Development of Styrene-Grafted Polyurethane by Radiation-Based Techniques
Abstract
:1. Introduction
2. Experimental
2.1. Materials
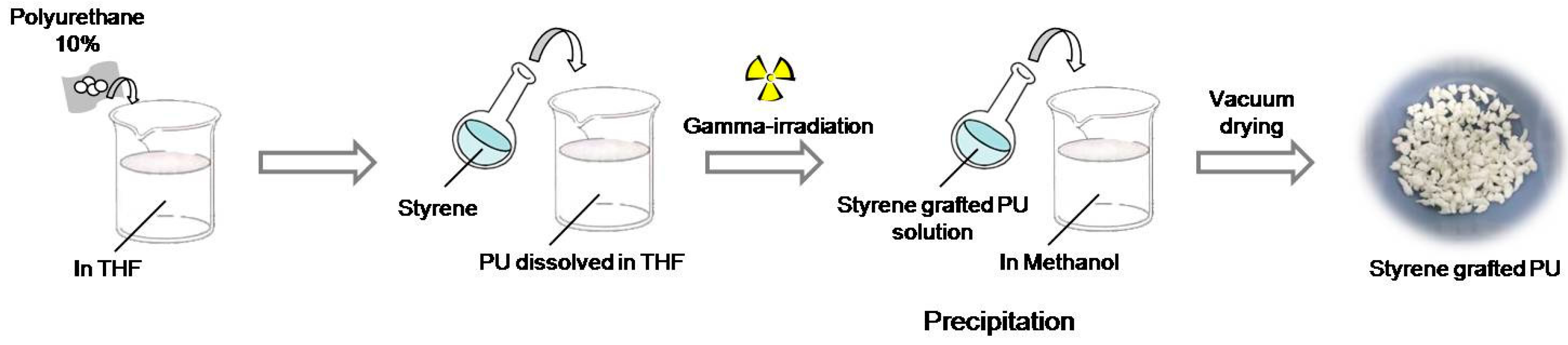
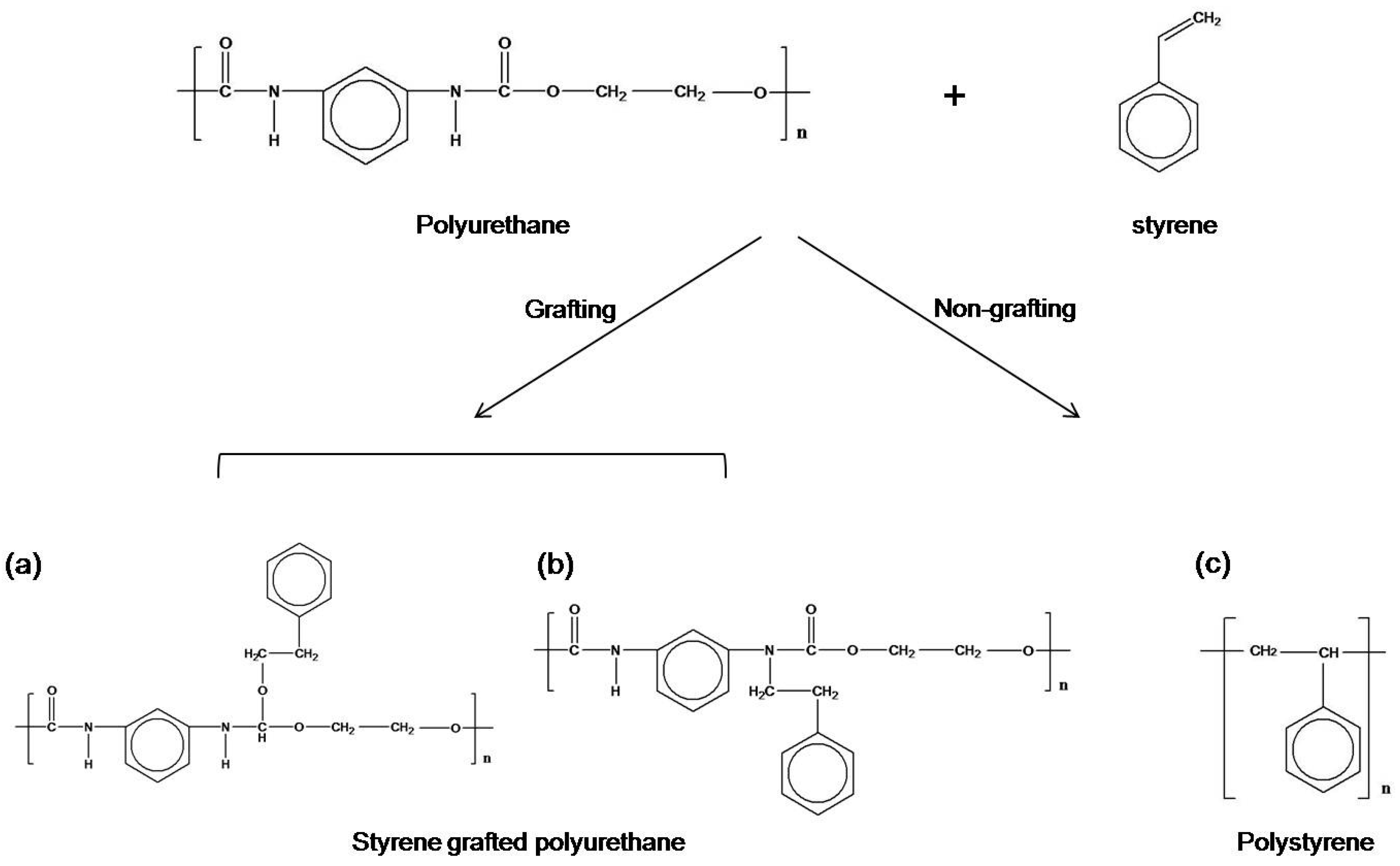
2.2. Preparation of Styrene Grafted Polyurethane
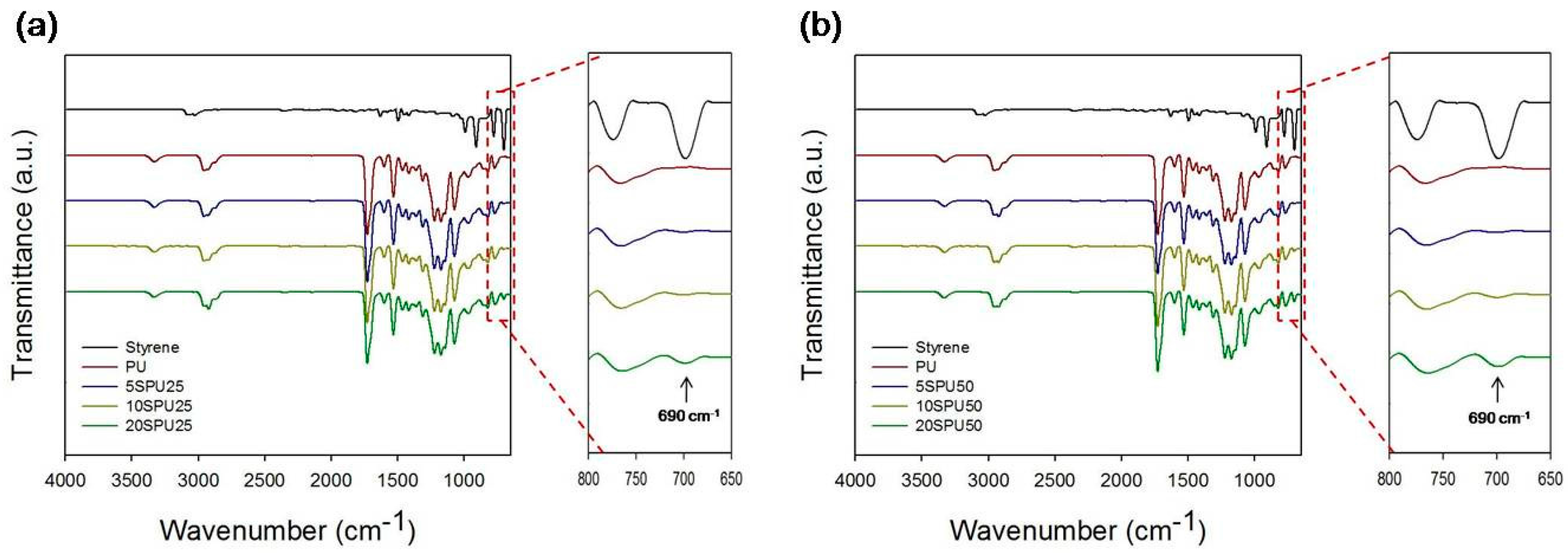
2.3. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4. Scanning Electron Microscope (SEM)
2.5. Blue Ink Staining
2.6. 1H-Nuclear Magnetic Resonance (1H-NMR)
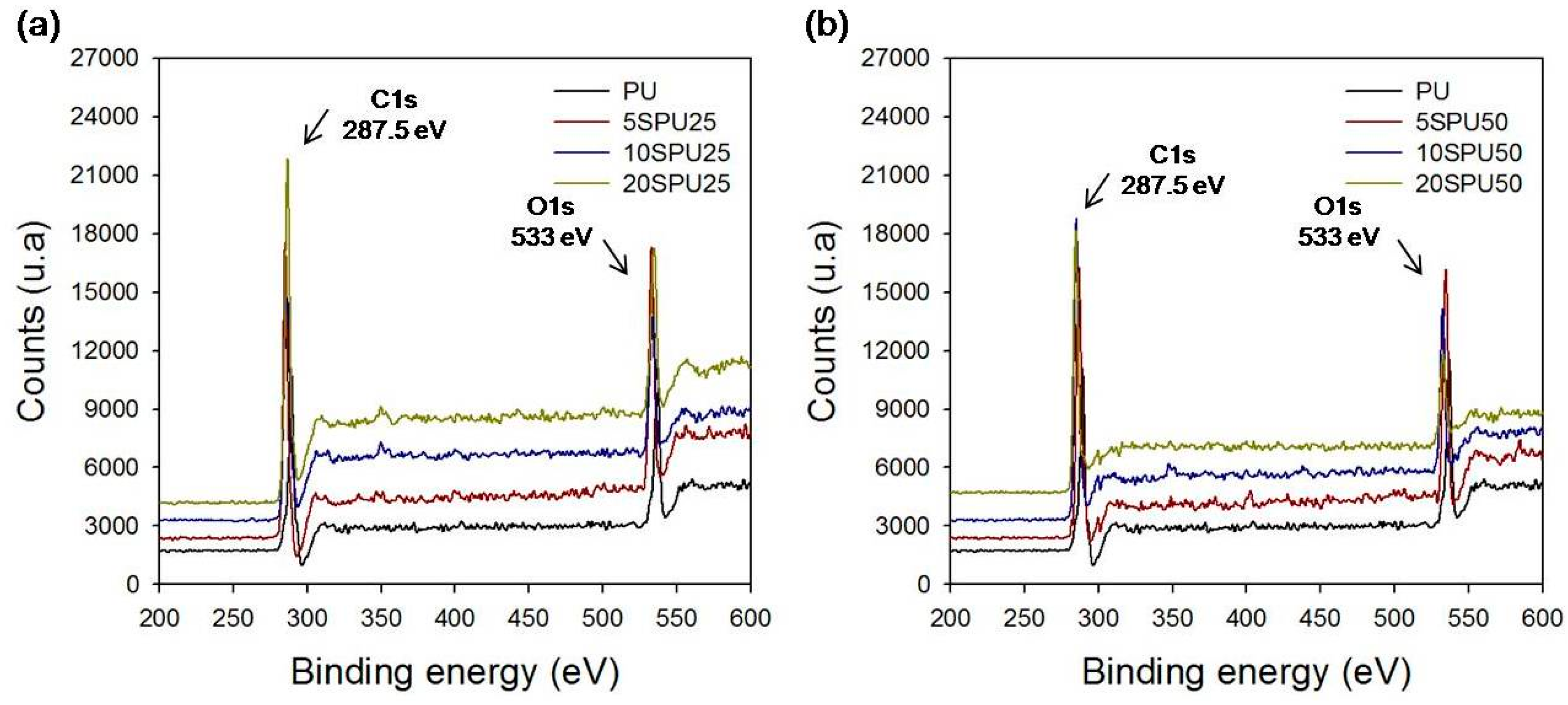
2.7. X-ray Photoelectron Spectroscopy (XPS)
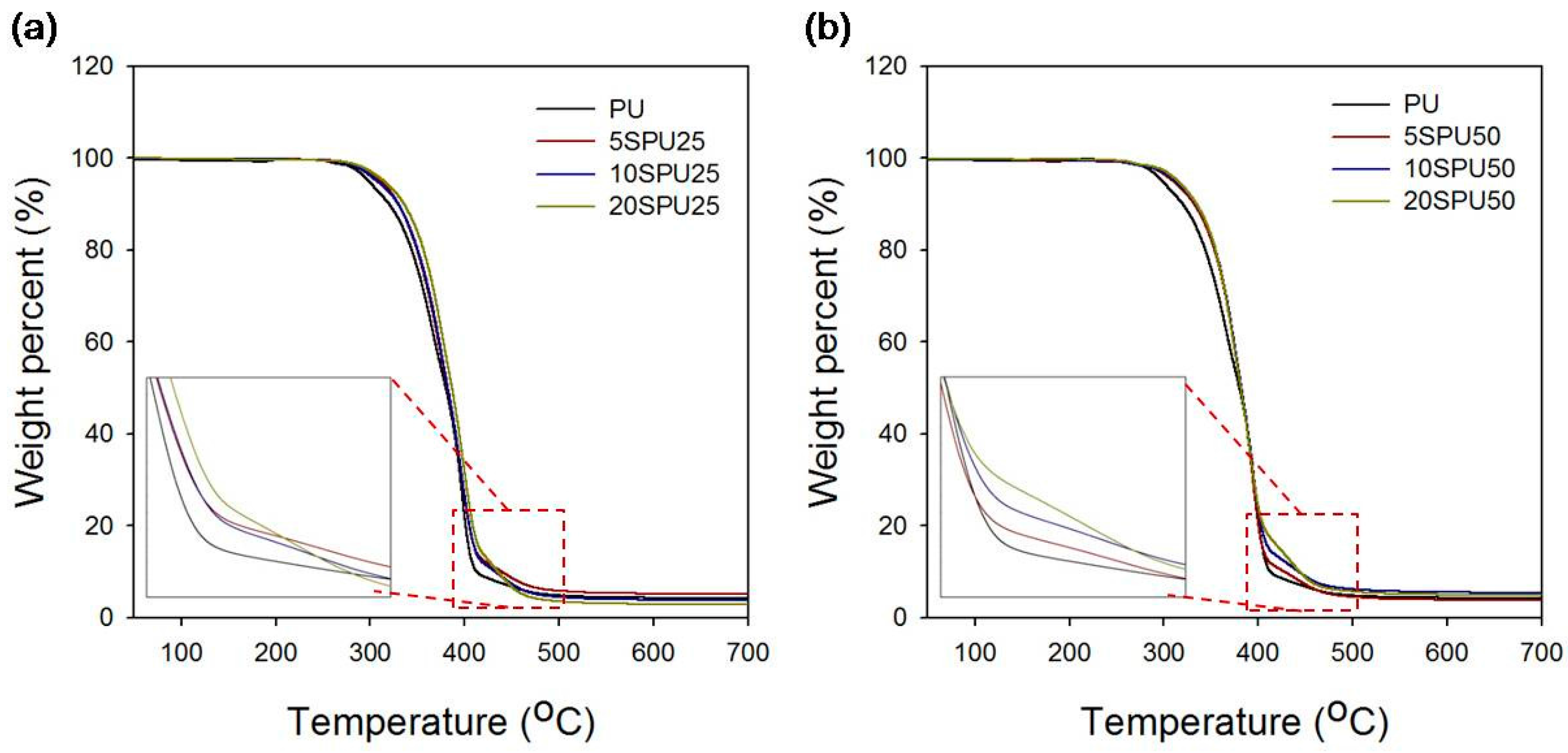
2.8. Thermogravimetric Analysis (TGA)
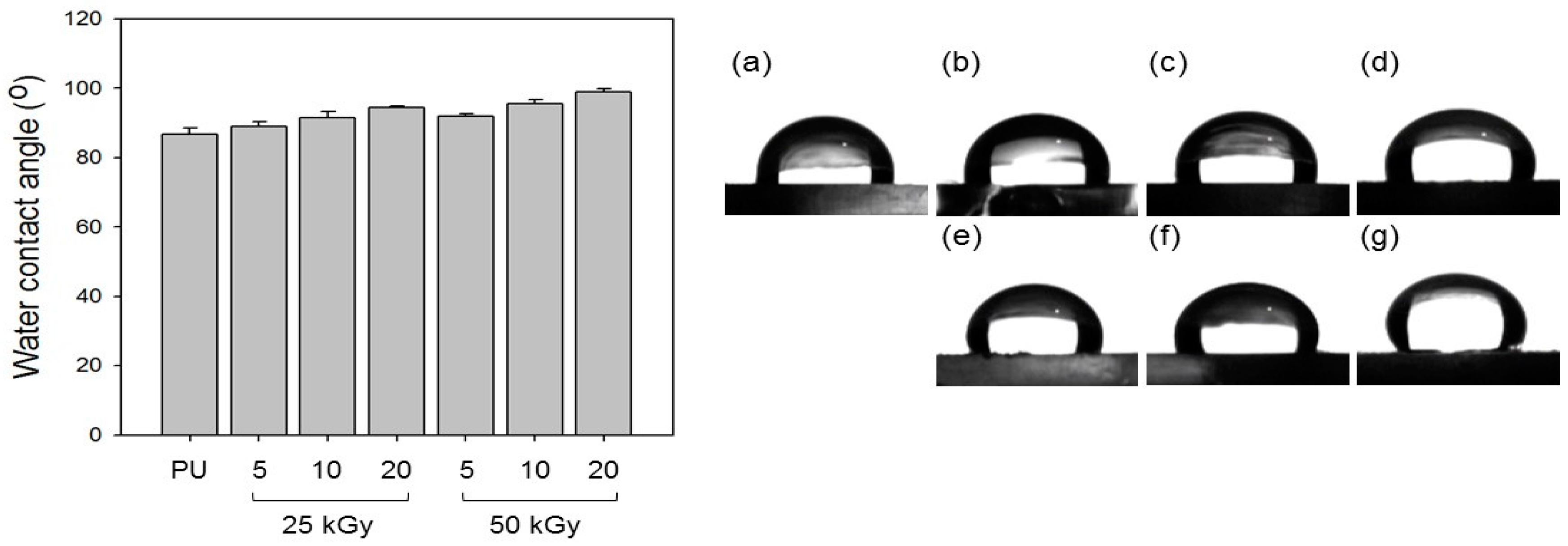
2.9. Contact Angle
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, J.S.; Lim, Y.M.; Nho, Y.C. Preparation of high density polyethylene/waste polyurethane blended compatibilized with polyethylene-graft-maleic anhydride by radiation. Materials 2015, 8, 1626–1635. [Google Scholar] [CrossRef]
- Park, J.S.; Lim, Y.M.; Nho, Y.C. Radiation-induced grafting with one-step process of waste polyurethane onto high-density polyethylene. Materials 2016, 9, 13. [Google Scholar] [CrossRef]
- Simon, D.; Garcia, M.T.; de Lucas, A.; Borreguero, A.M.; Rodriguez, J.F. Glycolysis of flexible polyurethane waste using stannous octoate as the catalyst: Study on the influence of reaction parameters. Polym. Degrad. Stab. 2013, 98, 144–149. [Google Scholar] [CrossRef]
- Beyer, G. Flame Retardant Properties of EVA-Nanocomposites and improvements by combination of nanofillers with aluminum trihydrate. Fire Mater. 2001, 25, 193–197. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.C. Controlling the morphology of polyurethane/polystyrene interpenetrating polymer networks for enhanced blood compatibility. J. Appl. Polym. Sci. 2002, 84, 379–387. [Google Scholar] [CrossRef]
- Tanobe, V.O.A.; Sydenstricker, T.H.D.; Amico, S.C.; Vargas, J.V.C.; Zawadzki, S.F. Evaluation of flexible postconsumed polyurethane foams modified by polystyrene grafting as sorbent material of oil spills. J. Appl. Polym. Sci. 2009, 111, 1842–1849. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Burford, R.P. Effect of foam density, oil viscosity, and temperature on oil sorption behavior of polyurethane. J. Appl. Polym. Sci. 2006, 99, 360–367. [Google Scholar] [CrossRef]
- Koenig, A.; Ziener, U.; Schaz, A.; Landfester, K. Polyurethane-block-polystyrene prepared by polymerization in miniemulsion. Macromol. Chem. Phys. 2007, 208, 155–163. [Google Scholar] [CrossRef]
- Calvete, D.P.; Holguin, D.F.R.; Luna, M.P. Grafting polymer based in active polyurethane matrixes via free radical. Procedia Mater. Sci. 2015, 9, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Jahny, K.; Adler, H.P.; Moritz, H. Kinetics of aqueous heterophase polymerization of styrene with polyurethane emulsifier. Macromol. Chem. Phys. 2001, 202, 2915–2920. [Google Scholar] [CrossRef]
- Gupta, B.; Srivastava, A.; Grover, N.; Saxena, S. Plasma induced graft polymerization of acrylic acid onto poly(ethylene terephthalate) monofilament. Indian J. Fibre Text. 2010, 35, 9–14. [Google Scholar]
- Rong, M.Z.; Ji, Q.L.; Zhang, M.Q.; Friedrich, K. Graft polymerization of vinyl monomers onto nanosized alumina particles. Eur. Polym. J. 2002, 38, 1573–1582. [Google Scholar] [CrossRef]
- Chan, C.M.; Ko, T.M. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Z.; Hu, Z.; Xi, H. Seeded-emulsion polymerization of styrene with waterborne polyurethane stabilizer via 60Co gamma ray. Colloids Surfaces A 2005, 265, 37–42. [Google Scholar] [CrossRef]
- Pierpoint, S.; Silverman, J.; Al-Sheikhly, M. Effects of ionizing radiation on the aging of polyester based polyurethane binder. Radiat. Phys. Chem. 2001, 62, 163–169. [Google Scholar] [CrossRef]
- Shin, Y.M.; Kim, K.S.; Lim, Y.M.; Nho, Y.C.; Shin, H. Modulation of spreading, proliferation, and differentiation of human mesenchymal stem cells on gelatin-immobilized poly(l-lactide-co-ɛ-caprolactone) substrates. Biomacromolecules 2008, 9, 1772–1881. [Google Scholar] [CrossRef] [PubMed]
- Ping, X.; Wang, M.; Ge, X. Surface modification of poly(ethylene terephthalate) (PET) film by gamma-ray induced grafting of poly(acrylic acid) and its application in antibacterial hybrid film. Radiat. Phys. Chem. 2011, 80, 567–572. [Google Scholar] [CrossRef]
- Wen, T.C.; Wu, M.S.; Yang, C.H. Spectroscopic investigations of poly(oxypropylene)glycol-based waterborne polyurethane doped with lithium perchlorate. Macromolecules 1999, 32, 2712–2720. [Google Scholar] [CrossRef]
- Queiroz, D.P.; de Rego, A.M.B.; de Pinho, M.N. Bi-soft segment polyurethane membranes: Surface studies by X-ray photoelectron spectroscopy. J. Membr. Sci. 2006, 281, 239–244. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, T.; Hui, Y.; Wang, W.; Yang, K.; Zhou, Q.; Wang, Y. Elaborate fabrication of well-defined side-chain liquid crystalline polyurethane networks with triple-shape memory capacity. J. Mater. Chem. A 2015, 3, 13435–13444. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.; Zhang, J. Preparation of thermal plastic polyurethane-polystyrene block copolymer via UV irradiation polymerization. J. Polym. Res. 2014, 21, 544. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Ge, X. Graft copolymers of polyurethane with various vinyl monomers via radiation-induced miniemulsion polymerization: Influential factors to grafting efficiency and particle morphology. Radiat. Phys. Chem. 2009, 78, 112–118. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Z.; Xu, C.; Ye, Q. Preparation of monodisperse polystyrene particles by radiation-induced dispersion polymerization using waterborne polyurethane as a stabilizer. Colloids Surfaces A 2006, 276, 72–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Z.; Huang, H.; Chen, H. Thermal degradation of polyurethane based on IPDI. J. Anal. Appl. Pyrol. 2009, 84, 89–94. [Google Scholar] [CrossRef]


| Samples | Concentration of Styrene (%) | Irradiation Dose (kGy) |
|---|---|---|
| 5SPU25 | 5 | 25 |
| 10SPU25 | 10 | 25 |
| 20SPU25 | 20 | 25 |
| 5SPU50 | 5 | 50 |
| 10SPU50 | 10 | 50 |
| 20SPU50 | 20 | 50 |
| Samples | Conversion Rate (%) |
|---|---|
| 5SPU25 | 56.6 |
| 10SPU25 | 60.6 |
| 20SPU25 | 82.8 |
| 5SPU50 | 59.8 |
| 10SPU50 | 74.4 |
| 20SPU50 | 87.1 |
| Samples | Carbon (%) | Oxygen (%) |
|---|---|---|
| PU | 76.4 | 21.2 |
| 5SPU25 | 78.1 | 19.6 |
| 10SPU25 | 80.1 | 17.0 |
| 20SPU25 | 81.1 | 15.4 |
| 5SPU50 | 77.6 | 20.3 |
| 10SPU50 | 80.5 | 16.5 |
| 20SPU50 | 87.4 | 10.6 |
| Samples | Weight Percent (%) |
|---|---|
| PU | 11.0 |
| 5SPU25 | 14.9 |
| 10SPU25 | 15.1 |
| 20SPU25 | 17.8 |
| 5SPU50 | 12.4 |
| 10SPU50 | 15.2 |
| 20SPU50 | 17.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-O.; Park, J.-S.; Lim, Y.-M. Development of Styrene-Grafted Polyurethane by Radiation-Based Techniques. Materials 2016, 9, 441. https://doi.org/10.3390/ma9060441
Jeong J-O, Park J-S, Lim Y-M. Development of Styrene-Grafted Polyurethane by Radiation-Based Techniques. Materials. 2016; 9(6):441. https://doi.org/10.3390/ma9060441
Chicago/Turabian StyleJeong, Jin-Oh, Jong-Seok Park, and Youn-Mook Lim. 2016. "Development of Styrene-Grafted Polyurethane by Radiation-Based Techniques" Materials 9, no. 6: 441. https://doi.org/10.3390/ma9060441
APA StyleJeong, J.-O., Park, J.-S., & Lim, Y.-M. (2016). Development of Styrene-Grafted Polyurethane by Radiation-Based Techniques. Materials, 9(6), 441. https://doi.org/10.3390/ma9060441






