Antimicrobial Properties of Diamond-Like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages
Abstract
:1. Introduction
2. Results
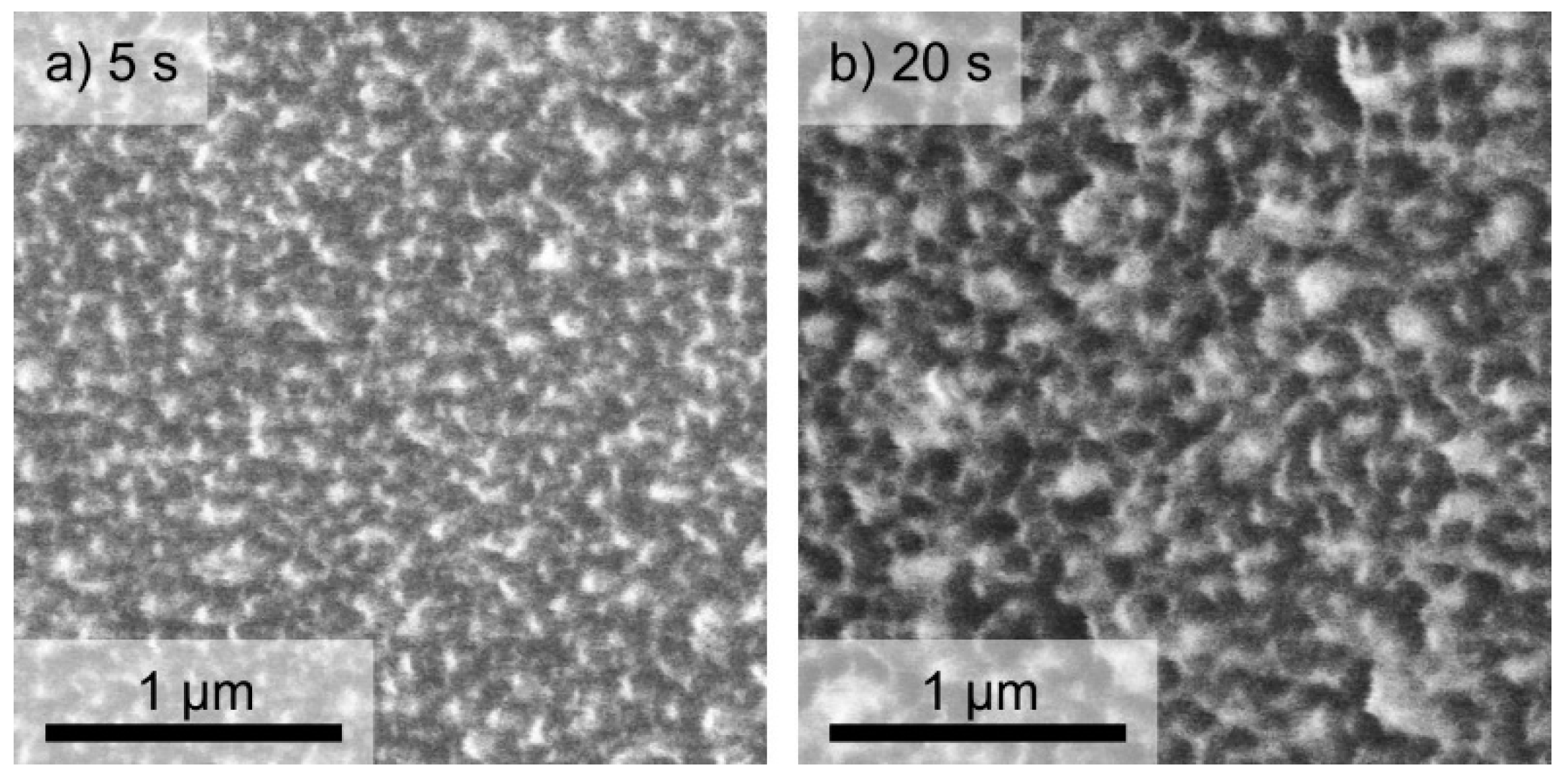
2.1. Structure and Composition of DLC:Ag Layers Deposited on Textile and Crystalline Silicon
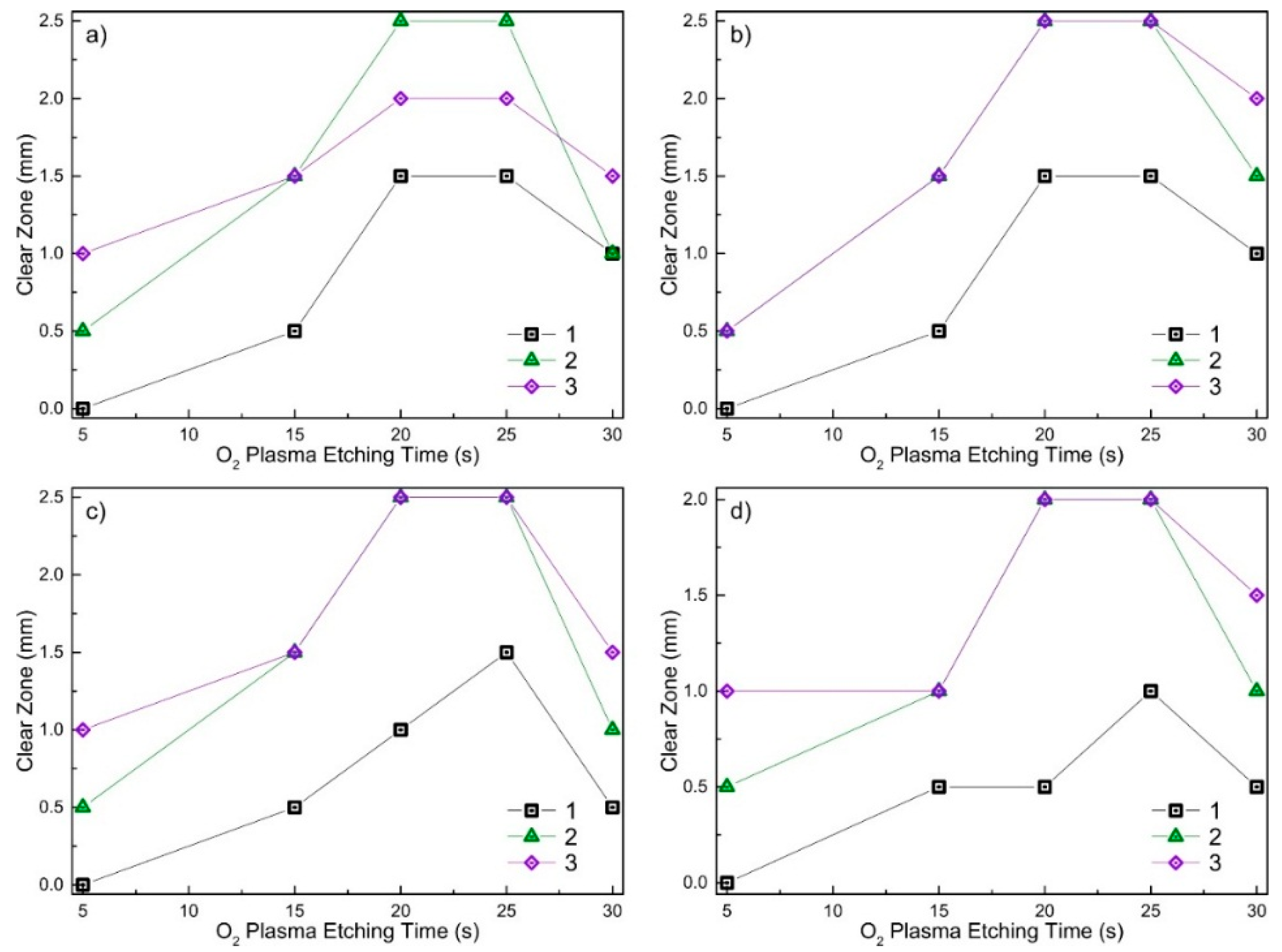
2.2. Antimicrobial Activity of Virgin and RF O2 Plasma Processed DLC:Ag Films
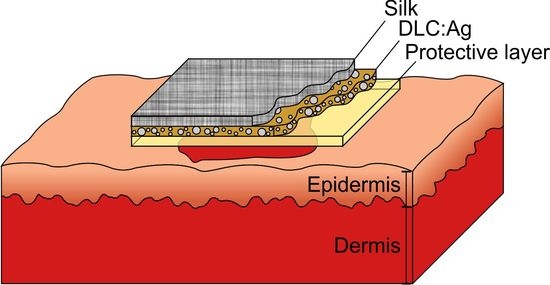
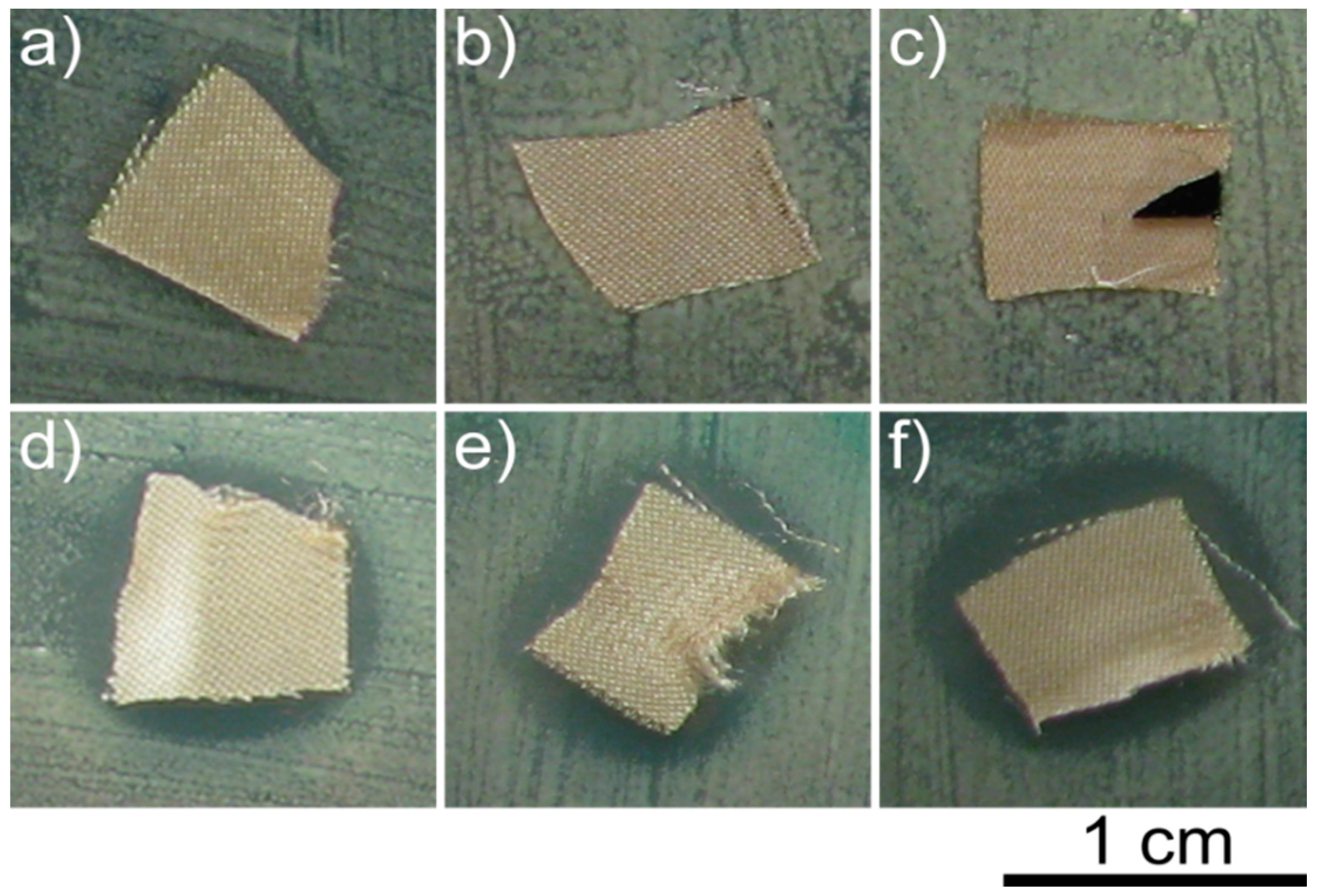
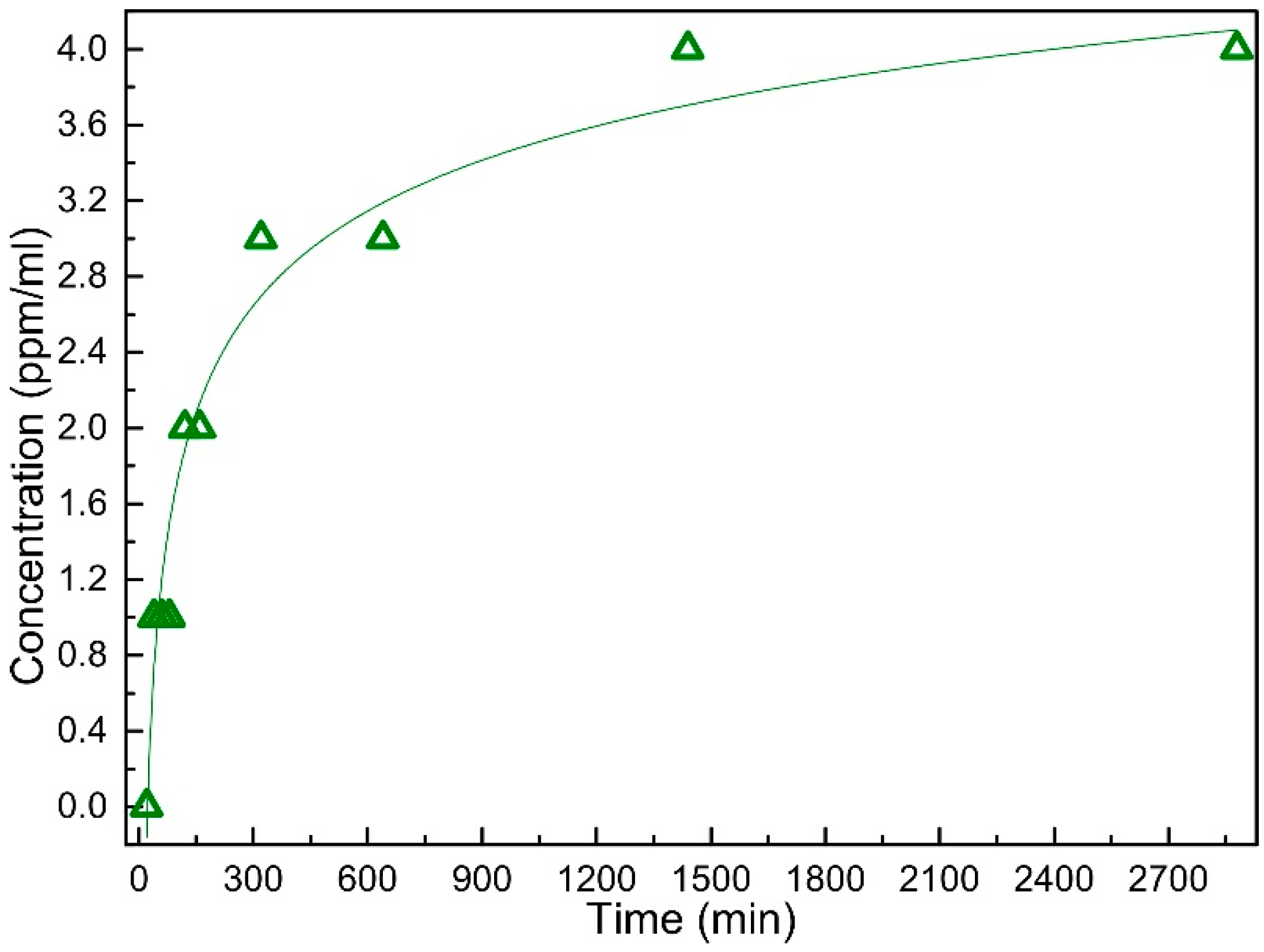
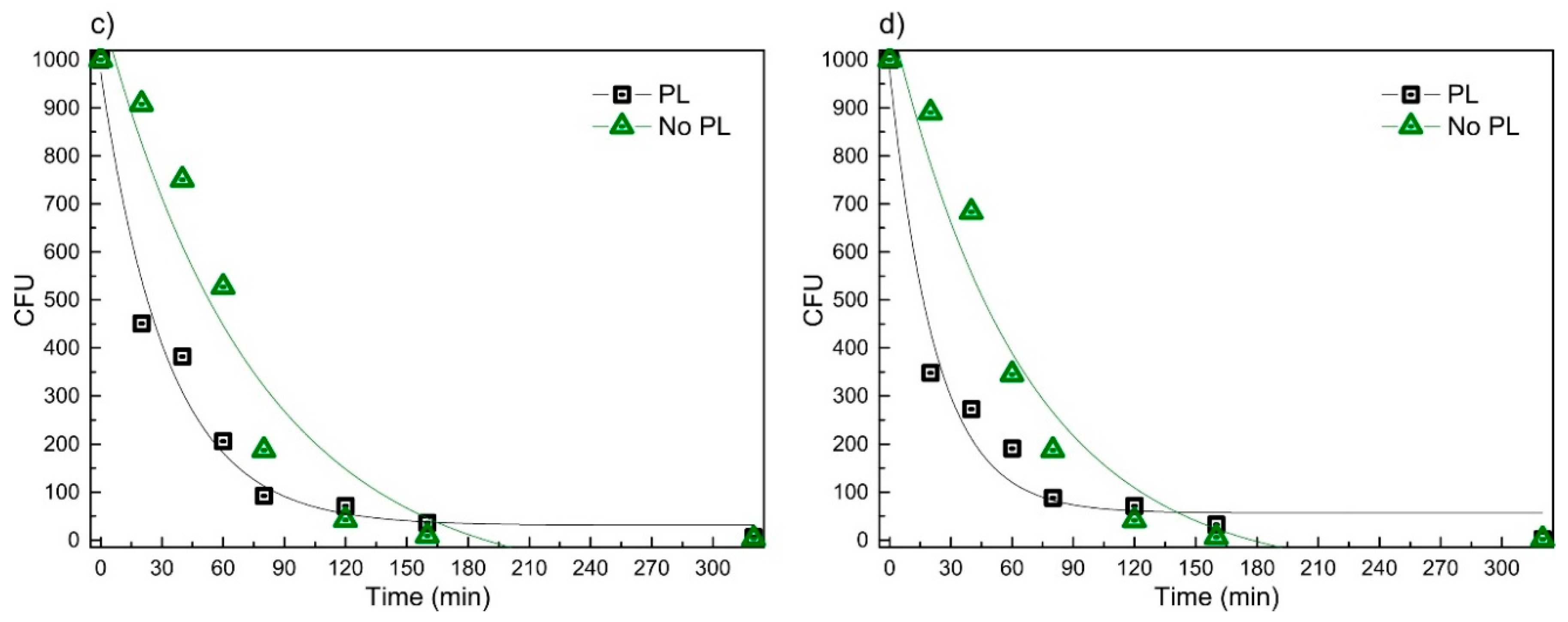
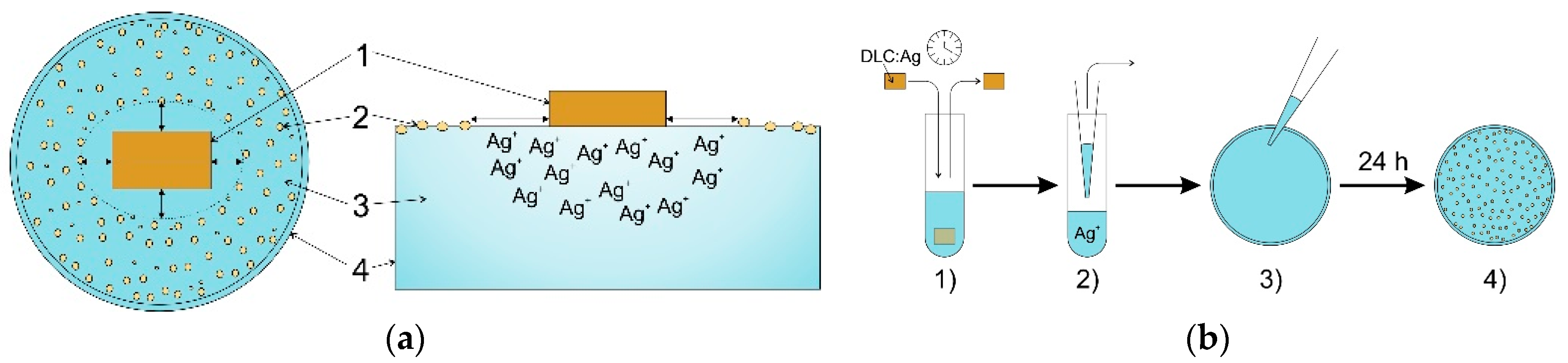
2.3. Antimicrobial Activity of Bandage Prototype
3. Discussion
4. Materials and Methods
4.1. Deposition, Characterization and O2 Plasma Processing of DLC:Ag Films
4.2. Microbiological Testing of Virgin and O2 Plasma-Processed DLC:Ag Films
4.3. Construction of the Bandage Prototype
4.4. Antimicrobial Testing of the Bandage Prototype
4.5. Antimicrobial Testing of the Bandage Prototype
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DLC | diamond-like carbon |
| DLC:Ag | diamond-like carbon with silver nanoparticles |
| RF | radio frequency |
| MRSA | methicillin-resistant Staphylococcus aureus |
| S. aureus | Staphylococcus aureus |
| Ag+ | silver ions |
| DNA | deoxyribonucleic acid |
| Ag NPs | silver nanoparticles |
| DC | direct current |
| PL | protective layer |
| SEM | scanning electron microscopy |
| EDS | energy dispersive X-ray spectroscopy |
| AAS | atomic absorption spectroscopy |
| N | number of analyzed particles |
| dav | average particle diameter |
| a | constant |
| b | constant |
| R2 | correlation coefficient |
| y0 | coefficient of extrapolation |
| A | coefficient of extrapolation |
| R0 | coefficient of extrapolation |
| S.E. | standard error |
References
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. MRSA and the Use of Silver Dressings: Overcoming Bacterial Resistance. Available online: http://www.worldwidewounds.com/2004/november/Thomas/Introducing-Silver-Dressings.html (accessed on 30 March 2016).
- Silver, L.L. Multi-targeting by monotherapeutic antibacterials. Nat. Rev. Drug Discov. 2007, 6, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.P.; Hauser-Gerspach, I.; Waltimo, T.; Stritzker, B. Antibacterial properties of silver containing diamond like carbon coatings produced by ion induced polymer densification. Surf. Coat. Technol. 2011, 205, 4850–4854. [Google Scholar] [CrossRef]
- Thet, N.T.; Alves, D.R.; Bean, J.E.; Booth, S.; Nzakizwanayo, J.; Young, A.E.R.; Jones, B.V.; Jenkins, A.T.A. Prototype development of the intelligent hydrogel wound dressing and its efficacy in the detection of model pathogenic wound biofilms. ACS Appl. Mater. Interfaces 2015. [Google Scholar] [CrossRef] [PubMed]
- Bociaga, D.; Komorowski, P.; Batory, D.; Szymanski, W.; Olejnik, A.; Jastrzebski, K.; Jakubowski, W. Silver-doped nanocomposite carbon coatings (Ag-DLC) for biomedical applications-physiochemical and biological evaluation. Appl. Surf. Sci. 2015, 355, 388–397. [Google Scholar] [CrossRef]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Katayama, Y.; Matsuo, M.; Sasaki, T.; Morimoto, Y.; Sekiguchi, A.; Baba, T. Multi-drug-resistant staphylococcus aureus and future chemotherapy. J. Infect. Chemother. 2014, 20, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Sannino, A.; Pollini, M. Surface chemical and biological characterization of flax fabrics modified with silver nanoparticles for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Owens, B. Silver makes antibiotics thousands of times more effective. Nature News, 19 June 2013. [Google Scholar] [CrossRef]
- Knetsch, M.L.W.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Koerner, E.; Aguirre, M.H.; Fortunato, G.; Ritter, A.; Ruehe, J.; Hegemann, D. Formation and distribution of silver nanoparticles in a functional plasma polymer matrix and related Ag+ release properties. Plasma Processes Polym. 2010, 7, 619–625. [Google Scholar] [CrossRef]
- Quelemes, P.V.; Araruna, F.B.; de Faria, B.E.F.; Kuckelhaus, S.A.S.; da Silva, D.A.; Mendonca, R.Z.; Eiras, C.; Soares, M.J.d.S.; Leite, J.R.S.A. Development and antibacterial activity of cashew gum-based silver nanoparticles. Int. J. Mol. Sci. 2013, 14, 4969–4981. [Google Scholar] [CrossRef] [PubMed]
- Lazic, V.; Saponjic, Z.; Vodnik, V.; Dimitrijevic, S.; Jovancic, P.; Nedeljkovic, J.; Radetic, M. A study of the antibacterial activity and stability of dyed cotton fabrics modified with different forms of silver. J. Serbian Chem. Soc. 2012, 77, 225–234. [Google Scholar] [CrossRef]
- Palomba, M.; Carotenuto, G.; Cristino, L.; Di Grazia, M.A.; Nicolais, F.; De Nicola, S. Activity of antimicrobial silver polystyrene nanocomposites. J. Nanomater. 2012. [Google Scholar] [CrossRef]
- Jamuna-Thevi, K.; Bakar, S.A.; Ibrahim, S.; Shahab, N.; Toff, M.R.M. Quantification of silver ion release, in vitro cytotoxicity and antibacterial properties of nanostuctured Ag doped TiO2 coatings on stainless steel deposited by rf magnetron sputtering. Vacuum 2011, 86, 235–241. [Google Scholar] [CrossRef]
- Alissawi, N.; Zaporojtchenko, V.; Strunskus, T.; Hrkac, T.; Kocabas, I.; Erkartal, B.; Chakravadhanula, V.S.K.; Kienle, L.; Grundmeier, G.; Garbe-Schoenberg, D.; et al. Tuning of the ion release properties of silver nanoparticles buried under a hydrophobic polymer barrier. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Larese, F.F.; D’Agostin, F.; Crosera, M.; Adami, G.; Renzi, N.; Bovenzi, M.; Maina, G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 2009, 255, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 2010, 408, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, L.; Ogino, A.; Nagatsu, M. Investigation into the antibacterial property of carbon films. Diam. Relat. Mater. 2008, 17, 1416–1419. [Google Scholar] [CrossRef]
- Silver as an Anti-Bacterial. Available online: https://www.silverinstitute.org/site/silver-in-technology/silver-in-medicine/bandages/ (accessed on 30 March 2016).
- Elastoplast-Antibacterial Waterproof. Available online: http://www.en.elastoplast.ca/Products/waterproof-antibacterial (accessed on 30 March 2016).
- Band-aid Plus Antibiotic Adhesive Bandages, Assorted Sizes. Available online: http://www.drugstore.com/band-aid-plus-antibiotic-adhesive-bandages-assorted-sizes/qxp149702 (accessed on 30 March 2016).
- Percival, S.L.; Bowler, P.G.; Dolman, J. Antimicrobial activity of silver-containing dressings on wound microorganisms using an in vitro biofilm model. Int. Wound J. 2007, 4, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.R.; Walker, M.; Hobot, J.A.; Bowler, P.G. Visualisation of bacterial sequestration and bactericidal activity within hydrating hydrofiber® wound dressings. Biomaterials 2006, 27, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Wach, R.A.; Mitomo, H.; Nagasawa, N.; Yoshii, F. Radiation crosslinking of carboxymethylcellulose of various degree of substitution at high concentration in aqueous solutions of natural pH. Radiat. Phys. Chem. 2003, 68, 771–779. [Google Scholar] [CrossRef]
- Meskinis, S.; Vasiliauskas, A.; Slapikas, K.; Gudaitis, R.; Andrulevicius, M.; Ciegis, A.; Niaura, G.; Kondrotas, R.; Tamulevicius, S. Bias effects on structure and piezoresistive properties of DLC:Ag thin films. Surf. Coat. Technol. 2014, 255, 84–89. [Google Scholar] [CrossRef]
- Choi, H.W.; Dauskardt, R.H.; Lee, S.-C.; Lee, K.-R.; Oh, K.H. Characteristic of silver doped DLC films on surface properties and protein adsorption. Diam. Relat. Mater. 2008, 17, 252–257. [Google Scholar] [CrossRef]
- Yaremchuk, I.; Meskinis, S.; Fitio, V.; Bobitski, Y.; Slapikas, K.; Ciegis, A.; Balevicius, Z.; Selskis, A.; Tamulevicius, S. Spectroellipsometric characterization and modeling of plasmonic diamond-like carbon nanocomposite films with embedded ag nanoparticles. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Meškinis, Š.; Vasiliauskas, A.; Šlapikas, K.; Gudaitis, R.; Yaremchuk, I.; Fitio, V.; Bobitski, Y.; Tamulevičius, S. Annealing effects on structure and optical properties of diamond-like carbon films containing silver. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of oxygen plasma nanotexturing of organic polymer surfaces: From stable super hydrophilic to super hydrophobic surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Creighton, J.A. Investigation by surface-enhanced raman spectroscopy of the effect of oxygen and hydrogen plasmas on adsorbate-covered gold and silver island films. Langmuir 1999, 15, 3545–3550. [Google Scholar] [CrossRef]
- Tamulevicius, T.; Tamuleviciene, A.; Virganavicius, D.; Vasiliauskas, A.; Kopustinskas, V.; Meskinis, S.; Tamulevicius, S. Structuring of DLC:Ag nanocomposite thin films employing plasma chemical etching and ion sputtering. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 2014, 341, 1–6. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Chen, L.H.; Zou, Y.L.; Nguyen, P.A.; Hansen, J.D.; Alford, T.L. Processing and encapsulation of silver patterns by using reactive ion etch and ammonia anneal. Mater. Chem. Phys. 2000, 66, 77–82. [Google Scholar] [CrossRef]
- Nguyen, P.; Zeng, Y.X.; Alford, T.L. Reactive ion etch of patterned and blanket silver thin films in Cl−2/O−2 and O−2 glow discharges. J. Vac. Sci. Technol. B 1999, 17, 2204–2209. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Wettability and antibacterial activity of modified diamond-like carbon films. Appl. Surf. Sci. 2009, 255, 8377–8382. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, Y.; Liu, X.; Lu, T.; Cui, T.; Meng, F.; Chu, P.K. Electron storage mediated dark antibacterial action of bound silver nanoparticles: Smaller is not always better. Acta Biomater. 2013, 9, 5100–5110. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Dai, S.A.; Fu, K.-Y.; Hsu, S.-H. Antibacterial properties of silver nanoparticles in three different sizes and their nanocomposites with a new waterborne polyurethane. Int. J. Nanomed. 2010, 5, 1017–1028. [Google Scholar]
- Ip, M.; Lui, S.L.; Poon, V.K.M.; Lung, I.; Burd, A. Antimicrobial activities of silver dressings: An in vitro comparison. J. Med. Microbiol. 2006, 55, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.B.; Lam, K.; Burrell, R.E. Wound management in an era of increasing bacterial antibiotic resistance: A role for topical silver treatment. Am. J. Infect. Control 1998, 26, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Yallapu, M.M.; Varaprasad, K.; Reddy, N.N.; Ravindra, S.; Naidu, N.S.; Raju, K.M. Fabrication of curcumin encapsulated chitosan-PVA silver nanocomposite films for improved antimicrobial activity. J. Biomater. Nanobiotechnol. 2011, 2, 55–64. [Google Scholar] [CrossRef]
- Yilma, A.N.; Singh, S.R.; Dixit, S.; Dennis, V.A. Anti-inflammatory effects of silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles in mouse macrophages infected with live chlamydia trachomatis. Int. J. Nanomed. 2013, 8, 2421–2432. [Google Scholar]
- Meskinis, S.; Vasiliauskas, A.; Slapikas, K.; Niaura, G.; Juskenas, R.; Andrulevicius, M.; Tamulevicius, S. Structure of the silver containing diamond like carbon films: Study by multiwavelength raman spectroscopy and xrd. Diam. Relat. Mater. 2013, 40, 32–37. [Google Scholar] [CrossRef]
- Yaremchuk, I.; Tamuleviciene, A.; Tamulevicius, T.; Slapikas, K.; Balevicius, Z.; Tamulevicius, S. Modeling of the plasmonic properties of DLC-Ag nanocomposite films. Phy. Status Solidi A-Appl. Mater. Sci. 2014, 211, 329–335. [Google Scholar] [CrossRef]
- Meškinis, Š.; Tamulevičius, T.; Niaura, G.; Šlapikas, K.; Vasiliauskas, A.; Ulčinas, O.; Tamulevičius, S. Surface enhanced raman scattering effect in diamond like carbon films containing ag nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 1–9. [Google Scholar]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
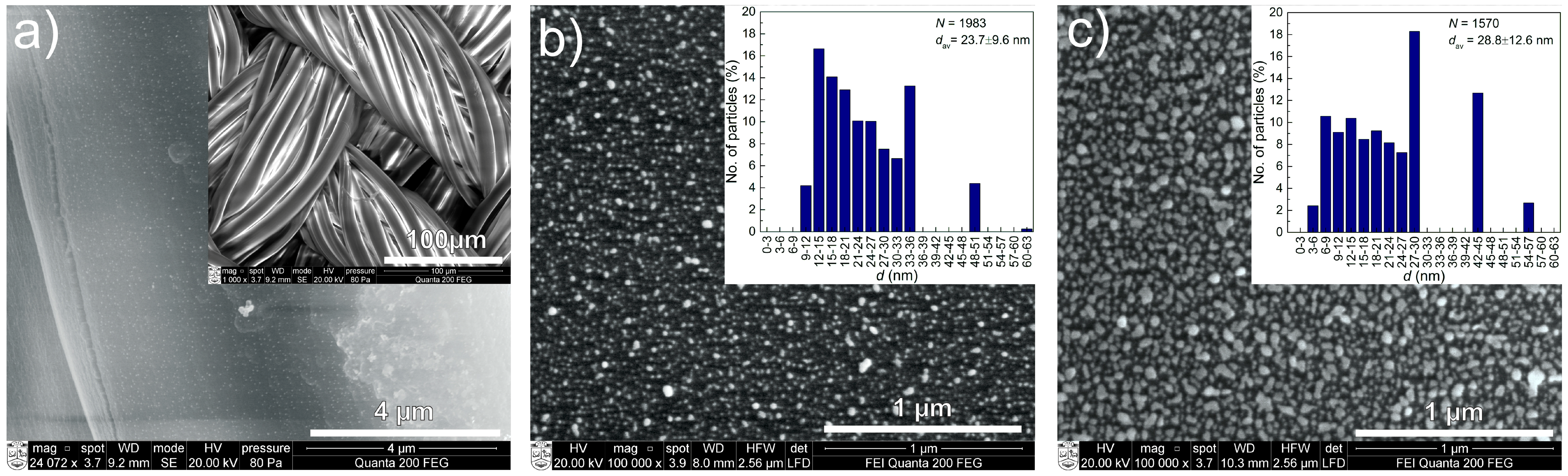
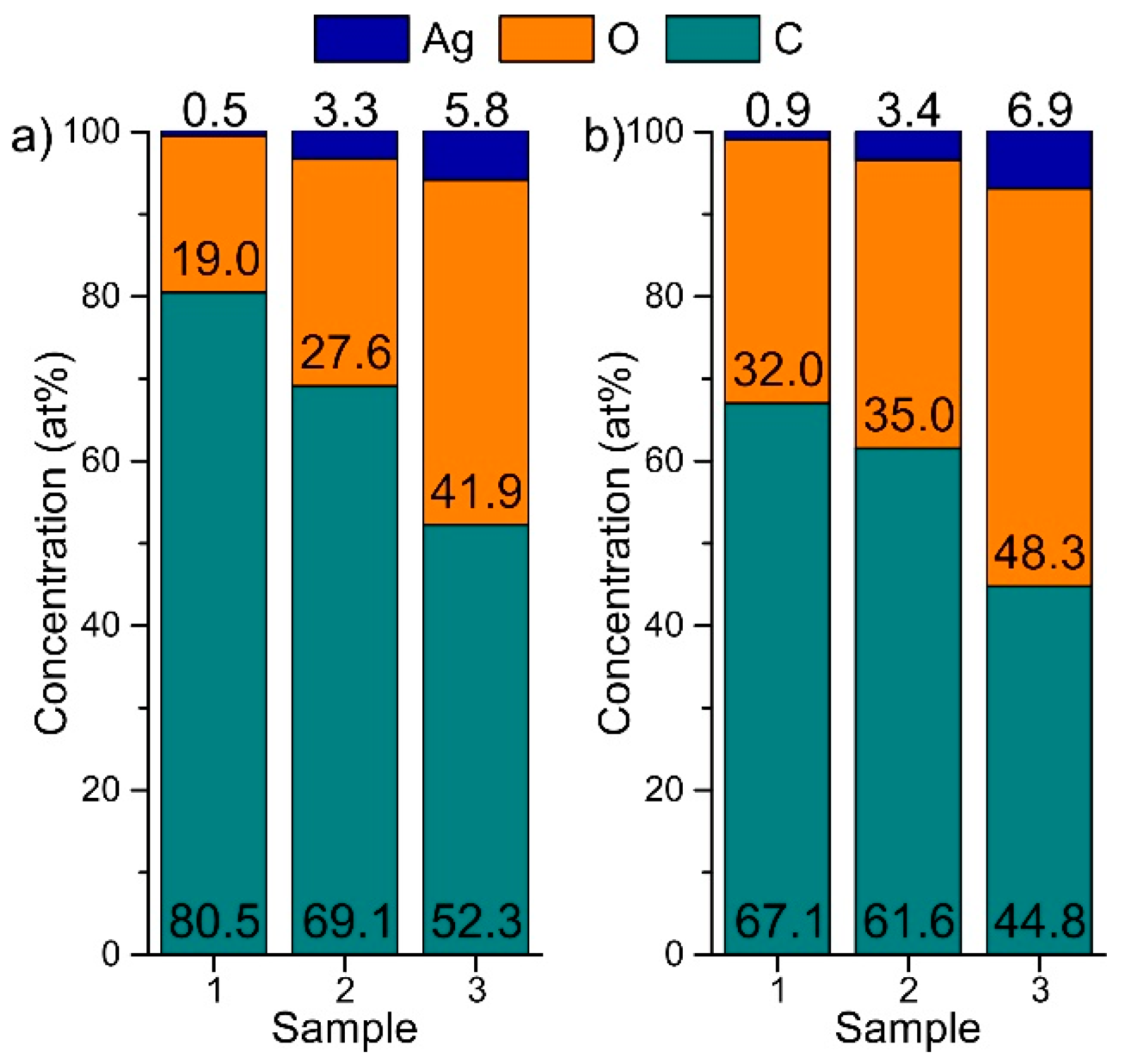


| Sample No. | Carbon (at %) | Silver (at %) | Oxygen (at %) |
|---|---|---|---|
| 1 | 67.54 ± 1.33 1 | 0.46 ± 0.02 | 32.01 ± 1.33 |
| 2 | 61.55 ± 0.58 | 3.12 ± 0.11 | 31.16 ± 4.65 |
| 3 | 48.92 ± 3.65 | 6.43 ± 0.65 | 44.65 ± 4.27 |
| Sample Structure | Fitting Coefficients | LTSaDA01 (a) | LTSaM01 (b) | LTSa635 (c) | LTSa603 (d) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Value | S.E. 1 | Value | S.E. | Value | S.E. | Value | S.E. | ||
| PL | y0 | 55.37 | 36.36 | 54.13 | 34.42 | 30.77 | 35.16 | 56.566 | 33.60 |
| A | 921.2 | 74.29 | 922.4 | 68.84 | 943.4 | 61.89 | 922.87 | 69.53 | |
| R0 | −0.0430 | 0.00826 | −0.0407 | 0.00717 | −0.0305 | 0.00463 | −0.0445 | 0.00803 | |
| R2 | 0.956 | 0.962 | 0.971 | 0.961 | |||||
| No PL | y0 | −83.45 | 110.3 | −75.39 | 97.95 | −101.1 | 130.6 | −77.21 | 95.29 |
| A | 1184.3 | 134.41 | 1157.7 | 121.45 | 1208.68 | 149.92 | 1170.6 | 117.74 | |
| R0 | −0.0149 | 0.00408 | −0.0154 | 0.0039 | −0.0132 | 0.00397 | −0.0153 | 0.00371 | |
| R2 | 0.919 | 0.930 | 0.908 | 0.936 | |||||
| Sample No. | Sputtering Duration (s) | Ar Gas Flow (sccm) | C2H2 Gas Flow (sccm) | Magnetron Voltage (V) | Magnetron Current (A) |
|---|---|---|---|---|---|
| 1 | 520 | 70 | 21.1 | 553–625 | 0.07–0.12 |
| 2 | 235 | 70 | 21.1 | 568–741 | 0.07–0.22 |
| 3 | 200 | 80 | 7.8 | 625–656 | 0.10–0.11 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juknius, T.; Ružauskas, M.; Tamulevičius, T.; Šiugždinienė, R.; Juknienė, I.; Vasiliauskas, A.; Jurkevičiūtė, A.; Tamulevičius, S. Antimicrobial Properties of Diamond-Like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages. Materials 2016, 9, 371. https://doi.org/10.3390/ma9050371
Juknius T, Ružauskas M, Tamulevičius T, Šiugždinienė R, Juknienė I, Vasiliauskas A, Jurkevičiūtė A, Tamulevičius S. Antimicrobial Properties of Diamond-Like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages. Materials. 2016; 9(5):371. https://doi.org/10.3390/ma9050371
Chicago/Turabian StyleJuknius, Tadas, Modestas Ružauskas, Tomas Tamulevičius, Rita Šiugždinienė, Indrė Juknienė, Andrius Vasiliauskas, Aušrinė Jurkevičiūtė, and Sigitas Tamulevičius. 2016. "Antimicrobial Properties of Diamond-Like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages" Materials 9, no. 5: 371. https://doi.org/10.3390/ma9050371
APA StyleJuknius, T., Ružauskas, M., Tamulevičius, T., Šiugždinienė, R., Juknienė, I., Vasiliauskas, A., Jurkevičiūtė, A., & Tamulevičius, S. (2016). Antimicrobial Properties of Diamond-Like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages. Materials, 9(5), 371. https://doi.org/10.3390/ma9050371