity hydroxyapatite foam based on the setting reaction of α-tricalcium phosphate foam. J. Biomed. Mater. Res. A 2009, 88, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Munar, M.L.; Udoh, K.; Ishikawa, K.; Matsuya, S.; Nakagawa, M. Effects of sintering temperature over 1,300 °C on the physical and compositional properties of porous hydroxyapatite foam. Dent. Mater. J. 2006, 25, 51–58. [Google Scholar] [CrossRef] [PubMed]
- James, W.D.; Berger, T.G.; Elston, D.M. Andrews’ Diseases of the Skin: Clinical Dermatology, 10th ed.; Saunders: Philadephia, PA, USA, 2005; p. 517. [Google Scholar]
- Rauch, F.; Glorieux, F.H. Osteogenesis imperfecta. Lancet 2004, 363, 1377–1385. [Google Scholar] [CrossRef]
- Munar, G.M.; Munar, M.L.; Tsuru, K.; Ishikawa, K. Influence of PLGA concentrations on structural and mechanical properties of carbonate apatite foam. Dent. Mater. J. 2013, 32, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Munar, G.M.; Munar, M.L.; Tsuru, K.; Ishikawa, K. Effect of PLGA reinforcement methods on mechanical property of carbonate apatite foam. Biomed. Mater. Eng. 2014, 24, 1817–1825. [Google Scholar] [PubMed]
- Wiria, F.E.; Leong, K.F.; Chua, C.K.; Liu, Y. Poly-ε-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomater. 2007, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.L.; Ayres, E.; Averous, L.; Schlatter, G.; Hebraud, A.; de Paula, A.C.; Viana, P.H.; Goes, A.M.; Orefice, R.L. Differentiation of human adipose-derived stem cells seeded on mineralized electrospun co-axial poly(ε-caprolactone) (PCL)/gelatin nanofibers. J. Mater. Sci. Mater. Med. 2014, 25, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. The Macromolecular Chemistry of Gelatin New York and London; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterals 2004, 25, 5675–5680. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, J.; Durance, T.D.; Wang, R. Porous scaffold of gelatin-starch with nanohydroxyapatite composite processed via novel microwave vacuum drying. Acta Biomater. 2008, 4, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Yaylaoglu, M.B.; Korkusuz, P.; Ors, U.; Korkusuz, F.; Hasirci, V. Development of a calcium phosphate-gelatin composite as a bone substitute and its use in drug relase. Biomaterials 1999, 20, 711–719. [Google Scholar] [CrossRef]
- Kim, S.M.; Yi, S.A.; Choi, S.H.; Kim, K.M.; Lee, Y.K. Gelatin-layered and multi-sized porous β-tricalcium phosphate for tissue engineering scaffold. Nanoscale Res. Lett. 2012, 7, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bhate, K.; Kulkarni, D.; Santhosh Kumar, S.N.; Katharina, R. The effect of alloplastic bone graft and absorbable gelatin sponge in prevention of periodontal defects on the distal aspect of mandibular second molars, after surgical removal of impacted mandibular third molar: A comparative prospective study. J. Maxillofac. Oral Surg. 2015, 14, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.L.; Sun, C.J.; Li, X.D.; Wang, Y.H.; Wang, C.E. Arterial embolization of massive hepatocellular carcinoma with lipiodol and gelatin sponge. Indian J. Cancer 2015, 51 (Suppl. 2), e49–e51. [Google Scholar] [PubMed]
- Sunarso, S.; Tsuru, K.; Ana, I.D.; Ishikawa, K. Effect of crosslinking to the mechanical property of apatite gelatin hybrid for bone substitution purposes. Indones. J. Chem. 2011, 11, 267–272. [Google Scholar]
- Rey, C.; Renugopalakrishnan, V.; Collins, B.; Glimcher, M.J. Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif. Tissue Int. 1991, 49, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.; Pearson, S.; LeGeros, R.Z. An infrared method for quantification of carbonate in carbonated apatites. Caries Res. 1984, 18, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Digenis, G.A.; Gold, T.B.; Shah, V.P. Cross-linking of gelatin capsules and its relevance to their in vitro-in vivo performance. J. Pharm. Sci. 1994, 83, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen engineering for biomaterial use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.; Krijgsveld, J.; Zaat, S.A.; Denkert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical application. J. Boimater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Gentile, P.; Saracino, S.; Chiono, V.; Nandagiri, V.K.; Muzio, G.; Canuto, R.A.; Ciardelli, G. Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int. J. Biol. Macromol. 2011, 49, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Broderick, E.P.; O’Halloran, D.M.; Rochev, Y.A.; Griffin, M.; Collighan, R.J.; Pandit, A.S. Enzymatic stabilization of gelatin-based scaffolds. J. Biomed. Mater. Res. B 2005, 72, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ciardelli, G.; Gentile, P.; Chiono, V.; Mattioli-Belmonte, M.; Vozzi, G.; Barbani, N.; Giusti, P. Enzymatically crosslinked porous composite matrices for bone tissue regeneration. J. Biomed. Mater. Res. A 2010, 92, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Cipriani, E.; Gnavi, S.; Chino, V.; Mattu, C.; Gentile, P.; Perroteau, I.; Zanetti, M.; Ciardelli, G. Crosslinked gelatin nanofibers: Preparation, characterisation and in vitro studies using glial-like cells. Mater. Sci. Eng. C 2013, 33, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, K.; Fujisawa, K.; Nagai, H.; Takamaru, N.; Ohe, G.; Tsuru, K.; Ishikawa, K.; Miyamoto, Y. Fabrication and Physical Evaluation of Gelatin-Coated Carbonate Apatite Foam. Materials 2016, 9, 711. https://doi.org/10.3390/ma9090711
Hara K, Fujisawa K, Nagai H, Takamaru N, Ohe G, Tsuru K, Ishikawa K, Miyamoto Y. Fabrication and Physical Evaluation of Gelatin-Coated Carbonate Apatite Foam. Materials. 2016; 9(9):711. https://doi.org/10.3390/ma9090711
Chicago/Turabian StyleHara, Kanae, Kenji Fujisawa, Hirokazu Nagai, Natsumi Takamaru, Go Ohe, Kanji Tsuru, Kunio Ishikawa, and Youji Miyamoto. 2016. "Fabrication and Physical Evaluation of Gelatin-Coated Carbonate Apatite Foam" Materials 9, no. 9: 711. https://doi.org/10.3390/ma9090711
APA StyleHara, K., Fujisawa, K., Nagai, H., Takamaru, N., Ohe, G., Tsuru, K., Ishikawa, K., & Miyamoto, Y. (2016). Fabrication and Physical Evaluation of Gelatin-Coated Carbonate Apatite Foam. Materials, 9(9), 711. https://doi.org/10.3390/ma9090711




