Role of Chloride in the Corrosion and Fracture Behavior of Micro-Alloyed Steel in E80 Simulated Fuel Grade Ethanol Environment
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Test Environments
2.2. Immersion Tests
2.3. Electrochemical Measurements
2.4. Visual Examination and Determination of Corrosion Rate
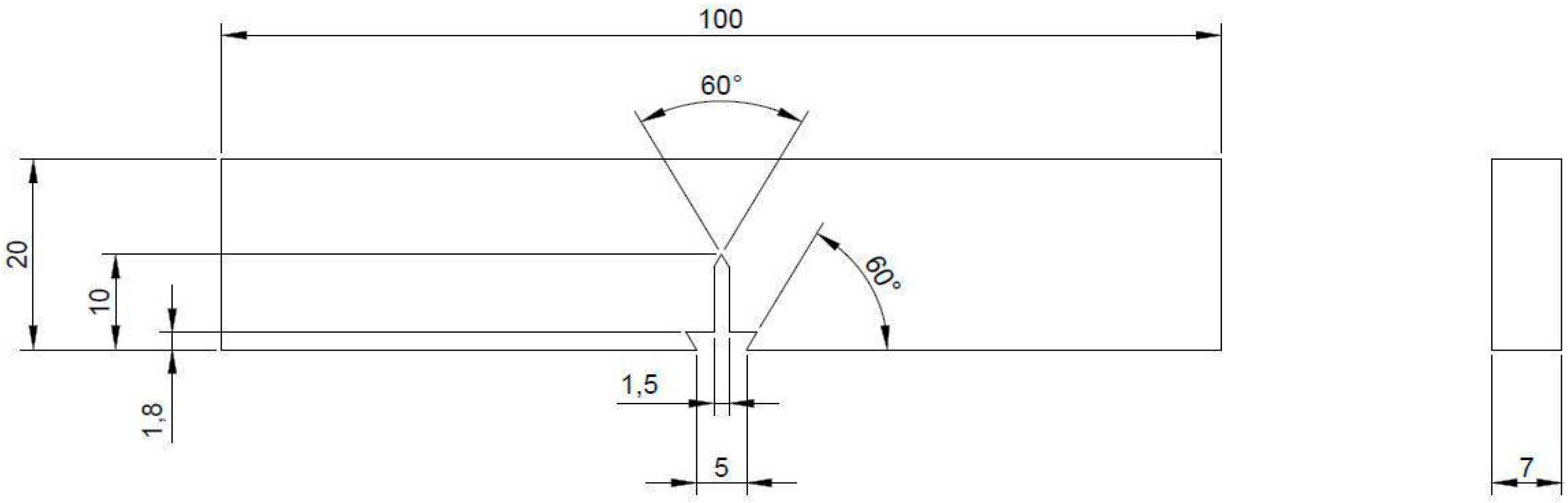
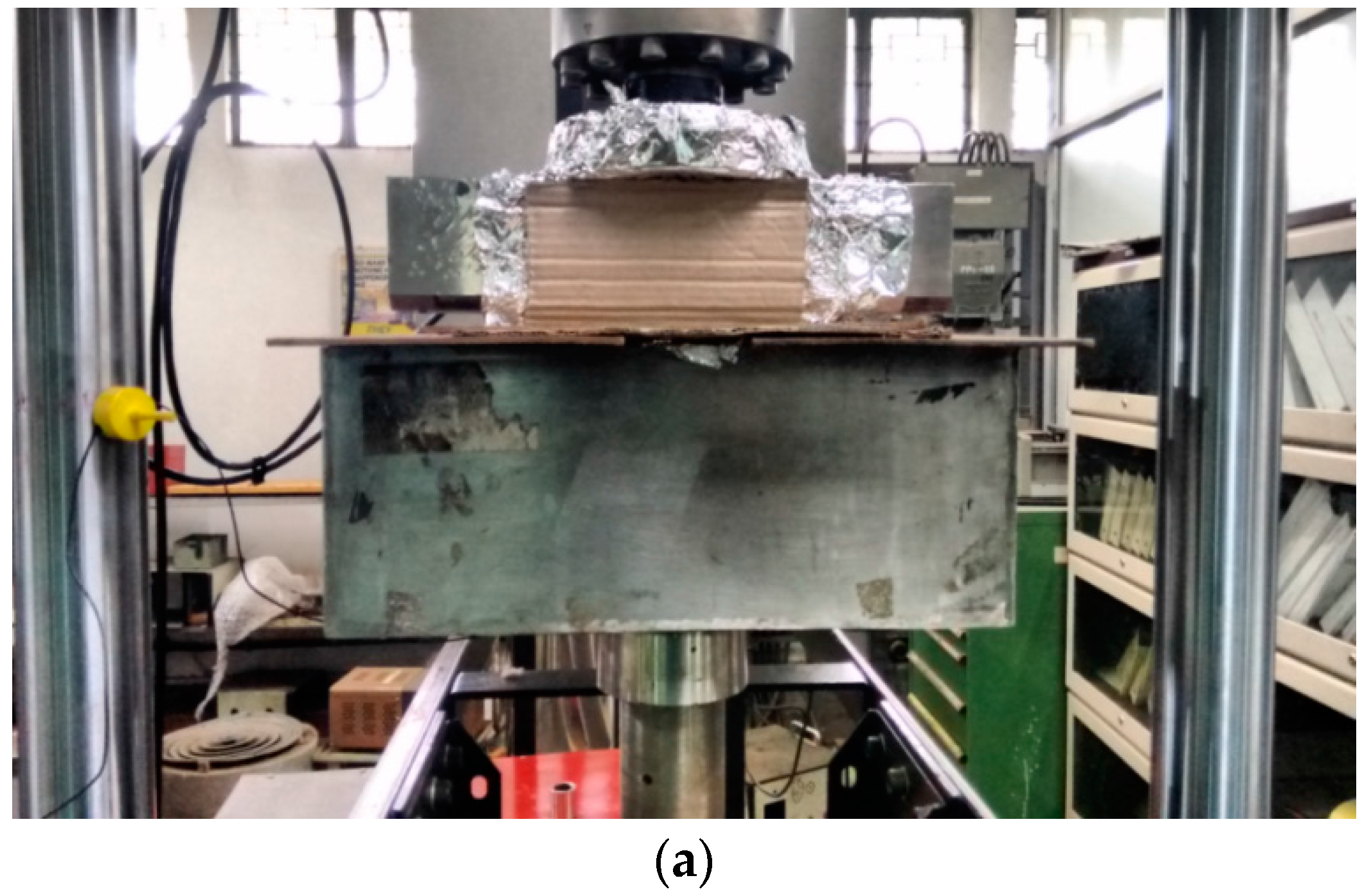
2.5. Tensile and Fracture Mechanics Tests
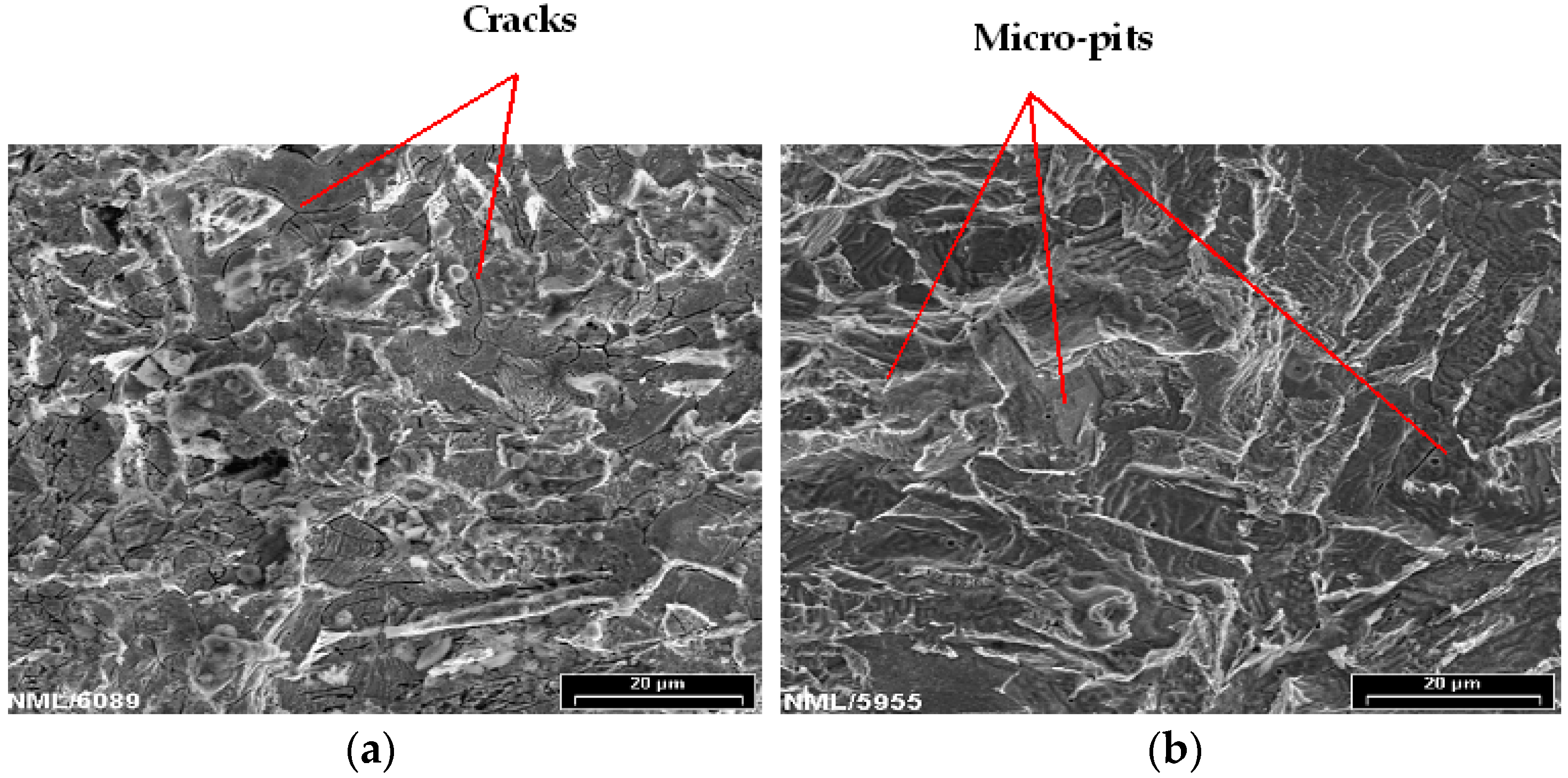
2.6. Microstructure, Fractography and Physical Characterizations
3. Results and Discussion
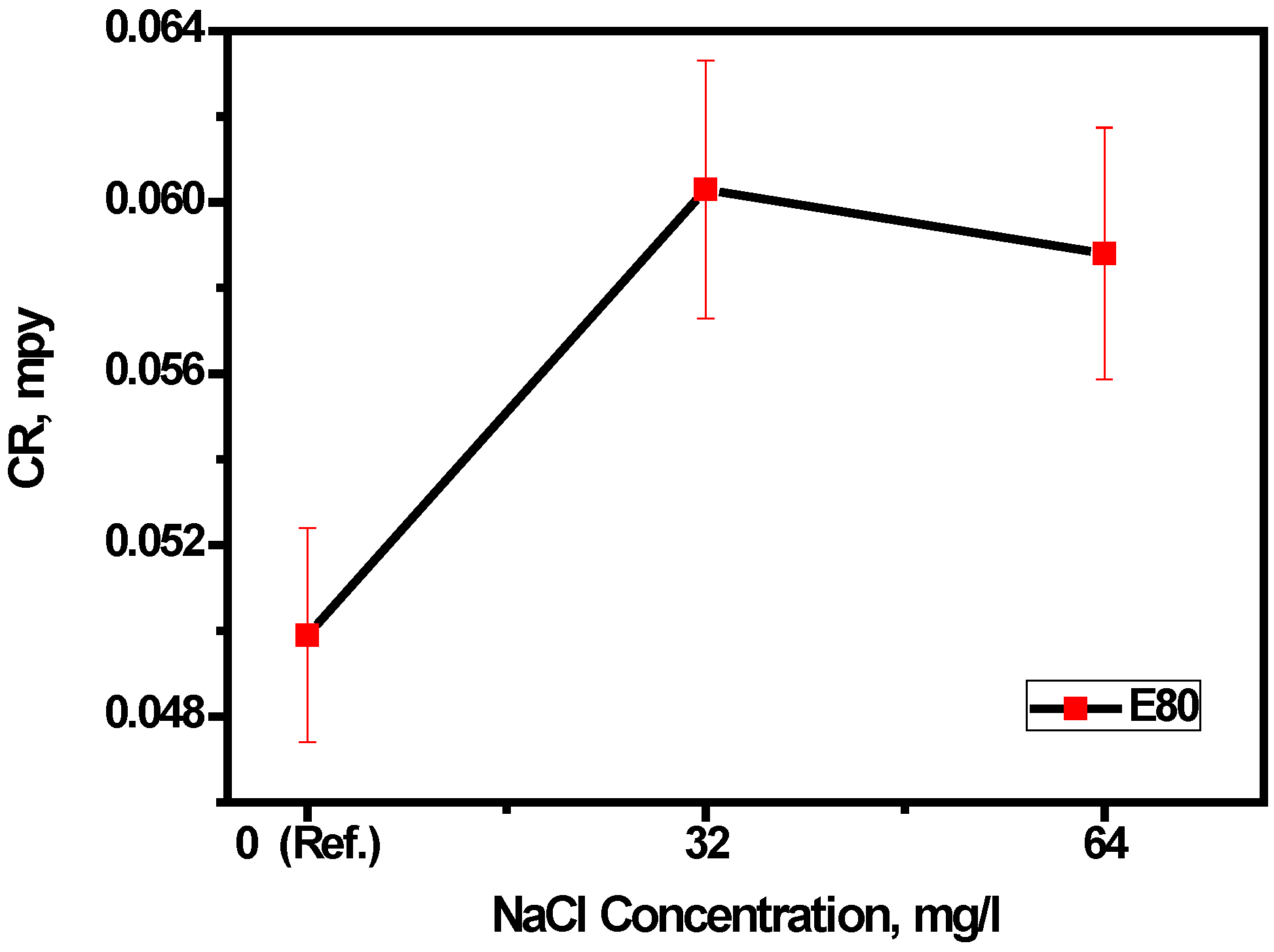
3.1. Effect of Chloride on Mass Loss of MAS in E80
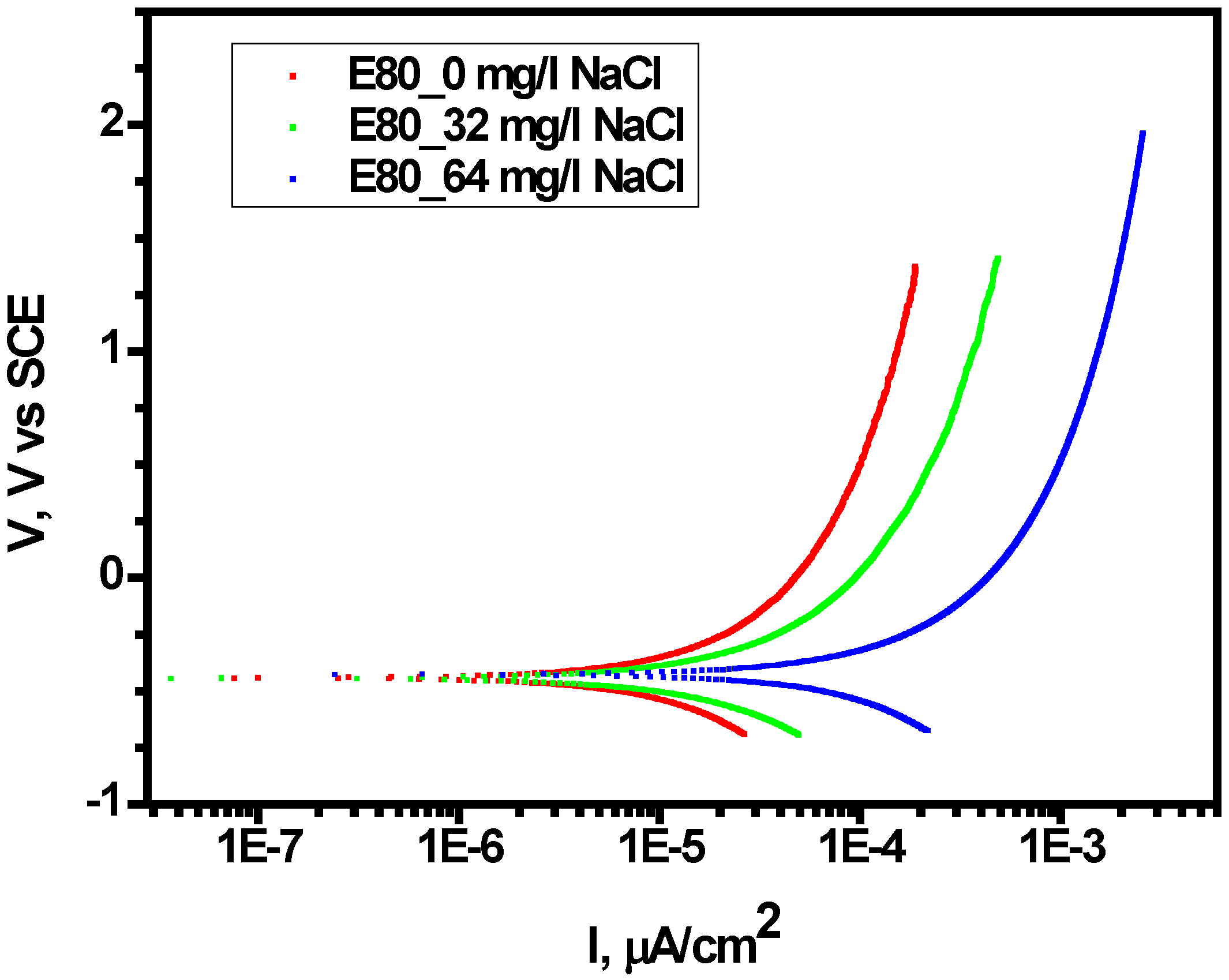
3.2. Effect of Chloride on Polarization Behavior of MAS in E80
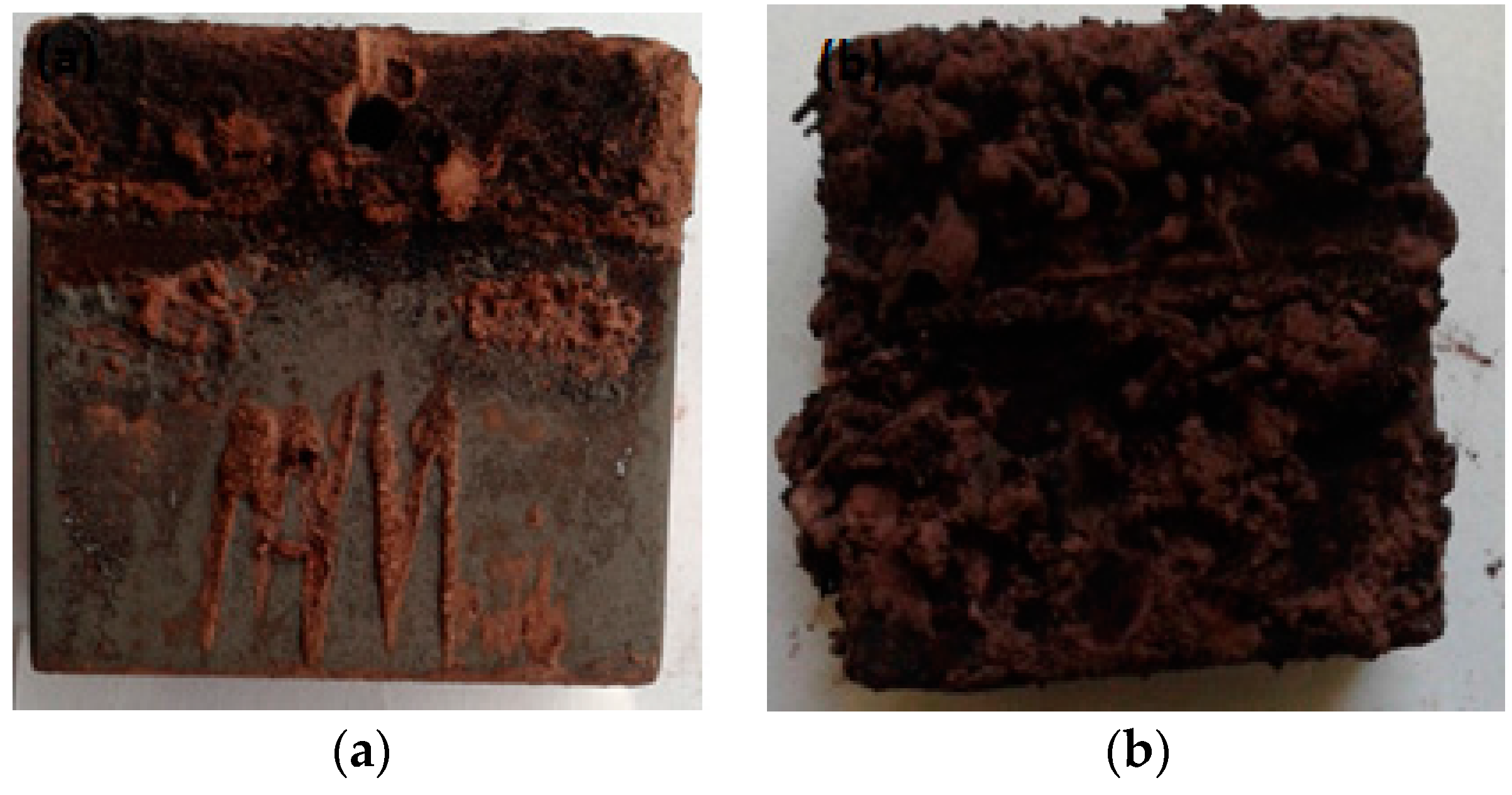
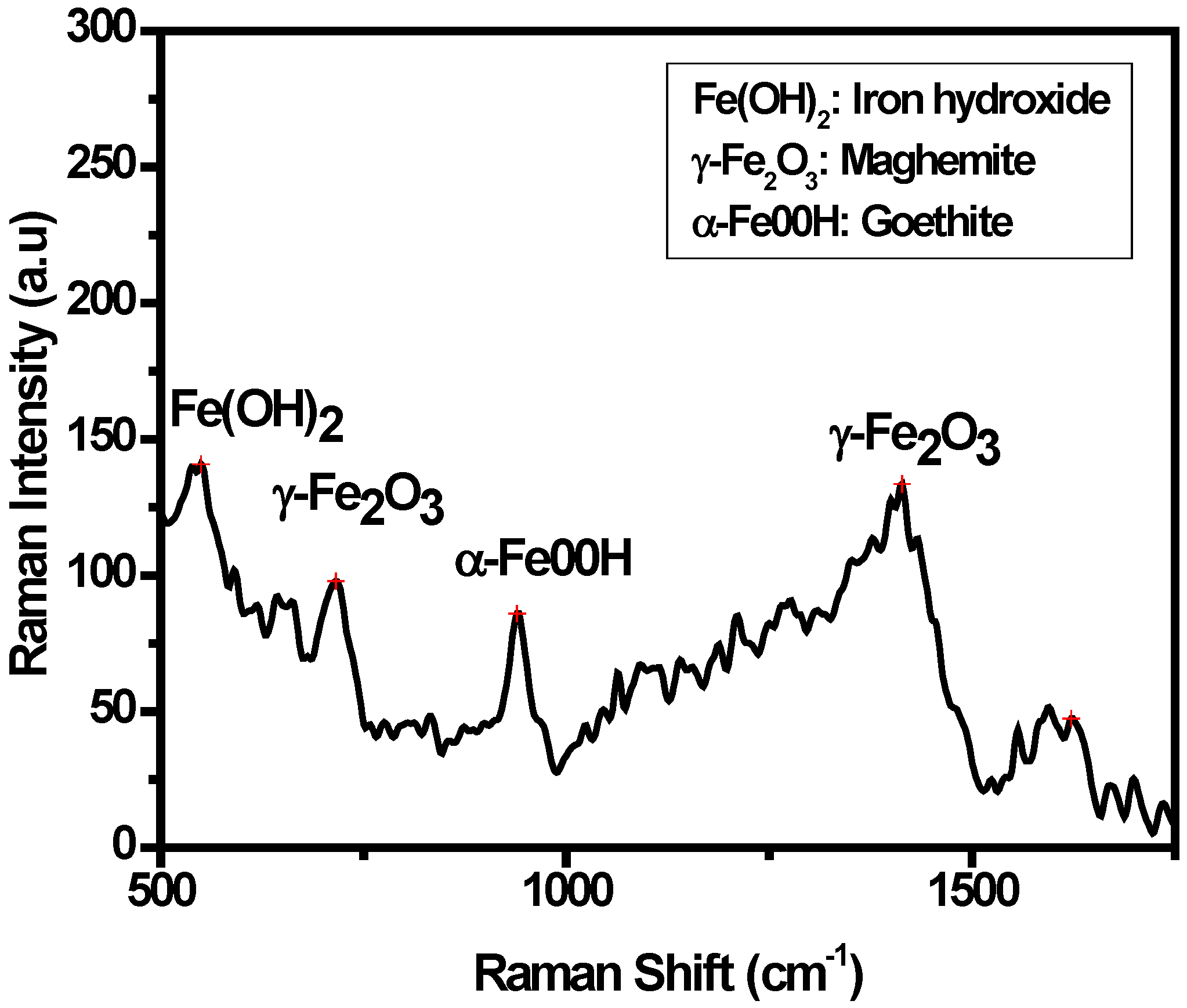
3.3. Characterization of the Oxide Layers Formed on MAS Exposed to E80
3.4. Effect of Chloride on Fracture Behavior of MAS in E80
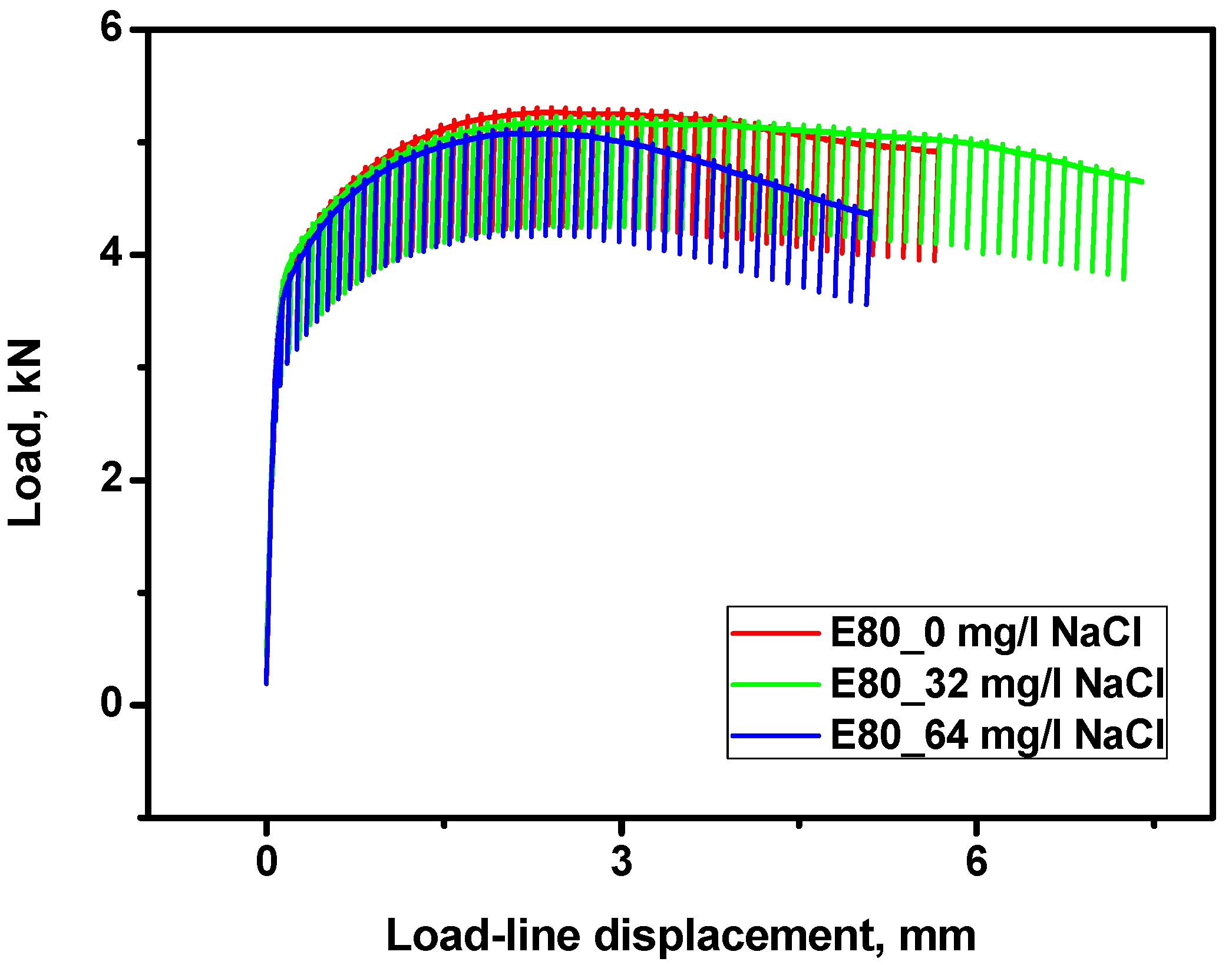
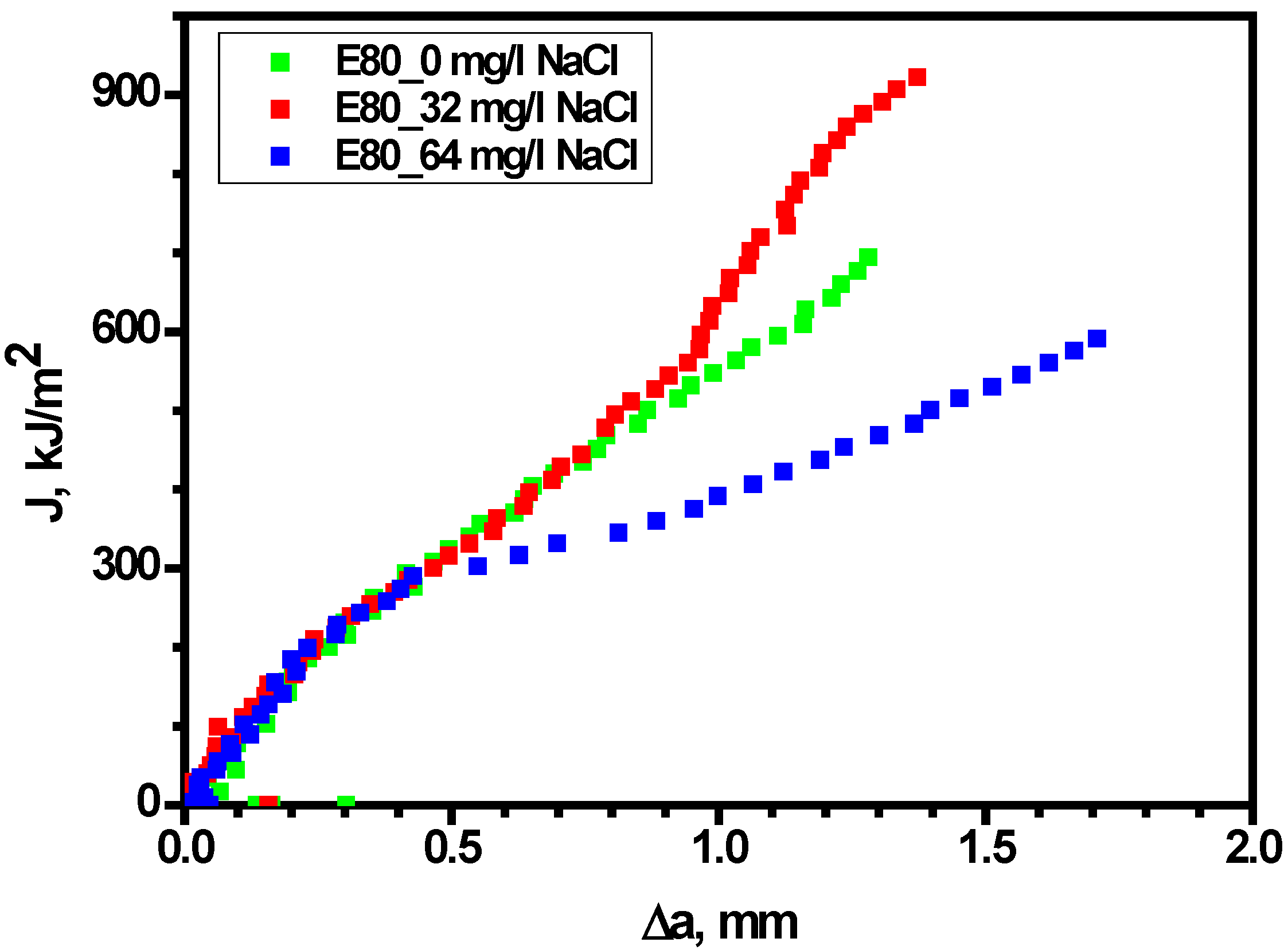
3.4.1. Effect of Chloride on the Load-Displacement Plots of MAS
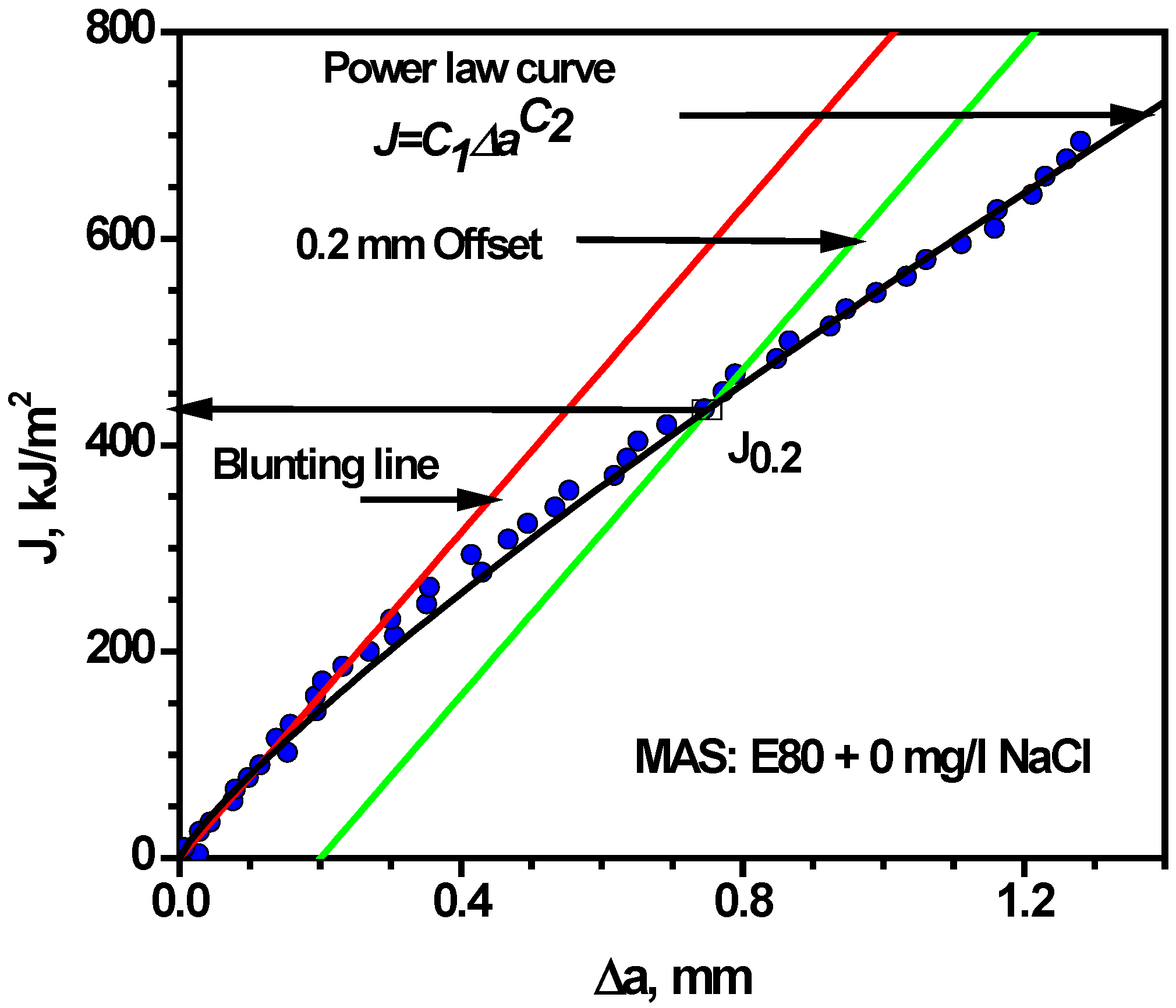
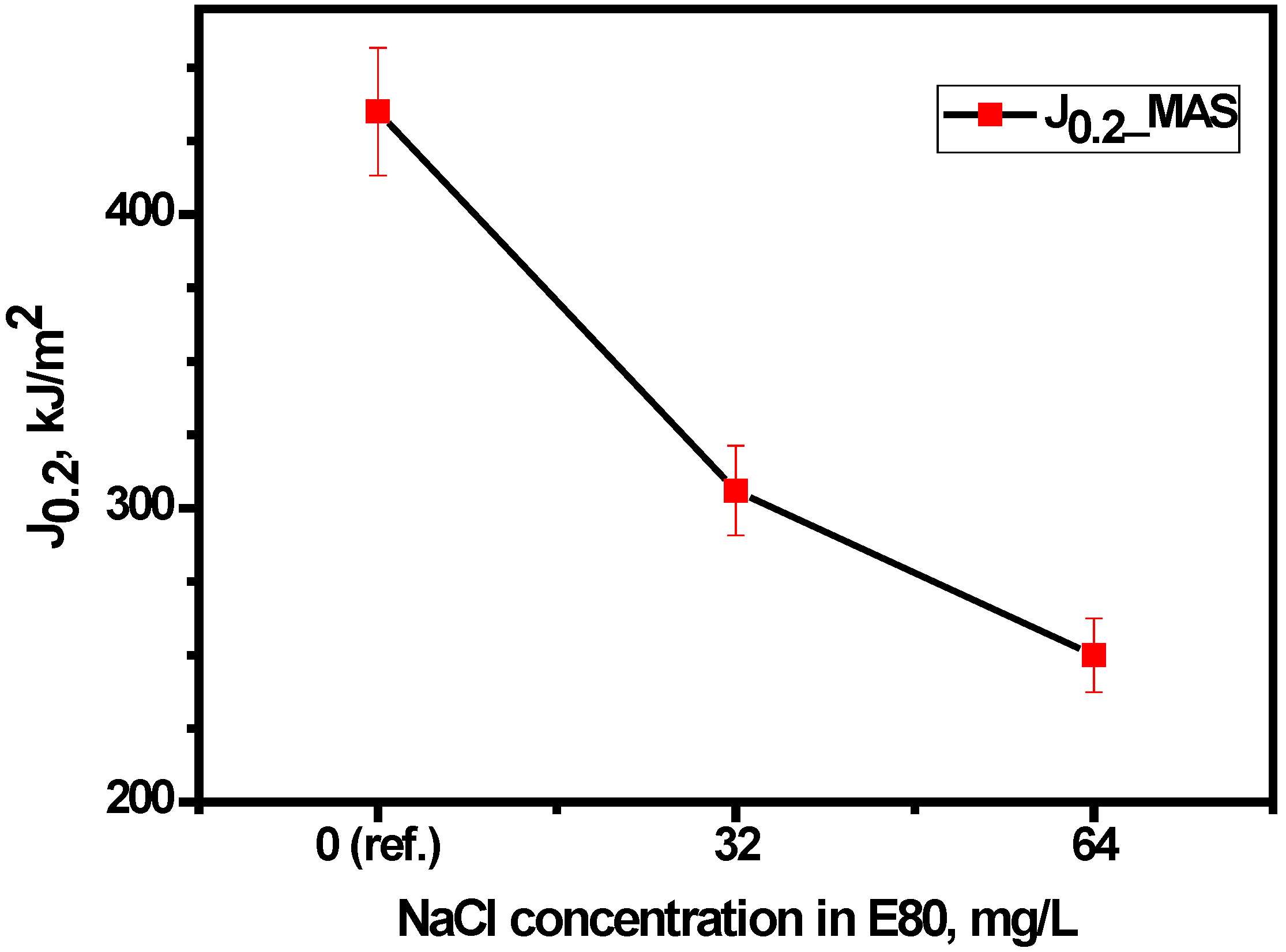
3.4.2. Effect of Chloride on Fracture Toughness of MAS
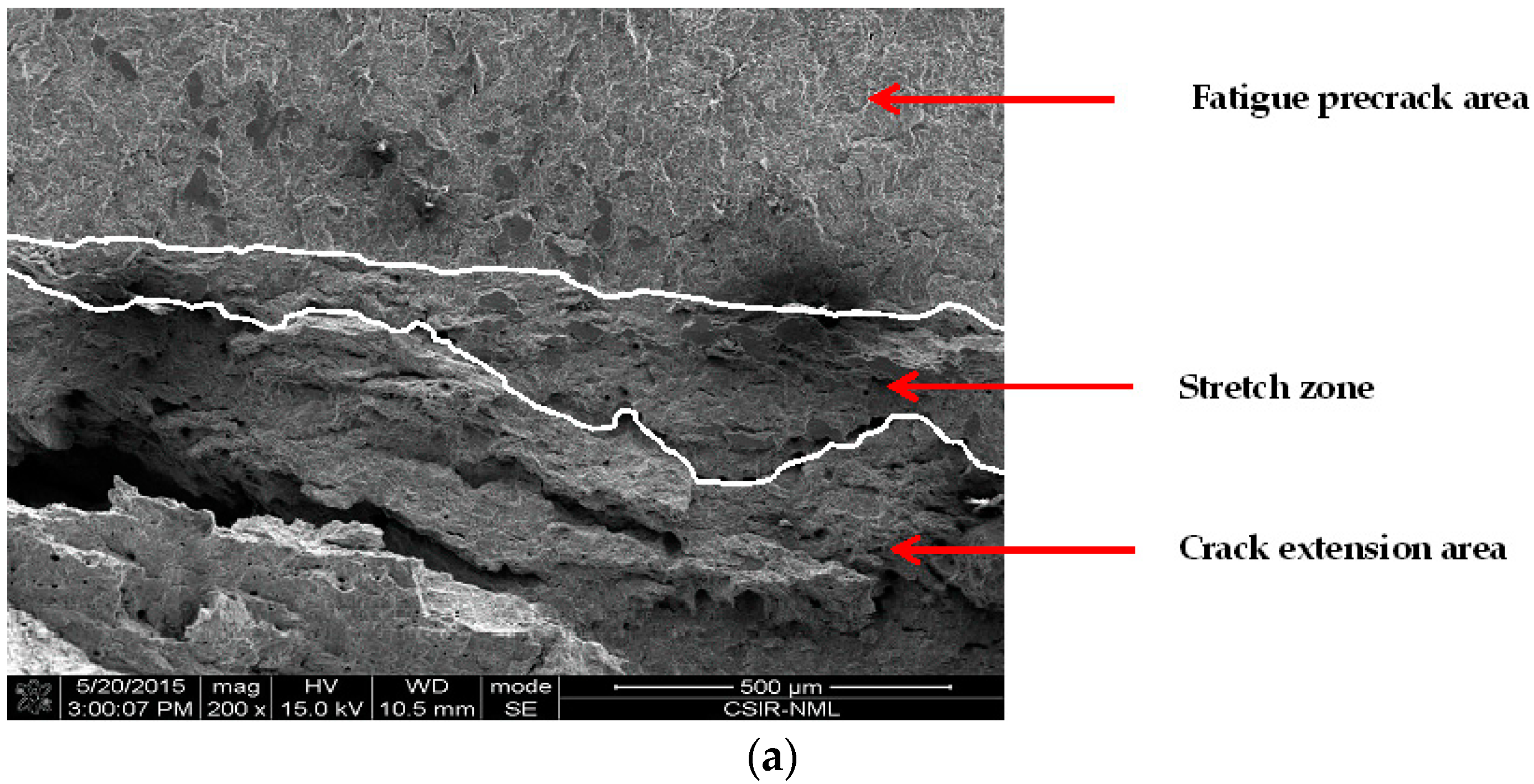
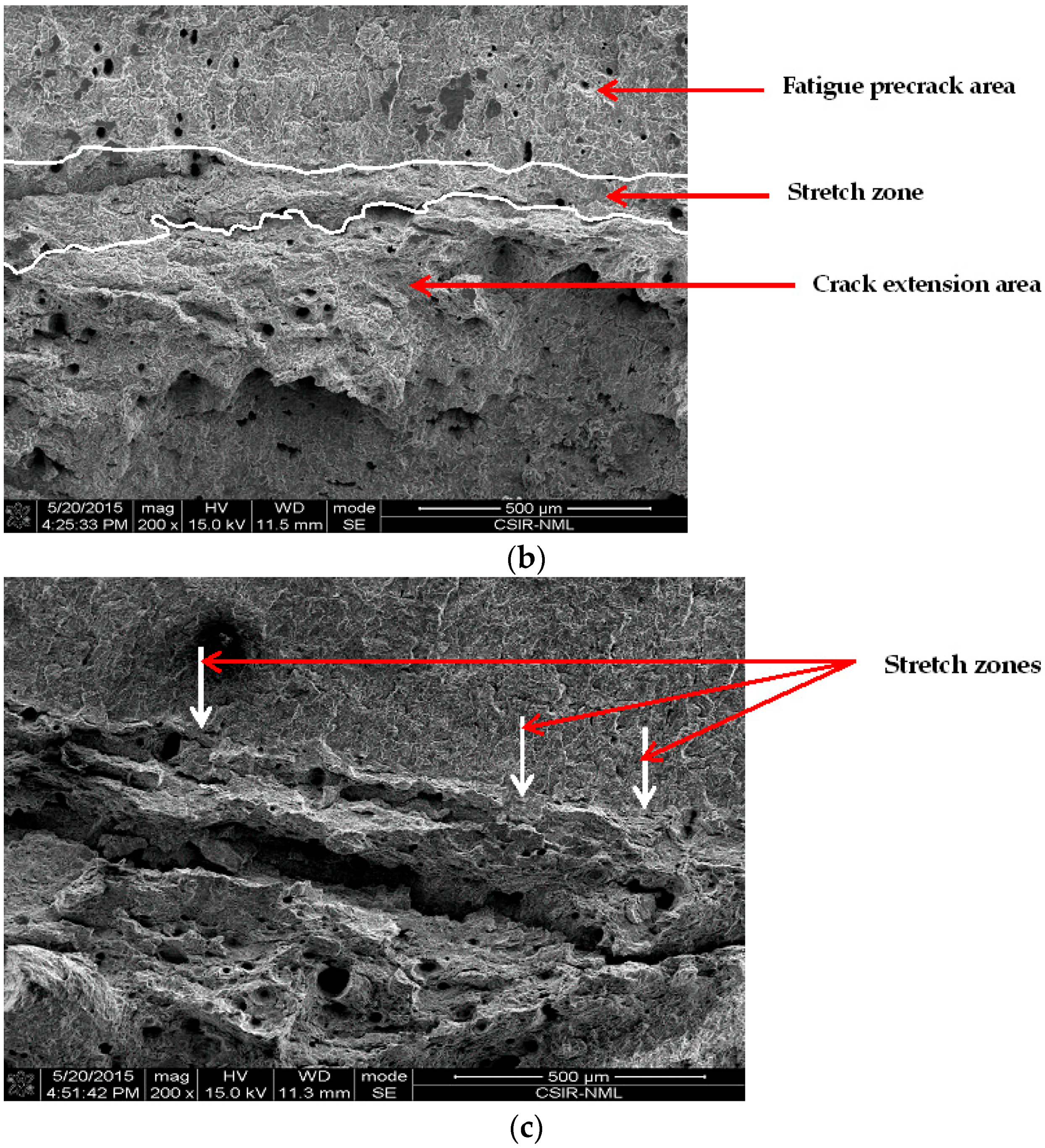
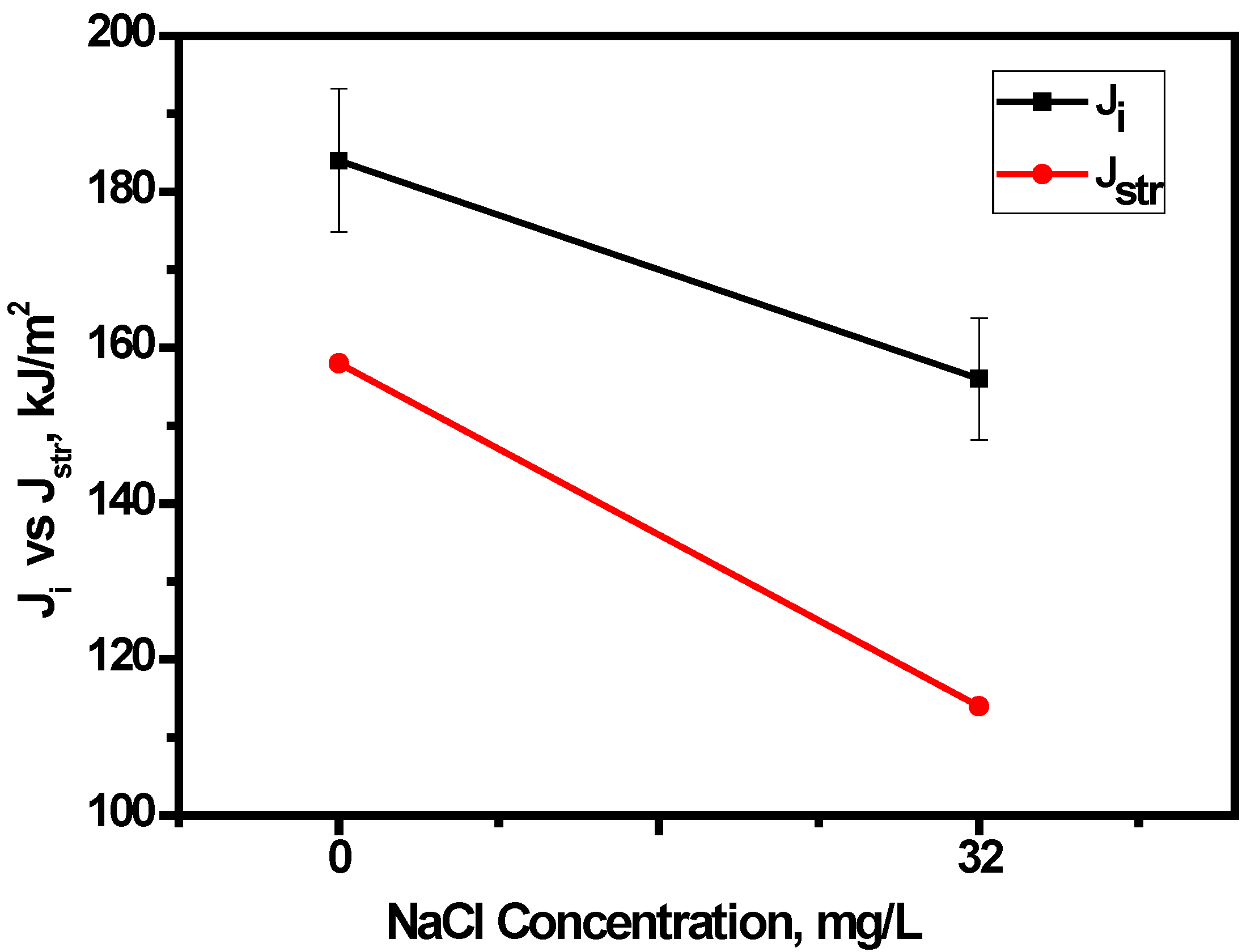
3.4.3. Effect of Chloride on Widths of Stretch Zones
4. Conclusions
- (1)
- The mass loss of MAS increased in the presence of chloride up to a threshold concentration of 32 mg/L. The ANOVA test further confirms, at 90% confidence, that there is no significant difference between 0, 32 and 64 mg/L NaCl concentrations on the corrosion rate of MAS.
- (2)
- Chloride caused pitting in MAS after immersion in E80 with chloride. In the absence of chloride, there was no pitting.
- (3)
- MAS did not demonstrate distinct passivation behavior as well as pitting potential with anodic polarization in the range of the ethanol-chloride ratio.
- (4)
- The fracture resistance of MAS reduced in E80 with increasing chloride and with respect to tests in the absence of chloride.
- (5)
- Crack tip blunting decreased with increasing chloride, thus accounting for a reduction in fracture toughness. In addition, the nature of the variation of Jstr with the chloride concentration in E80 is similar to that of Ji, which therefore qualifies the use of SZW in determining the initiation fracture toughness of MAS in E80.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| API | American Petroleum Institute |
| ASTM | American Standard for Testing Materials |
Instantaneous area under the load-plastic load line displacement curve in fracture toughness test | |
| , | Instantaneous crack length, original crack length, crack extension |
| B, BN | Specimen thickness, Net specimen thickness |
Un-cracked ligament, at the start of test and at (i − 1)th step | |
| COD | Crack opening displacement |
| CTOD | Crack tip opening displacement |
Tearing slope at critical crack extension | |
| E | Elastic modulus |
| EDM | electro-discharge machining |
| EPFM | Elastic-plastic fracture mechanics |
| eu, eT | Uniform elongation, Total elongation |
| Hv | Vickers hardness |
| J0.2, JIC, Jpl, Jstr | An energy based fracture parameter determined at 0.2 mm crack extension, qualified as plane strain fracture toughness, plastic part of fracture toughness, fracture toughness measured from stretch zone |
| Ki | Instantaneous stress intensity factor |
| KISCC | Threshold stress intensity factor for SCC |
| LLD | Load-line displacement |
| MAS | Micro-alloyed steel |
| n | Strain hardening exponent |
| N-SSR | Notched- slow strain rate |
| OCP | Open circuit potential |
| Pi | Instantaneous load |
| RFA | Renewable fuels association |
| S | Specimen span |
| SCC | Stress corrosion cracking |
| SCE | Saturated calomel electrode |
| SEM | Scanning electron microscope |
| SFGE | Simulated fuel grade ethanol |
| SSRT | Slow strain rate testing |
| SZW | Stretch zone width |
| TPB | Three-point bend |
Specimen width | |
| ∆K | Stress intensity factor range |
| , σYS, σUTS | Flow stress, Yield stress, Ultimate tensile stress |
Poisson’s ratio |
References
- Part V–VIII. In Stress Corrosion Cracking of Carbon Steel in Fuel-grade Ethanol: Review, Experience Survey, Field Monitoring and Laboratory Testing, 2nd ed.; API Technical Report 939-D; API: Washington, DC, USA, 2013.
- Micic, V.; Jotanovic, M. Bioethanol as fuel for internal combustion engines. Zastita Mater. 2015, 56, 403–408. [Google Scholar] [CrossRef]
- Highina, B.K.; Bugaje, I.M.; Umar, B. Liquid biofuels as alternative transport fuels in Nigeria. J. Pet. Tech. Dev. 2012, 1, 1–15. [Google Scholar]
- Munoz, M.; Moreno, F.; Morea, J. Emissions of an automobile diesel engine fuelled with sunflower methyl ester. Trans. ASAE 2004, 47, 5–11. [Google Scholar] [CrossRef]
- Baena, L.M.; Gomez, M.; Calderon, J.A. Aggressiveness of a 20% Bioethanol-80% gasoline mixture on autoparts: I behavior of metallic materials and evaluation of their electrochemical properties. Fuel 2012, 95, 320–328. [Google Scholar] [CrossRef]
- API Bulletin 939-E. In Identification, Repair and Mitigation of Cracking of Steel Equipment in Ethanol Service, 2nd ed.; API: Washington, DC, USA, 2013.
- Sharma, S.K. Green Corrosion Chemistry and Engineering: Opportunities and Challenges, 1st ed.; Wiley-VCH Verlag GmBH & Co. KgaA: Hoboken, NJ, USA, 2011; pp. 1–10. [Google Scholar]
- Kane, R.D.; Maldonado, J.G.; Klein, L.J. Stress corrosion cracking in fuel ethanol: A newly recognized phenomenon. In Proceedings of the Corrosion 2004, San Antonio, TX, USA, 28 March–2 April 2004.
- De Souza, J.P.; Mattos, O.R.; Sathler, L.; Takenouti, H. Impedance measurements of corroding mild steel in an automotive fuel ethanol with and without inhibitor in a two and three electrode cell. Corros. Sci. 1987, 27, 1351–1364. [Google Scholar] [CrossRef]
- Kane, R.D. Part I–IV. In Stress Corrosion Cracking of Carbon Steel in Fuel-Grade Ethanol: Review, Experience Survey, Field Monitoring and Laboratory Testing, 2nd ed.; API Technical Report 939-D, No. C939D2; API: Washington, DC, USA, 2007; p. 172. [Google Scholar]
- Beavers, J.A.; Brongers, M.P.; Agrawal, A.K.; Tallarida, F.A. Prevention of internal SCC in ethanol pipelines. In Proceedings of the Corrosion 2008, New Orleans, LA, USA, 16–20 March 2008.
- Venkatesh, A.; Chambers, B.; Kane, R.D.; Kirkham, K. Evaluation of stress corrosion cracking behavior of steel in multiple ethanol environments. In Proceedings of the Corrosion 2010, San Antonio, TX, USA, 14–18 March 2010.
- Veritas, D.N. Biofuels infrastructure, managing in an uncertain future. In DNV Research and Innovation, Position Paper 03; Sridhar, N., Gui, F., Beavers, J.A., James, J., Eds.; DNV: Hovik, Norway, 2010; pp. 1–24. [Google Scholar]
- Standard Specification for Denatured Fuel ethanol for Blending with Gasolines for Use as Automotive Spark-Ignition Engine Fuel, ASTM D-4806-01a. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2001.
- Standard Practice for Preparing, Cleaning and Evaluating Corrosion Test Specimens, ASTM G1-03. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2003.
- Lou, X.; Yang, D.; Singh, P.M. Effect of ethanol chemistry on stress corrosion cracking of carbon steel in fuel-grade ethanol. Corrosion 2009, 65, 785–797. [Google Scholar] [CrossRef]
- Lou, X.; Singh, P.M. Role of water, acetic acid and chloride on corrosion and pitting behavior of carbon steel in fuel-grade ethanol. Corros. Sci. 2010, 52, 2303–2315. [Google Scholar] [CrossRef]
- Standard Test Method for Tension Testing of Metallic Materials, ASTM E8/E8M-15a. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2015.
- Standard Test Method for Measurement of Fracture Toughness, ASTM E1820-08a. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2008.
- Tarafder, S.; Sivaprasad, S.; Ranganath, V.R. Comparative assessment of fatigue and fracture behavior of cast and forged railway wheels. Fatigue Fract. Eng. Mater. Struct. 2007, 30, 863–876. [Google Scholar] [CrossRef] [Green Version]
- Joseph, O.O.; Alo, F.I. An assessment of the microstructure and mechanical properties of 0.26% low carbon steel under different cooling media: Analysis by one-way ANOVA. Ind. Eng. Lett. 2014, 4, 39–45. [Google Scholar]
- Loto, C.A.; Joseph, O.O.; Loto, R.T.; Popoola, A.P.I. Corrosion inhibitive behavior of camellia sinensis on aluminium alloy in H2SO4. Int. J. Electrochem. Sci. 2014, 9, 1221–1231. [Google Scholar]
- Loto, C.A.; Joseph, O.O.; Loto, R.T.; Popoola, A.P.I. Inhibition effect of vernonia amygdalina extract on the corrosion of mild steel reinforcement in concrete in 0.2M H2SO4 environment. Eur. J. Environ. Civ. Eng. 2013, 17, 1026–1038. [Google Scholar] [CrossRef]
- Sowards, J.W.; Weeks, T.S.; McColskey, J.D. The influence of simulated fuel-grade ethanol on fatigue crack propagation in pipeline and storage-tank steels. Corros. Sci. 2013, 75, 415–425. [Google Scholar] [CrossRef]
- Ma, F.-Y. Chapter 7: Corrosive Effects of Chlorides on Metals. In Pitting Corrosion; ISBN 978-953-51-0275-5. Bensaiah, N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 140–178. [Google Scholar]
- Sei, J.; Cook, D.; Townsend, H. Characterization of iron oxides commonly formed as corrosion products on steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Int. J. Geophys. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Balasubramaniam, R.; Kumar, A.V.R.; Dillmann, P. Characterization of rust on ancient Indian iron. Curr. Sci. 2003, 58, 1546–1555. [Google Scholar]
- Das, S.K.; Sivaprasad, S.; Das, S.; Chatterjee, S.; Tarafder, S. The effect of variation of microstructure on fracture mechanics parameters of HSLA-100 steel. Mater. Sci. Eng. A Struct. 2006, 431, 68–79. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Tarafder, S.; Ranganath, V.R.; Das, S.K.; Ray, K.K. Can stretch zone measurements provide a good estimate of fracture toughness? In Proceedings of the 10th International Conference on Fracture (ICF), Honolulu, HI, USA, 2–6 December 2001.
- Ranganath, V.R.; Kumar, A.N.; Pandey, R.K. Fracture toughness characterization of a weldment in a microalloyed steel using resistance curves. Mater. Sci. Eng. A Struct. 1991, 132, 153–160. [Google Scholar] [CrossRef]
- Bassim, M.N.; Mathews, J.R.; Hyatt, C.V. Evaluation of fracture toughness of HSLA 80 steel at high loading rates using stretch zone measurements. Eng. Fract. Mech. 1992, 43, 297–303. [Google Scholar] [CrossRef]
- Sreenivasan, P.R.; Ray, S.K.; Vaidyanathan, S.; Rodriguez, P. Measurement of stretch zone height and its relationship to crack tip opening displacement and initiation J-value in an AISI 316 stainless steel. Fatigue Fract. Eng. Mater. Struct. 1996, 19, 855–868. [Google Scholar] [CrossRef]


| Element | C | Mn | Si | Cr | Ni | Al | Ti | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Micro-alloyed | 0.13 | 0.77 | 0.012 | 0.027 | 0.015 | 0.042 | 0.0025 | 0.0017 | 0.006 | balance |
| Ethanol (Vol %) | Methanol (Vol %) | Water (Vol %) | NaCl (mg/L) | Acetic Acid (mg/L) |
|---|---|---|---|---|
| 98.5 | 0.5 | 1 | 32 | 56 |
| Sample | σYS (MPa) | σUTS (MPa) | eu (%) | eT (%) | n# | Log k | Hv* |
|---|---|---|---|---|---|---|---|
| MAS | 301.54 | 458.83 | 18.27 | 38.89 | 0.13 | 2.52 | 111.8 |
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | Mean Square Ratio (MSR) | Min. MSR at 90% Confidence |
|---|---|---|---|---|---|
| Chloride concentration | 83,041.44 | 2 | 41,520.72 | 1.20 | 5.46 |
| Residual | 103,408.71 | 3 | 34,469.57 | ||
| Total | 186,450.15 | 5 |
| Solution Chemistry | Ecorr (mV) | icorr-estimate (A/cm2) | Corrosion Rate (mpy) |
|---|---|---|---|
| E80 + 0 mg/L NaCl | −3.93 × 102 | 7.99 × 105 | 3.56 × 101 |
| E80 + 32 mg/L NaCl | −4.13 × 102 | 8.27 × 105 | 3.69 × 101 |
| E80 + 64 mg/L NaCl | −4.38 × 102 | 7.61 × 108 | 3.39 × 101 |
| Environmental | Temperature | σo | J0.2 | B | |||
|---|---|---|---|---|---|---|---|
| Condition | (°C) | (MPa) | (kJ/m2) | (mm) | (mm) | ||
| E80 + 0 mg/L NaCl | 27 | 379 | 435 | 7.94 | 9.14 | 11.48 | 0.85 |
| E80 + 32 mg/L NaCl | 27 | 379 | 306 | 8.00 | 9.06 | 8.07 | 0.97 |
| E80 + 64 mg/L NaCl | 27 | 379 | 250 | 7.94 | 9.15 | 6.60 | 0.74 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, O.O.; Loto, C.A.; Sivaprasad, S.; Ajayi, J.A.; Tarafder, S. Role of Chloride in the Corrosion and Fracture Behavior of Micro-Alloyed Steel in E80 Simulated Fuel Grade Ethanol Environment. Materials 2016, 9, 463. https://doi.org/10.3390/ma9060463
Joseph OO, Loto CA, Sivaprasad S, Ajayi JA, Tarafder S. Role of Chloride in the Corrosion and Fracture Behavior of Micro-Alloyed Steel in E80 Simulated Fuel Grade Ethanol Environment. Materials. 2016; 9(6):463. https://doi.org/10.3390/ma9060463
Chicago/Turabian StyleJoseph, Olufunmilayo O., Cleophas A. Loto, Seetharaman Sivaprasad, John A. Ajayi, and Soumitra Tarafder. 2016. "Role of Chloride in the Corrosion and Fracture Behavior of Micro-Alloyed Steel in E80 Simulated Fuel Grade Ethanol Environment" Materials 9, no. 6: 463. https://doi.org/10.3390/ma9060463
APA StyleJoseph, O. O., Loto, C. A., Sivaprasad, S., Ajayi, J. A., & Tarafder, S. (2016). Role of Chloride in the Corrosion and Fracture Behavior of Micro-Alloyed Steel in E80 Simulated Fuel Grade Ethanol Environment. Materials, 9(6), 463. https://doi.org/10.3390/ma9060463





