Recovery of Butanol by Counter-Current Carbon Dioxide Fractionation with its Potential Application to Butanol Fermentation
Abstract
:1. Introduction
2. Results
2.1. Effect of Solvent-to-Feed Ratio
2.2. Effect of Pressure and Temperature
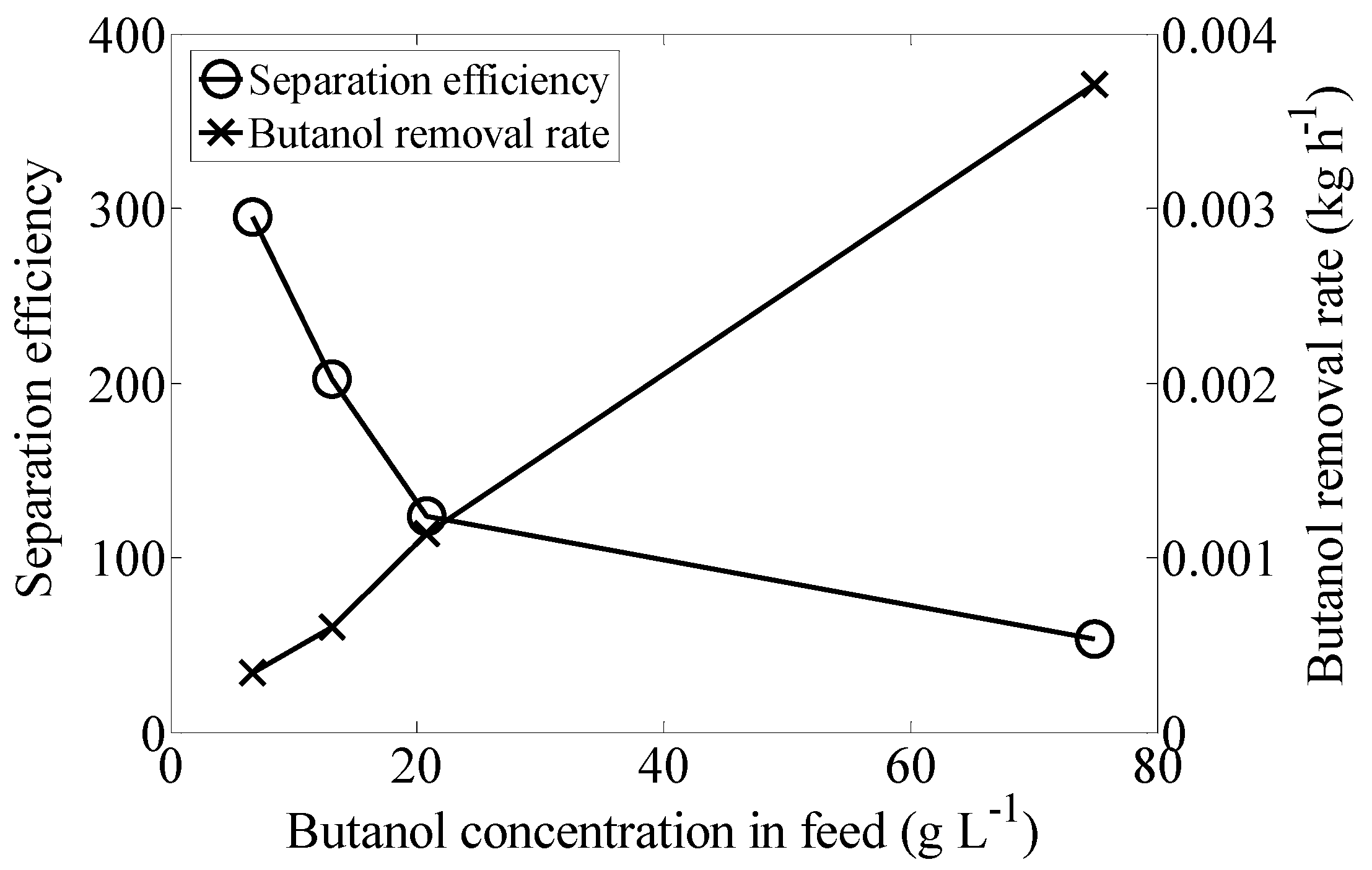
2.3. Butanol Concentration
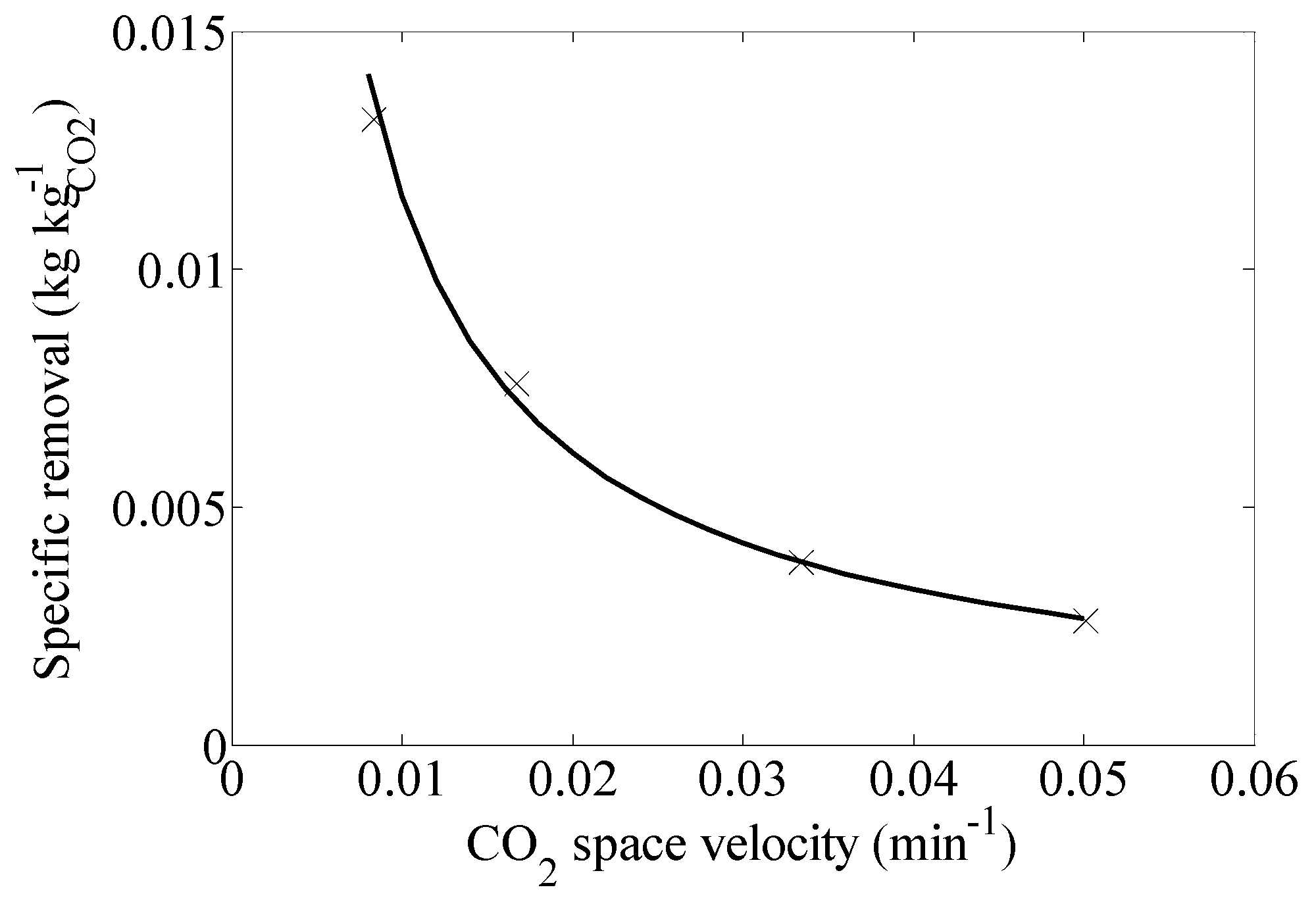
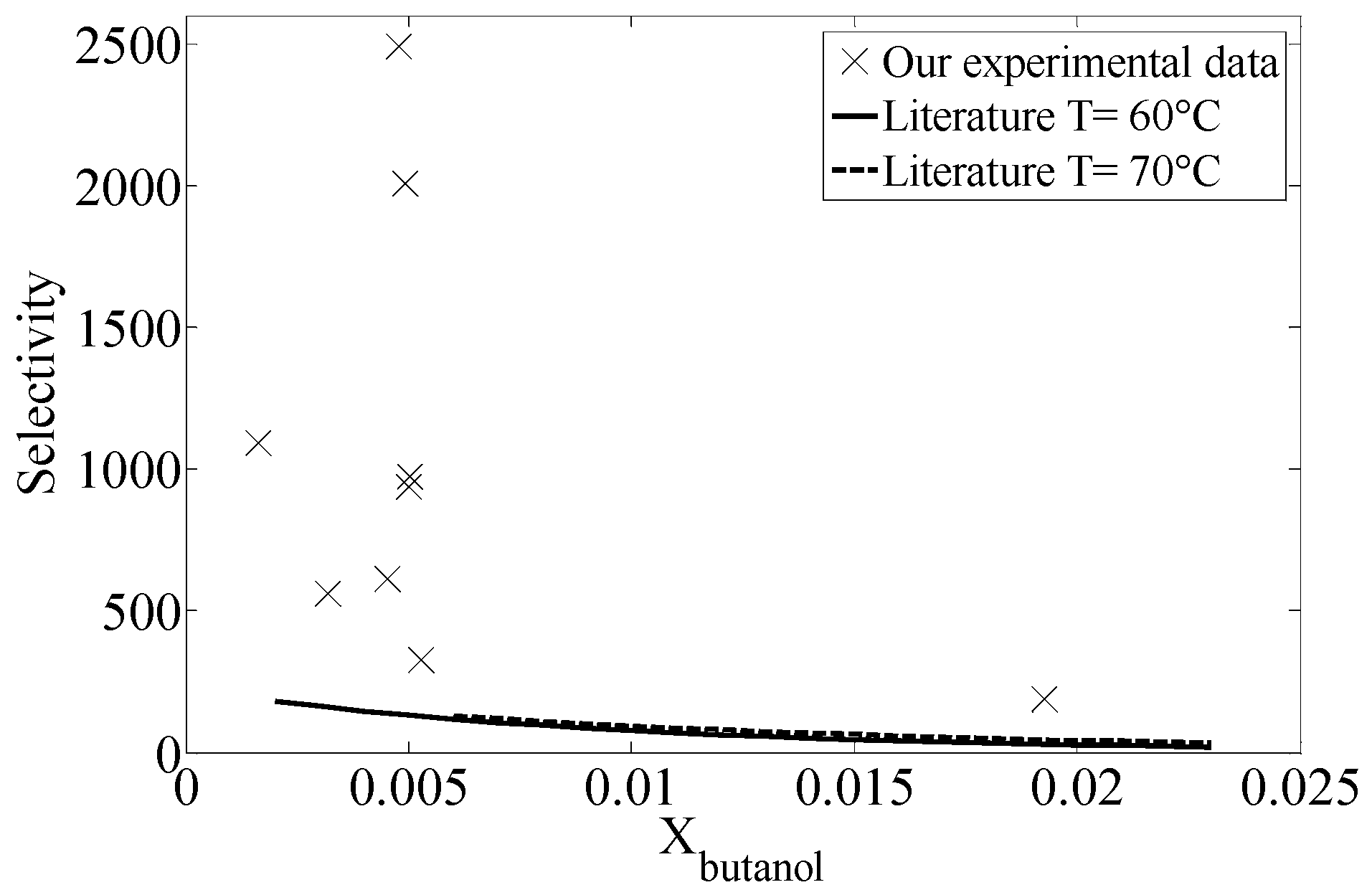
2.4. Comparison with Equilibrium Data
2.5. Butanol, Acetone and Ethanol
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fractionation Column
4.3. Analytical Method
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Águeda, V.I.; Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; García, Á. Column dynamics of an adsorption-drying-desorption process for butanol recovery from aqueous solutions with silicalite pellets. Sep. Purif. Technol. 2013, 104, 307–321. [Google Scholar] [CrossRef]
- Pikeresearch. Available online: www.pikeresearch.%20com/research/biofuels-markets-and-technologies (accessed on 17 September 2015).
- Carvalho, D.L.; De Avillez, R.R.; Rodrigues, M.T.; Borges, L.E.P.; Appel, L.G. Mg and Al mixed oxides and the synthesis of n-butanol from ethanol. Appl. Catal. A Gen. 2012, 415–416, 96–100. [Google Scholar] [CrossRef]
- Green, E.M. Fermentative production of butanol—The industrial perspective. Curr. Opin. Biotechnol. 2011, 22, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Anton, D.; Dobson, I. DuPont and BP biobutanol update. Ind. Bioprocess. 2008, 30, 7. [Google Scholar]
- Balasubramanian, S.; Allen, J.D.; Kanitkar, A.; Boldor, D. Oil extraction from Scenedesmus obliquus using a continuous microwave system—Design, optimization, and quality characterization. Bioresour. Technol. 2011, 102, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R.L. Challenges in biobutanol production: How to improve the efficiency? Renew. Sustain. Energy Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Luyben, W.L. Control of the Heterogeneous Azeotropic n-Butanol/Water Distillation System. Energy Fuels 2008, 22, 4249–4258. [Google Scholar] [CrossRef]
- Qureshi, N.; Cotta, M.A.; Saha, B.C. Bioconversion of barley straw and corn stover to butanol (a biofuel) in integrated fermentation and simultaneous product recovery bioreactors. Food Bioprod. Process. 2014, 92, 298–308. [Google Scholar] [CrossRef]
- Kraemer, K.; Harwardt, A.; Bronneberg, R.; Marquardt, W. Separation of butanol from acetone-butanol-ethanol fermentation by a hybrid extraction-distillation process. Comput. Chem. Eng. 2010, 35, 949–963. [Google Scholar] [CrossRef]
- Saravanan, V.; Waijers, D.A.; Ziari, M.; Noordermeer, M.A. Recovery of 1-butanol from aqueous solutions using zeolite ZSM-5 with a high Si/Al ratio; suitability of a column process for industrial applications. Biochem. Eng. J. 2010, 49, 33–39. [Google Scholar] [CrossRef]
- Fouad, E.A.; Feng, X. Pervaporative separation of n-butanol from dilute aqueous solutions using silicalite-filled poly(dimethyl siloxane) membranes. J. Membr. Sci. 2009, 339, 120–125. [Google Scholar] [CrossRef]
- Liu, F.; Liu, L.; Feng, X. Separation of acetone-butanol-ethanol (ABE) from dilute aqueous solutions by pervaporation. Sep. Purif. Technol. 2005, 42, 273–282. [Google Scholar] [CrossRef]
- Huang, J.; Meagher, M.M. Pervaporative recovery of n-butanol from aqueous solutions and ABE fermentation broth using thin-film silicalite-filled silicone composite membranes. J. Membr. Sci. 2001, 192, 231–242. [Google Scholar] [CrossRef]
- Qureshi, N.; Meagher, M.M.; Huang, J.; Hutkins, R.W. Acetone butanol ethanol (ABE) recovery by pervaporation using silicalite-silicone composite membrane from fed-batch reactor of Clostridium acetobutylicum. J. Membr. Sci. 2001, 187, 93–102. [Google Scholar] [CrossRef]
- Fadeev, A.G.; Selinskaya, Y.A.; Kelley, S.S.; Meagher, M.M.; Litvinova, E.G.; Khotimsky, V.S.; Volkov, V.V. Extraction of butanol from aqueous solutions by pervaporation through poly(1-trimethylsilyl-1-propyne). J. Membr. Sci. 2001, 186, 205–217. [Google Scholar] [CrossRef]
- Fouad, E.A.; Feng, X. Use of pervaporation to separate butanol from dilute aqueous solutions: Effects of operating conditions and concentration polarization. J. Membr. Sci. 2008, 323, 428–435. [Google Scholar] [CrossRef]
- Fadeev, A.G.; Kelley, S.S.; McMillan, J.D.; Selinskaya, Y.A.; Khotimsky, V.S.; Volkov, V.V. Effect of yeast fermentation by-products on poly[1-(trimethylsilyl)-1-propyne] pervaporative performance. J. Membr. Sci. 2003, 214, 229–238. [Google Scholar] [CrossRef]
- Qureshi, N. Integrated Bioprocessing and simultaneous product recovery for butanol production. In Biorefineries: Integrated Biochemical Processes for Liquid Biofuels; Qureshi, A.V.N., Hodge, D., Vertès, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 205–223. [Google Scholar]
- Laitinen, A.; Kaunisto, J. Supercritical fluid extraction of 1-butanol from aqueous solutions. J. Supercrit. Fluids 1999, 15, 245–252. [Google Scholar] [CrossRef]
- Brunner, G. Counter-current separations. J. Supercrit. Fluids 2009, 47, 574–582. [Google Scholar] [CrossRef]
- Ennis, B.M.; Maddox, I.S. Use of Clostridium acetobutylicum P262 for production of solvents from whey permeate. Biotechnol. Lett. 1985, 7, 601–606. [Google Scholar] [CrossRef]
- Frick, C.; Schügerl, K. Continuous acetone-butanol production with free and immobilized Clostridium acetobutylicum. Appl. Microbiol. Biotechnol. 1986, 25, 186–193. [Google Scholar] [CrossRef]
- Maddox, I.S. The acetone-butanol-ethanol fermentation: Recent progress in technology. Biotechnol. Genet. Eng. Rev. 1989, 7, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C.; Qureshi, N.; Liu, S. Cellulosic butanol production from agricultural biomass and residues: Recent advances in technology. In Advanced Biofuels and Bioproducts; Lee, J.W., Ed.; Springer: New York, NY, USA, 2013; pp. 247–265. [Google Scholar]
- Qureshi, N.; Maddox, I.S. Reactor design for the ABE fermentation using cells of Clostridium acetobutylicum immobilized by adsorption onto bonechar. Bioprocess Eng. 1988, 3, 69–72. [Google Scholar] [CrossRef]
- Qureshi, N.; Singh, V.; Liu, S.; Ezeji, T.C.; Saha, B.C.; Cotta, M.A. Process integration for simultaneous saccharification, fermentation, and recovery (SSFR): Production of butanol from corn stover using Clostridium beijerinckii P260. Bioresour. Technol. 2014, 154, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, H.; Chen, P. High-Pressure Phase Equilibria of Carbon Dioxide + 1-Butanol, and Carbon Dioxide + Water + 1-Butanol Systems. J. Chem. Eng. Data 2002, 47, 776–780. [Google Scholar] [CrossRef]
- Huang, W.; Ramey, D.; Yang, S. Continuous Production of Butanol by Clostridium acetobutylicum Immobilized in a Fibrous Bed Bioreactor. Appl. Biochem. Biotechnol. 2004, 113, 887–898. [Google Scholar] [CrossRef]
- Afschar, A.S.; Biebl, H.; Schaller, K.; Schiigerl, K. Production of acetone and butanol by Clostridium acetobutylicum in continuous culture with cell recycle. Appl. Microbiol. Biotechnol. 1985, 22, 394–398. [Google Scholar] [CrossRef]
- Qureshi, N.; Schripsema, J.; Lienhardt, J.; Blaschek, H.P. Continuous solvent production by Clostridium beijerinckii BA101 immobilized by adsorption onto brick. World J. Microbiol. Biotechnol. 2000, 16, 377–382. [Google Scholar] [CrossRef]
- Qureshi, N.; Meagher, M.; Hutkins, R. Recovery of butanol from model solutions and fermentation broth using a silicalite/silicone membrane. J. Membr. Sci. 1999, 158, 115–125. [Google Scholar] [CrossRef]
- Qureshi, N.; Singh, V. Process economics of renewable biorefineries: Butanol and ethanol production in integrated bioprocesses from lignocellulosics and other industrial by-products. In Biorefineries: Integrated Biochemical Processes for Liquid Biofuels; Qureshi, N., Hodge, D., Vertès, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 237–254. [Google Scholar]
- Eller, F.J.; Taylor, S.L.; Laszlo, J.A.; Compton, D.L.; Teel, J.A. Counter-Current Carbon Dioxide Purification of Partially Deacylated Sunflower Oil. J. Am. Oil Chem. Soc. 2008, 86, 277–282. [Google Scholar] [CrossRef]
- Dunford, N.T.; Teel, J.A.; King, J.W. A continuous countercurrent supercritical fluid deacidification process for phytosterol ester fortification in rice bran oil. Food Res. Int. 2003, 36, 175–181. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
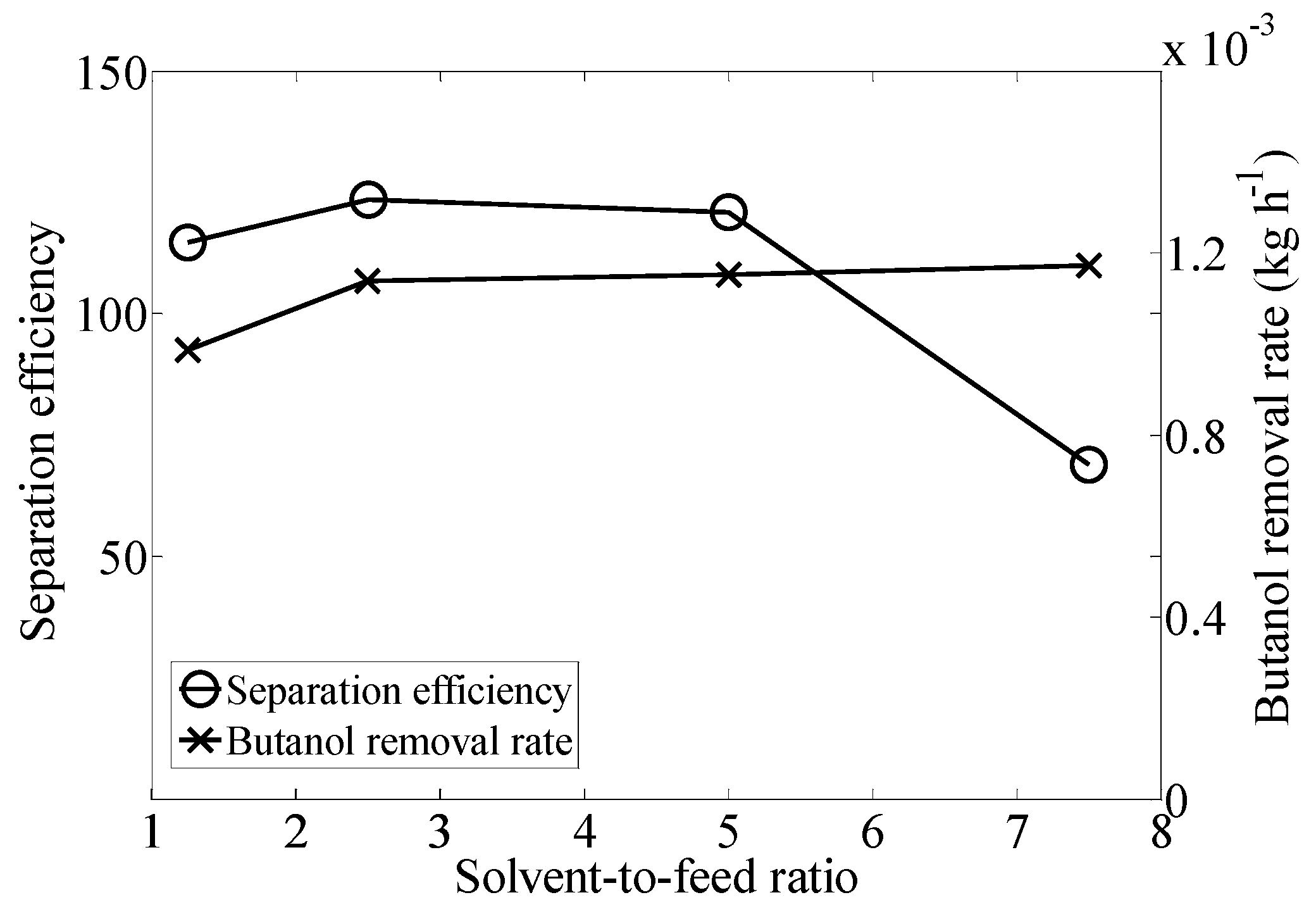

| Solvent to Feed Rate | Butanol Concentration in Extract (g·L−1) | Total Removal (%) |
|---|---|---|
| 1.25 | 603.15 ± 13.22 | 76.87 ± 7.72 |
| 2.5 | 571.15 ± 42.21 | 93.01 ± 0.79 |
| 5 | 548.00 ± 82.80 | 94.01 ± 2.83 |
| 7.5 | 423.06 ± 39.26 | 96.14 ± 0.17 |
| Temperature (°C) | Pressure (MPa) | Separation Efficiency | Removal Rate (kg·h−1) | Butanol Concentration in Extract (g·L−1) | Total Removal (%) |
|---|---|---|---|---|---|
| 25 | 10.34 | 123.49 ± 34.34 | 0.0011 ± 0.0002 | 571.15 ± 42.21 | 93.01 ± 0.79 |
| 35 | 10.34 | 351.48 ± 5.74 | 0.0011 ± 2.04 × 10−5 | 787.50 ± 3.54 | 92.33 ± 0.71 |
| 50 | 25.16 | 119.15 ± 6.09 | 0.0012 ± 4.16 × 10−5 | 552.4 ± 17.11 | 96.90 ± 0.90 |
| Butanol Concentration in Feed (g·L−1) | Butanol Concentration in Extract (g·L−1) | Total Removal (%) |
|---|---|---|
| 6.7 ± 0.6 | 526.99 ± 58.28 | 84.46 ± 2.38 |
| 13.1 ± 0.7 | 609.2 ± 48.01 | 77.15 ± 1.50 |
| 20.8 ± 1.1 | 571.15 ± 42.21 | 93.01 ± 0.79 |
| 74.9 ± 5.3 | 712.5 ± 80.26 | 85.63 ± 4.93 |
| Concentration in Feed (g·L−1) | Concentration in Raffinate (g·L−1) | Concentration in Extract (g·L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Butanol | Acetone | Ethanol | Butanol | Acetone | Ethanol | Butanol | Acetone | Ethanol |
| 19.83 ± 2.83 | - | - | 1.49 ± 0.12 | - | - | 787.50 ± 3.54 | - | - |
| 20.09 ± 1.48 | 6.37 ± 1.37 | - | 1.74 ± 0.15 | 1.14 ± 0.11 | - | 740.73 ± 10.47 | 24.01 ± 1.34 | - |
| 19.34 ± 0.99 | 4.71 ± 0.38 | 1.01 ± 0.07 | 1.61 ± 0.08 | 0.57 ± 0.04 | 0.75 ± 0.01 | 829 ± 85 | 69.09 ± 0.81 | 0.00 ± 0.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solana, M.; Qureshi, N.; Bertucco, A.; Eller, F. Recovery of Butanol by Counter-Current Carbon Dioxide Fractionation with its Potential Application to Butanol Fermentation. Materials 2016, 9, 530. https://doi.org/10.3390/ma9070530
Solana M, Qureshi N, Bertucco A, Eller F. Recovery of Butanol by Counter-Current Carbon Dioxide Fractionation with its Potential Application to Butanol Fermentation. Materials. 2016; 9(7):530. https://doi.org/10.3390/ma9070530
Chicago/Turabian StyleSolana, Miriam, Nasib Qureshi, Alberto Bertucco, and Fred Eller. 2016. "Recovery of Butanol by Counter-Current Carbon Dioxide Fractionation with its Potential Application to Butanol Fermentation" Materials 9, no. 7: 530. https://doi.org/10.3390/ma9070530
APA StyleSolana, M., Qureshi, N., Bertucco, A., & Eller, F. (2016). Recovery of Butanol by Counter-Current Carbon Dioxide Fractionation with its Potential Application to Butanol Fermentation. Materials, 9(7), 530. https://doi.org/10.3390/ma9070530






