Design and Validity of Randomized Controlled Dental Restorative Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Information Sources, Search, and Selection
2.4. Data Collection Process and Items
- -
- Restorative and adhesive material: class, name, manufacturer.
- -
- Trial design and methods: setting (primary or secondary care), number of patients; number of teeth; number of lesions; follow-up and drop-out, outcome measure (USPHS; Federation Dentaire International (FDI); Hickel; Vanherle; other), trial registration, unit of randomization (single teeth/patients, tooth pairs, i.e., splitmouth, clusters of teeth or patients), method of analysis (analysis of participants regardless of whether they received the intervention or were available for follow-up, or analysis of participants based on the intervention they received and their availability for follow-up).
- -
- Included teeth: dentition (primary or permanent), cavity location (cervical, i.e., Black class V), load-bearing (i.e., class I/II), or non-cervical and non-load-bearing (i.e., class III/IV), indication for treatment (caries/replacement or non-caries lesions).
- -
- Failures: number of failures per group, statistically significant different failure rates between groups. Failure was defined as restorations being lost or requiring restorative re-intervention (due to secondary caries, fracture etc.).
- -
- Risk of bias: as recommended by the Cochrane Risk of Bias tool [1], the domains of sequence generation, allocation concealment, operator blinding, examiner blinding, attrition bias and selective reporting were used. Note that for operator and examiner blinding, we assessed both reported blinding and possibility of blinding, i.e., blinding was not assumed when materials were clearly distinguishable when placing and evaluating them, even if studies reported on blinding.
2.5. Analysis
3. Results
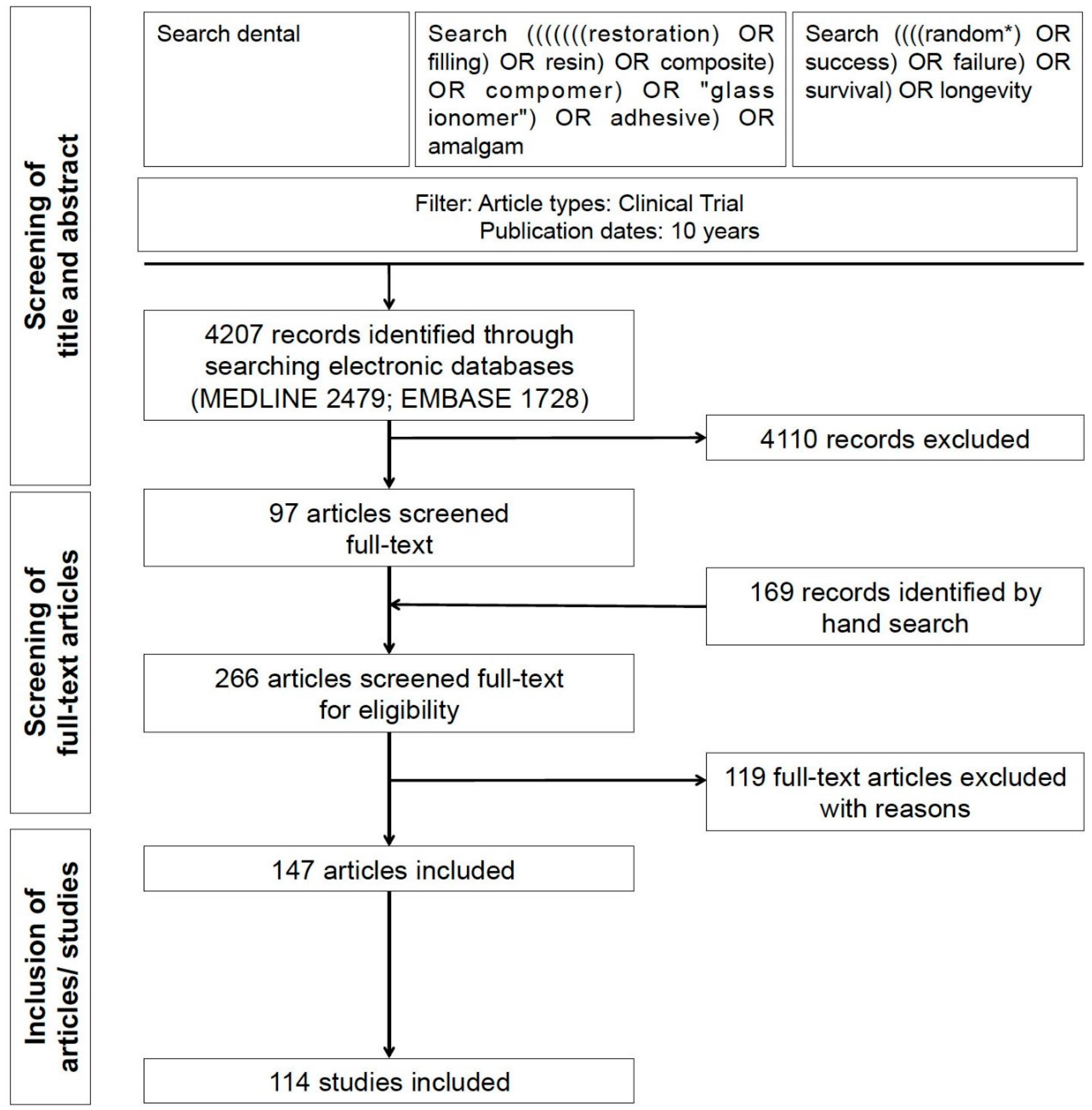
3.1. Search and Studies
3.2. Trial Properties
3.3. Changes of Trial Properties with Time
3.4. Factors Associated with Significant Differences
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FDI | Federation Dentaire International |
| OR | Odds Ratio |
| USPHS | United States Public Health Services |
References
- Higgins, J.; Altman, D.; Gotzsche, P.; Juni, P.; Moher, D.; Oxman, A.; Savovic, J.; Schulz, K.; Weeks, L.; Sterne, J.; et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F. Consort 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Altman, D.; Moher, D.; Group, T.C. Consort 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 340, c332. [Google Scholar]
- Koletsi, D.; Karagianni, A.; Pandis, N.; Makou, M.; Polychronopoulou, A.; Eliades, T. Are studies reporting significant results more likely to be published? Am. J. Orthod. Dentofac. Orthop. 2009, 136, 632.e1–632.e5. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Greenland, S.; Hlatky, M.A.; Khoury, M.J.; Macleod, M.R.; Moher, D.; Schulz, K.F.; Tibshirani, R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014, 383, 166–175. [Google Scholar] [CrossRef]
- Hobdell, M.; Petersen, P.; Clarkson, J.; Johnson, N. Global goals for oral health 2020. Int. Dent. J. 2003, 53, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar] [PubMed]
- Frencken, J.E.; Peters, M.C.; Manton, D.J.; Leal, S.C.; Gordan, V.V.; Eden, E. Minimal intervention dentistry for managing dental caries—A review: Report of a FDI task group. Int. Dent. J. 2012, 62, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Pandis, N.; Polychronopoulou, A.; Eliades, T. An assessment of quality characteristics of randomised control trials published in dental journals. J. Dent. 2010, 38, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.10; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Cairo, F.; Sanz, I.; Matesanz, P.; Nieri, M.; Pagliaro, U. Quality of reporting of randomized clinical trials in implant dentistry. A systematic review on critical aspects in design, outcome assessment and clinical relevance. J. Clin. Periodontol. 2012, 39 (Suppl. 12), 81–107. [Google Scholar] [CrossRef] [PubMed]
- Rattinger, G.; Bero, L. Factors associated with results and conclusions of trials of thiazolidinediones. PLoS ONE 2009, 4, e5826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulz, K.F.; Grimes, D.A. Blinding in randomised trials: Hiding who got what. Lancet 2002, 359, 696–700. [Google Scholar] [CrossRef]
- Moher, D.; Cook, D.; Jadad, A.; Tugwell, P.; Moher, M.; Jones, A.; Pham, B.; Klassen, T. Assessing the quality of reports of randomised trials: Implications for the conduct of meta-analyses. Health Technol. Assess. 1999, 3, 1–4. [Google Scholar]
- Gluud, L.L. Bias in clinical intervention research. Am. J. Epidemiol. 2006, 163, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Fricton, J.R.; Ouyang, W.; Nixdorf, D.R.; Schiffman, E.L.; Velly, A.M.; Look, J.O. Critical appraisal of methods used in randomized controlled trials of treatments for temporomandibular disorders. J. Orofac. Pain 2010, 24, 139–151. [Google Scholar] [PubMed]
- Veiga, D.F.; Veiga-Filho, J.; Pellizzon, R.F.; Juliano, Y.; Ferreira, L.M. Evolution of reports of randomised clinical trials in plastic surgery. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Smail-Faugeron, V.; Fron-Chabouis, H.; Durieux, P. Clinical trial registration in oral health journals. J. Dent. Res. 2015, 94, 8s–13s. [Google Scholar] [CrossRef] [PubMed]
- Huic, M.; Marusic, M.; Marusic, A. Completeness and changes in registered data and reporting bias of randomized controlled trials in ICMJE journals after trial registration policy. PLoS ONE 2011, 6, e25258. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; Boutron, I.; Moher, D.; Altman, D.G.; Ravaud, P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009, 302, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Killeen, S.; Sourallous, P.; Hunter, I.A.; Hartley, J.E.; Grady, H.L. Registration rates, adequacy of registration, and a comparison of registered and published primary outcomes in randomized controlled trials published in surgery journals. Ann. Surg. 2014, 259, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Lochner, H.V.; Bhandari, M.; Tornetta, P. Type-II error rates (beta errors) of randomized trials in orthopaedic trauma. J. Bone Joint Surg. Am. 2001, 83, 1650–1655. [Google Scholar] [PubMed]
- Weaver, C.S.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Bath, P.M. Sample size calculations in acute stroke trials: A systematic review of their reporting, characteristics, and relationship with outcome. Stroke J. Cereb. Circ. 2004, 35, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Brunthaler, A.; Konig, F.; Lucas, T.; Sperr, W.; Schedle, A. Longevity of direct resin composite restorations in posterior teeth. Clin. Oral Investig. 2003, 7, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hickel, R.; Peschke, A.; Tyas, M.; Mjor, I.; Bayne, S.; Peters, M.; Hiller, K.A.; Randall, R.; Vanherle, G.; Heintze, S.D. FDI world dental federation—Clinical criteria for the evaluation of direct and indirect restorations. Update and clinical examples. J. Adhes. Dent. 2010, 12, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Hickel, R.; Roulet, J.F.; Bayne, S.; Heintze, S.D.; Mjor, I.A.; Peters, M.; Rousson, V.; Randall, R.; Schmalz, G.; Tyas, M.; et al. Recommendations for conducting controlled clinical studies of dental restorative materials. Clin. Oral Investig. 2007, 11, 5–33. [Google Scholar] [CrossRef] [PubMed]
- Fleming, P.S.; Koletsi, D.; Seehra, J.; Pandis, N. Systematic reviews published in higher impact clinical journals were of higher quality. J. Clin. Epidemiol. 2014, 67, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, Z.; Galandiuk, S.; Abby, M.; Shannon, K.V.; Dajani, D.; Hicks, N.; Rai, S.N. The value of lesser-impact-factor surgical journals as a source of negative and inconclusive outcomes reporting. Ann. Surg. 2011, 253, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, E.; Siontis, K.C.; Pfeiffer, T.; Ioannidis, J.P. Perceived information gain from randomized trials correlates with publication in high-impact factor journals. J. Clin. Epidemiol. 2012, 65, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, M.; Krauthammer, M.; Gershoni, A.; Mimouni, F.; Nesher, R. Positive results bias and impact factor in ophthalmology. Curr. Eye Res. 2015, 40, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.M.; Akl, E.A.; Sun, X.; Bassler, D.; Mertz, D.; Mejza, F.; Vandvik, P.O.; Malaga, G.; Johnston, B.C.; Dahm, P.; et al. Randomized trials published in higher vs. Lower impact journals differ in design, conduct, and analysis. J. Clin. Epidemiol. 2013, 66, 286–295. [Google Scholar] [CrossRef] [PubMed]
| Variable | Median (25/75th Percentiles) or Number (Percentages) |
|---|---|
| Patients per trial | 37 (30/51; range: 8–456) |
| Lesions per patient | 3.0 (2.1/4.0; range: 1.0–9.0) |
| Number of lesions per trial | 105 (83/144; range: 36–1108) |
| Follow-up per trial | 24 (20/48; range: 6–156) |
| Percentage of lesions retained at follow-up | 91 (77/99; range: 53–100) |
| Trials with load-bearing/cervical/other cavities | 53 (47%)/57 (50%)/4 (3%) |
| Trials in permanent/primary dentition/not reported | 104 (91%)/9 (8%)/1 (1%) |
| Trials in primary/secondary care | 3 (3%)/111 (97%) |
| Trials published in journals with impact factor >2 | 30 (26%) |
| Trials which stated a hypothesis | 6 (5%) |
| Trials which described sample size calculation | 19 (17%) |
| Trials which randomized a tooth/a tooth pair/a cluster | 82 (72%)/30 (26%)/2 (2%) |
| Trials which performed intention-to-treat analysis/did not require such analysis as no attrition | 1 (1%)/20 (18%) |
| Registered trials | 2 (2%) |
| Trials using outcome measure other than USPHS | 11 (10%) |
| Trials with unclear/high risk of bias | 112 (98%) |
| Trials with unclear/high risk of sequence generation | 70 (61%) |
| Trials with unclear/high risk of allocation concealment | 106 (93%) |
| Trials with unclear/high risk of operator or participant blinding | 113 (99%) |
| Trials with unclear/high risk of examiner blinding | 52 (46%) |
| Trials with unclear/high risk of missing data | 19 (17%) |
| Trials with unclear/high risk of selective | 3 (3%) |
| Trials yielding significant differences between groups | 26 (23%) |
| Continuous Variables | β (95% CI) |
|---|---|
| Number of patients | 0.19 (−0.98/4.44) |
| Lesions per patient | 0.05 (−0.05/0.13) |
| Number of lesions | 8.70 (−1.53/18.9) |
| Follow-up time (months) | 1.40 (−0.48/3.19) |
| Lesions retained at follow-up | 0.00 (−0.01/0.01) |
| Binary variables | OR (95% CI) |
| Trials on load-bearing cavities (ref.: cervical) | 1.03 (0.91/1.17) |
| Trials in permanent dentition (ref.: primary) | 1.22 (0.96/1.55) |
| Trials in secondary care (ref.: primary care) | 0.91 (0.61/1.35) |
| Trials which stated a hypothesis (ref.: not stated) | 1.27 (0.92/1.77) |
| Trials which described a sample size calculation (ref.: not described) | 1.35 (1.10/1.65) |
| Trials which randomized tooth pairs (ref.: randomized teeth) | 0.90 (0.76/1.00) |
| Registered trials (ref.: not registered) | 1.91 (0.69/5.32) |
| Trials using outcome measure other than USPHS (ref.: USPHS) | 1.31 (1.02/1.69) |
| Trials with sequence generation unclear/high (ref.: low) | 0.77 (0.67/0.89) |
| Trials with allocation concealment unclear/high (ref.: low) | 0.49 (0.28/0.86) |
| Trials with blinding of operators unclear/high (ref.: low) | 0.35 (0.04/2.96) |
| Trials with blinding of examiners unclear/high (ref.: low) | 0.83 (0.73/0.94) |
| Trials with missing data unclear/high (ref.: low) | 1.03 (0.87/1.21) |
| Trials with selective reporting unclear/high (ref.: low) | 0.91 (0.62/1.33) |
| Trials yielding significant differences between groups (ref.: no significant differences) | 0.89 (0.76/1.03) |
| Intention-to-treat analysis performed/not required (ref.: required, but not performed) | 0.97 (0.83/1.14) |
| Variable | Model 1 (Simulttaneously) | Model 2 (Backwards) |
|---|---|---|
| Fit | R2 = 0.31, p < 0.001 | R2 = 0.19, p < 0.001 |
| OR (95% CI) | OR (95% CI) | |
| Year of publication of trial | 0.78 (0.62/0.99) | 0.81 (0.68/0.96) |
| Journal impact >2 (ref.: ≤2) | 3.53 (0.72/17.3) | 3.26 (1.25/10.8) |
| Number of patients | 1.02 (0.98/1.07) | |
| Lesions per patient | 0.66 (0.26/1.71) | |
| Follow-up time (months) | 1.01 (0.98/1.04) | 1.02 (1.00/1.03) |
| Retained lesions (%) | ||
| Trials with load-bearing cavities (ref.: cervical) | 0.29 (0.12/7.32) | 0.29 (0.09/0.94) |
| Trials in permanent dentition (ref.: primary care) | 3.3 (0.04/3.00) | |
| Trials describing sample size calculation (ref.: not described) | 2.48 (0.26/23.7) | |
| Registered trials (ref.: not registered) | n/a | |
| Trials using outcome measure other than USPHS (ref.: USPHS) | 7.46 (8.69/64.0) | 4.77 (1.07/21.3) |
| Trials with sequence generation unclear/high (ref.: low) | 3.61 (0.78/16.8) | |
| Trials with allocation concealment unclear/high (ref.: low) | n/a | |
| Trial with blinding of operators unclear/high (ref.: low) | n/a | |
| Trials with blinding of examiners unclear/high (ref.: low) | 0.51 (0.14/1.87) | |
| Trials with missing data unclear/high (ref.: low) | 0.44 (0.05/3.92) | |
| Trials with selective reporting unclear/high (ref.: low) | 557 (5.6/55,442) | 12.1 (0.90/123) |
| Trials accounting for attrition in analysis/trails without attrition a (ref.: attrition not accounted for) | 1.75 (0.64/4.78) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göstemeyer, G.; Blunck, U.; Paris, S.; Schwendicke, F. Design and Validity of Randomized Controlled Dental Restorative Trials. Materials 2016, 9, 372. https://doi.org/10.3390/ma9050372
Göstemeyer G, Blunck U, Paris S, Schwendicke F. Design and Validity of Randomized Controlled Dental Restorative Trials. Materials. 2016; 9(5):372. https://doi.org/10.3390/ma9050372
Chicago/Turabian StyleGöstemeyer, Gerd, Uwe Blunck, Sebastian Paris, and Falk Schwendicke. 2016. "Design and Validity of Randomized Controlled Dental Restorative Trials" Materials 9, no. 5: 372. https://doi.org/10.3390/ma9050372
APA StyleGöstemeyer, G., Blunck, U., Paris, S., & Schwendicke, F. (2016). Design and Validity of Randomized Controlled Dental Restorative Trials. Materials, 9(5), 372. https://doi.org/10.3390/ma9050372






