Immobilization of Trypsin in Lignocellulosic Waste Material to Produce Peptides with Bioactive Potential from Whey Protein
Abstract
:1. Introduction
2. Results and Discussion
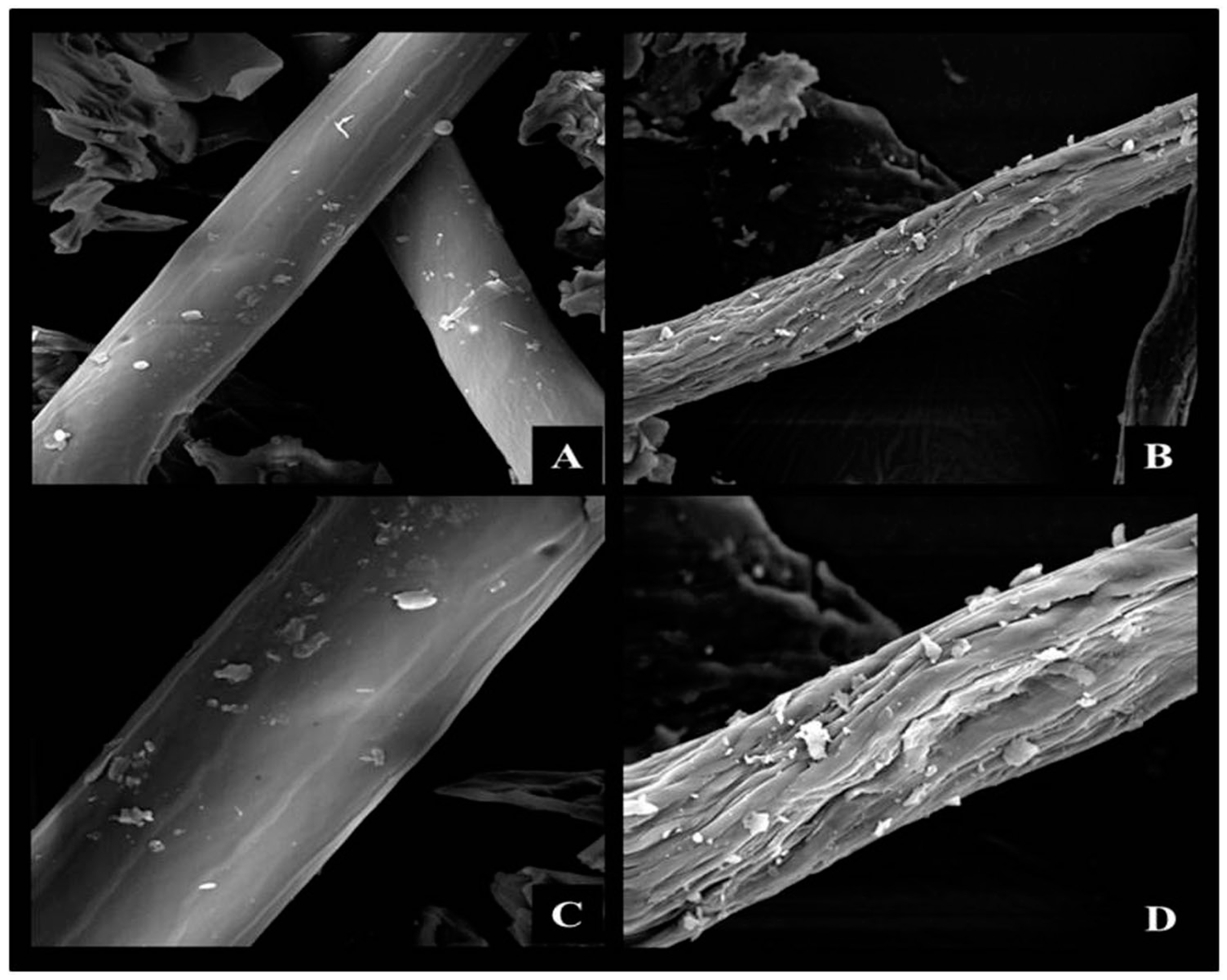
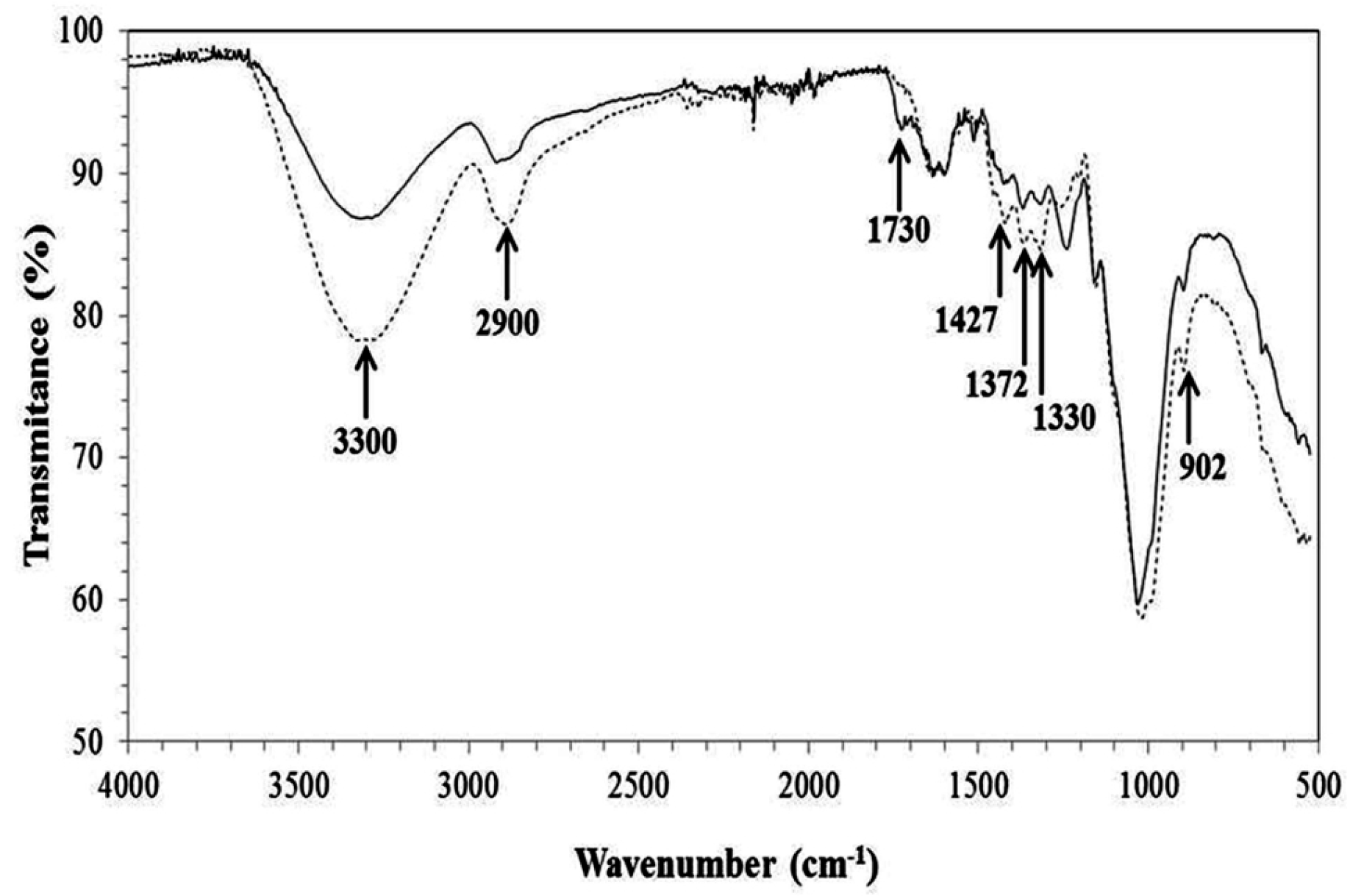
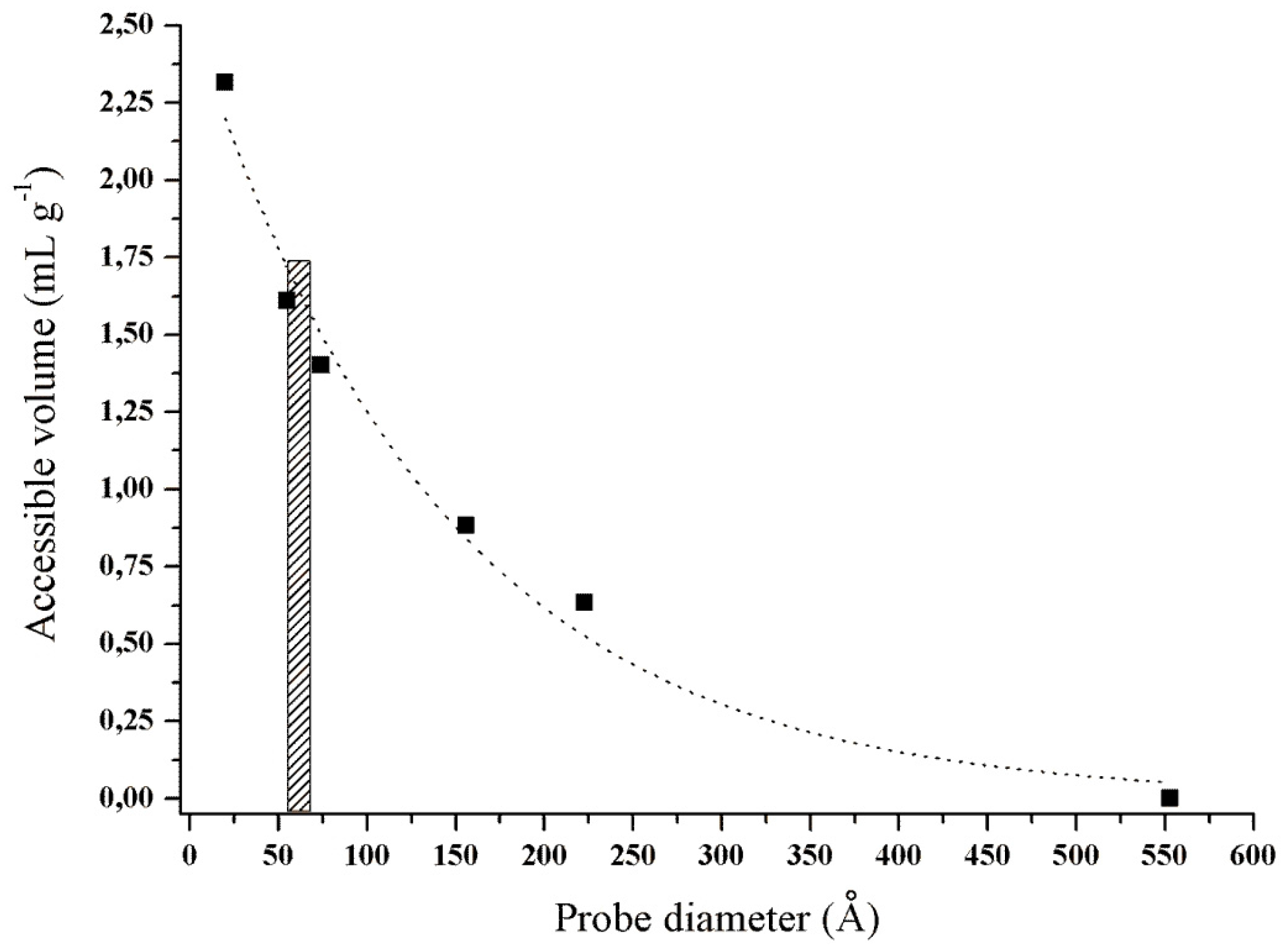
2.1. Pretreatment and Characterization of Corn Cob Powder
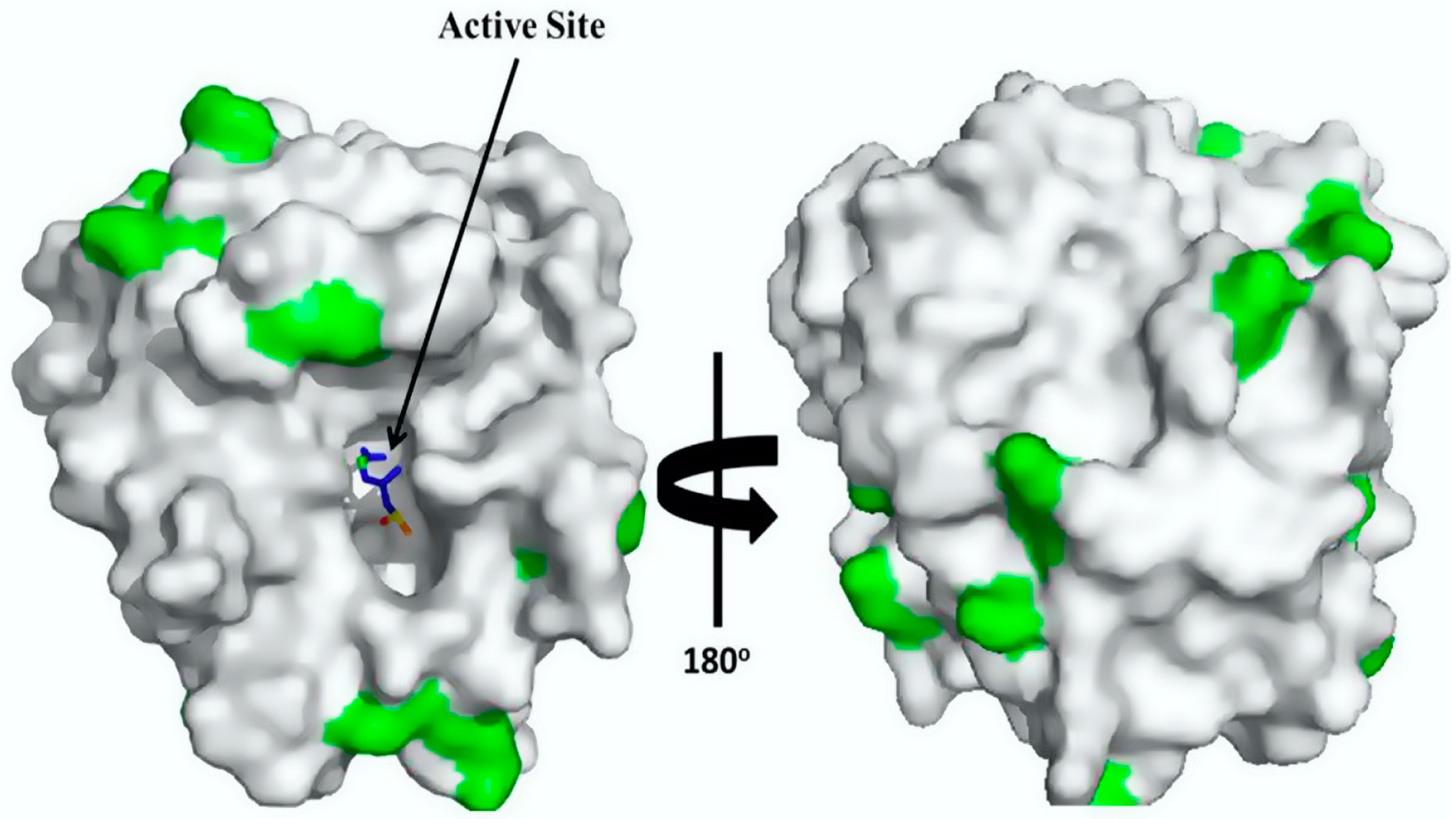
2.2. Immobilization and Biochemical Characterization of Trypsin
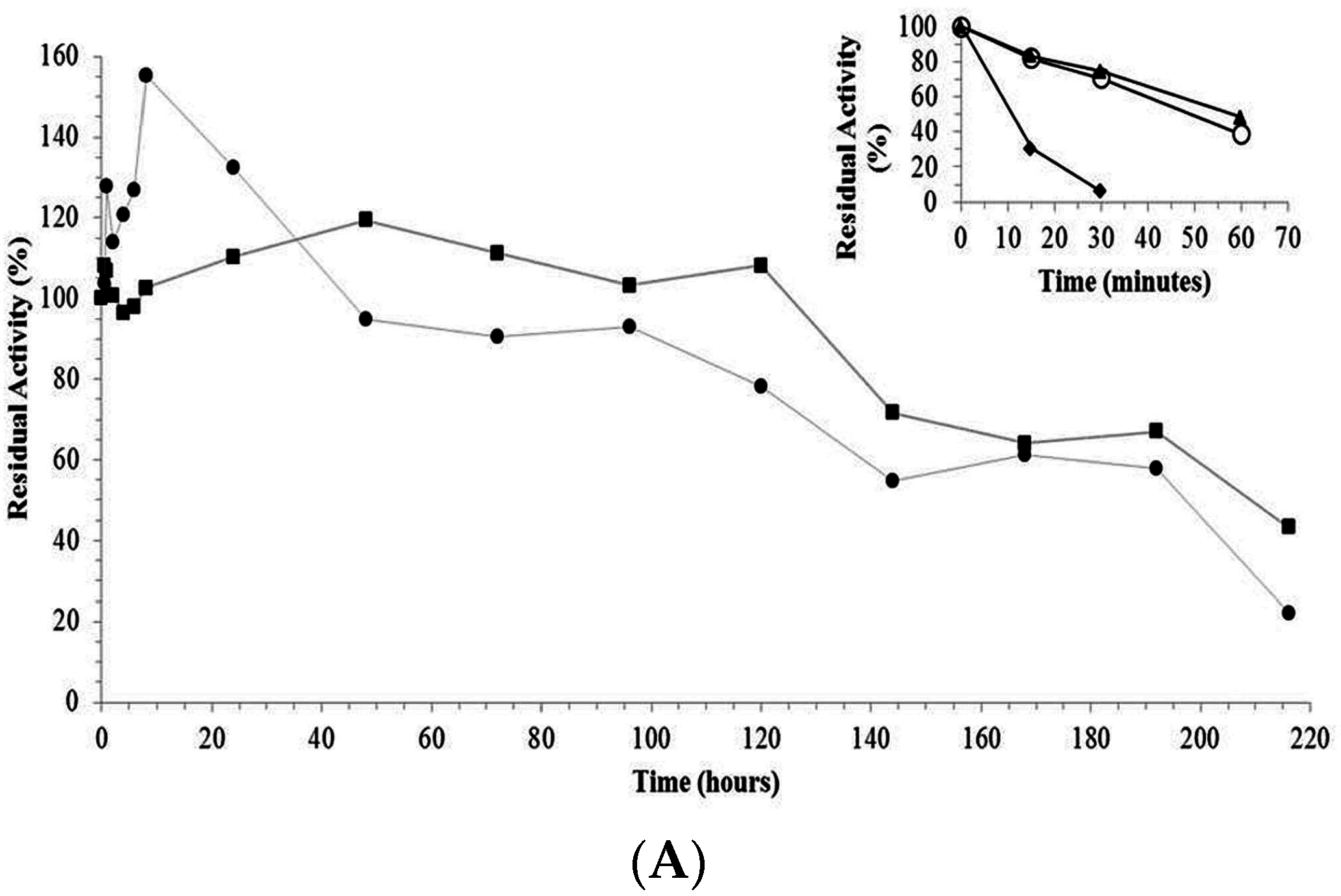
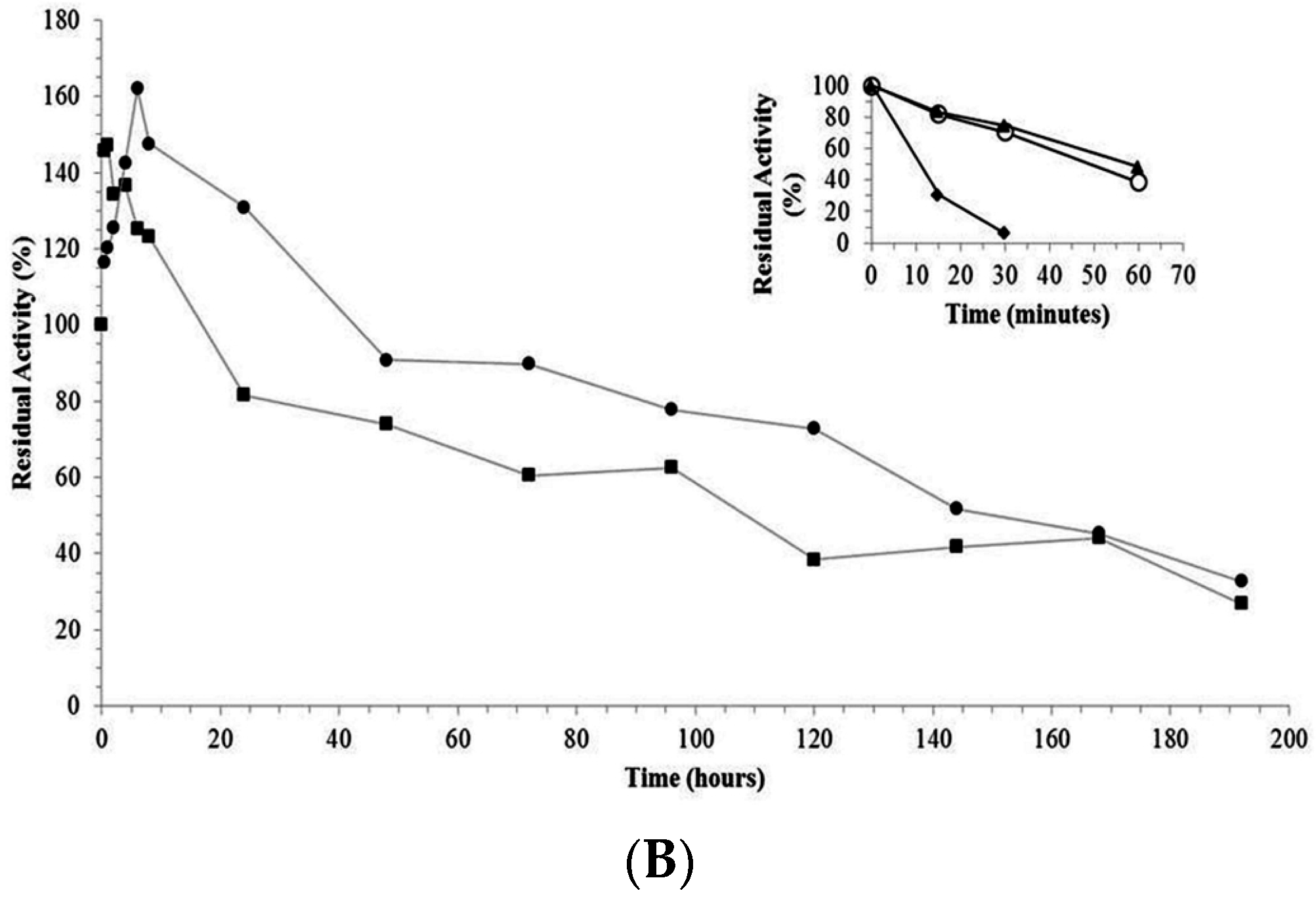
2.3. Thermal Stability of Derivatives
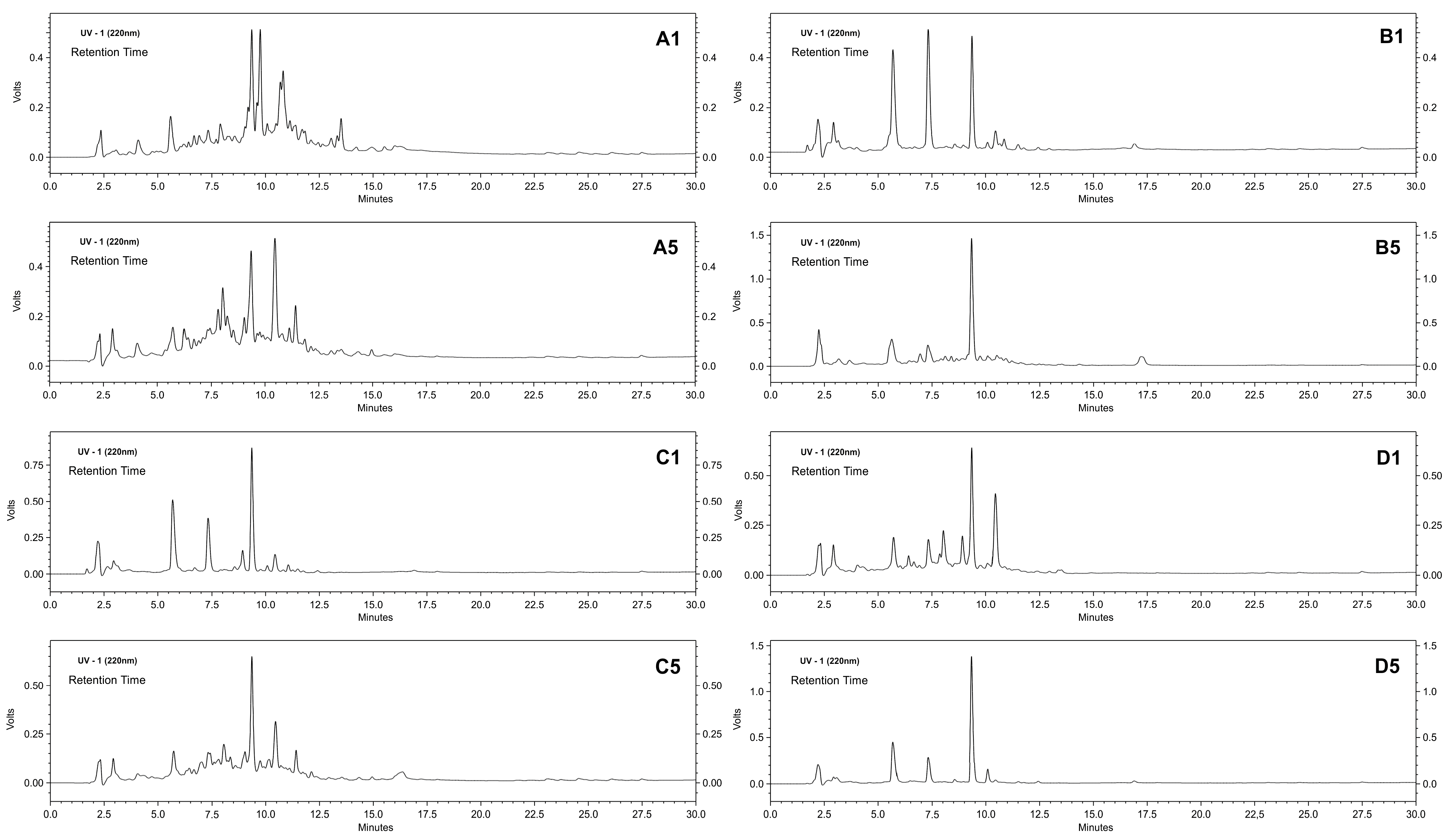
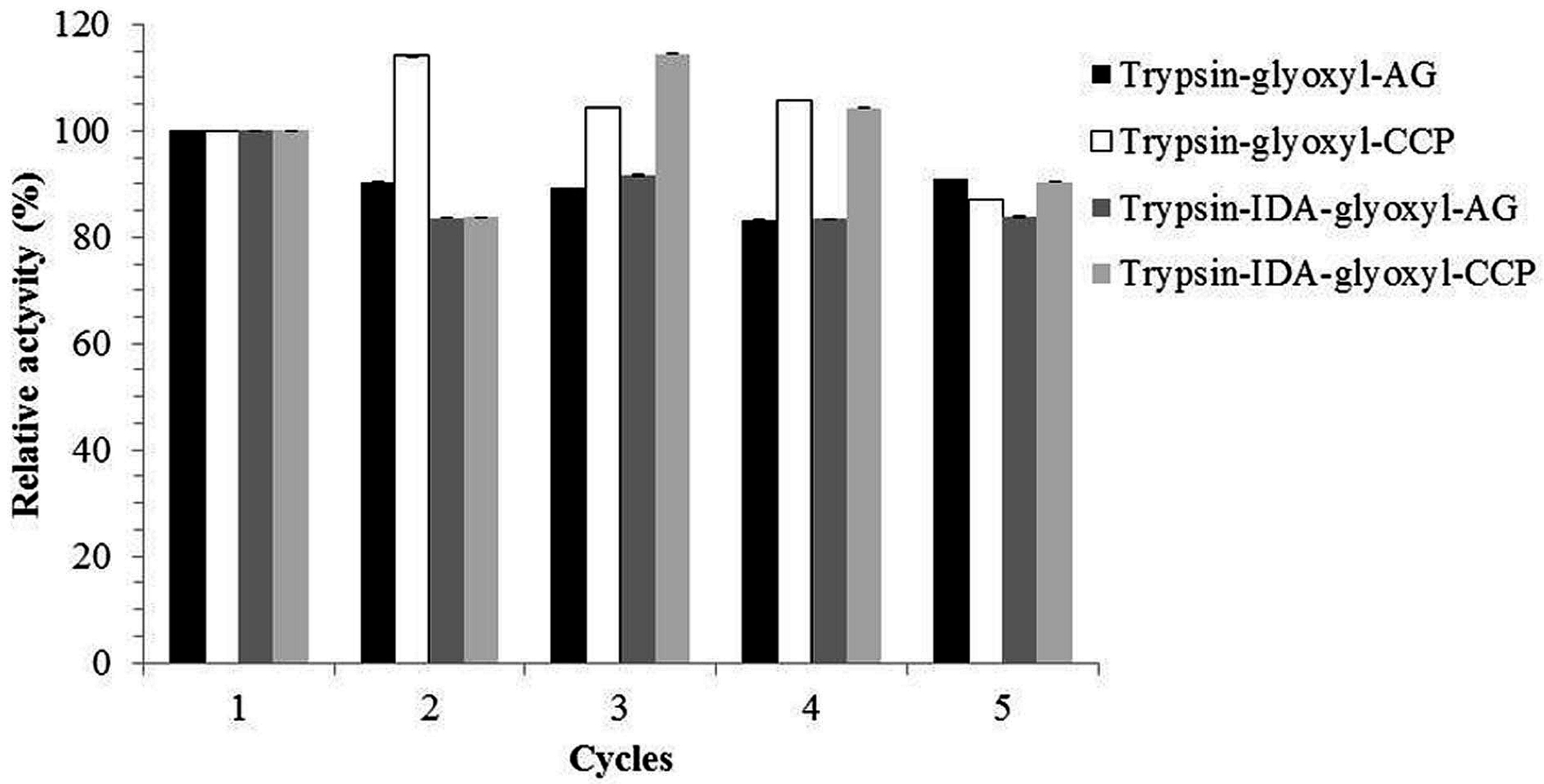
2.4. Application of Derivatives to Produce Peptides
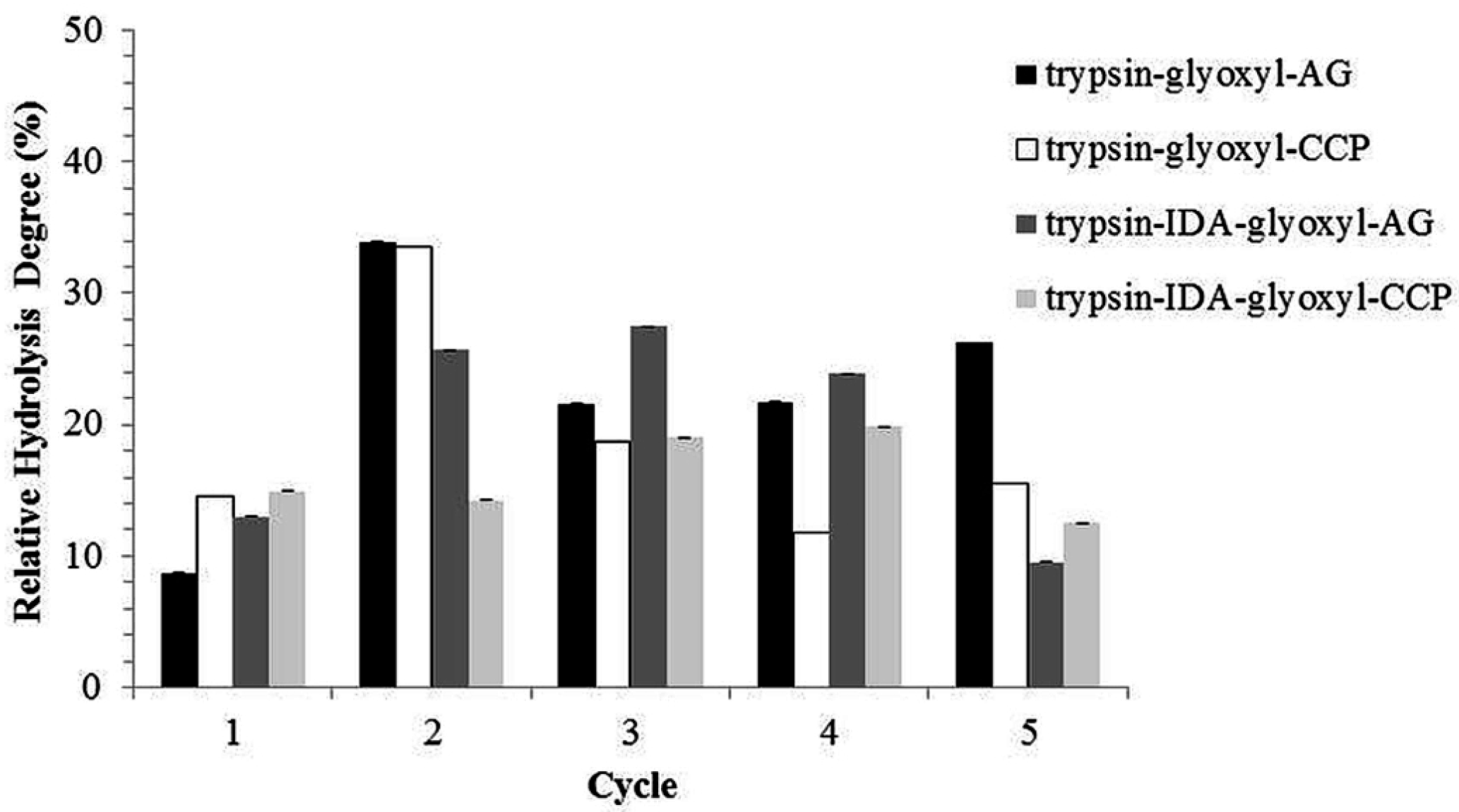
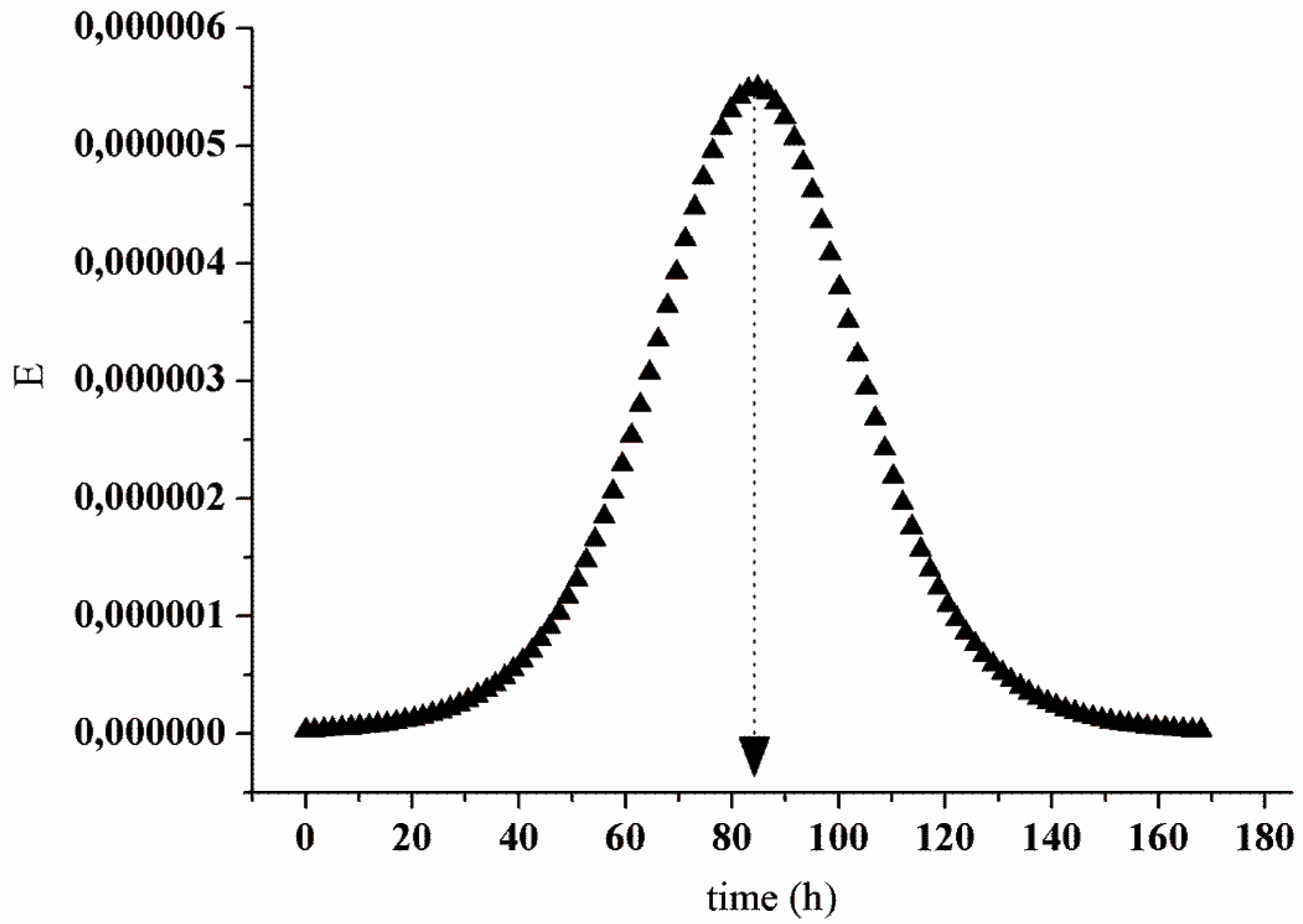
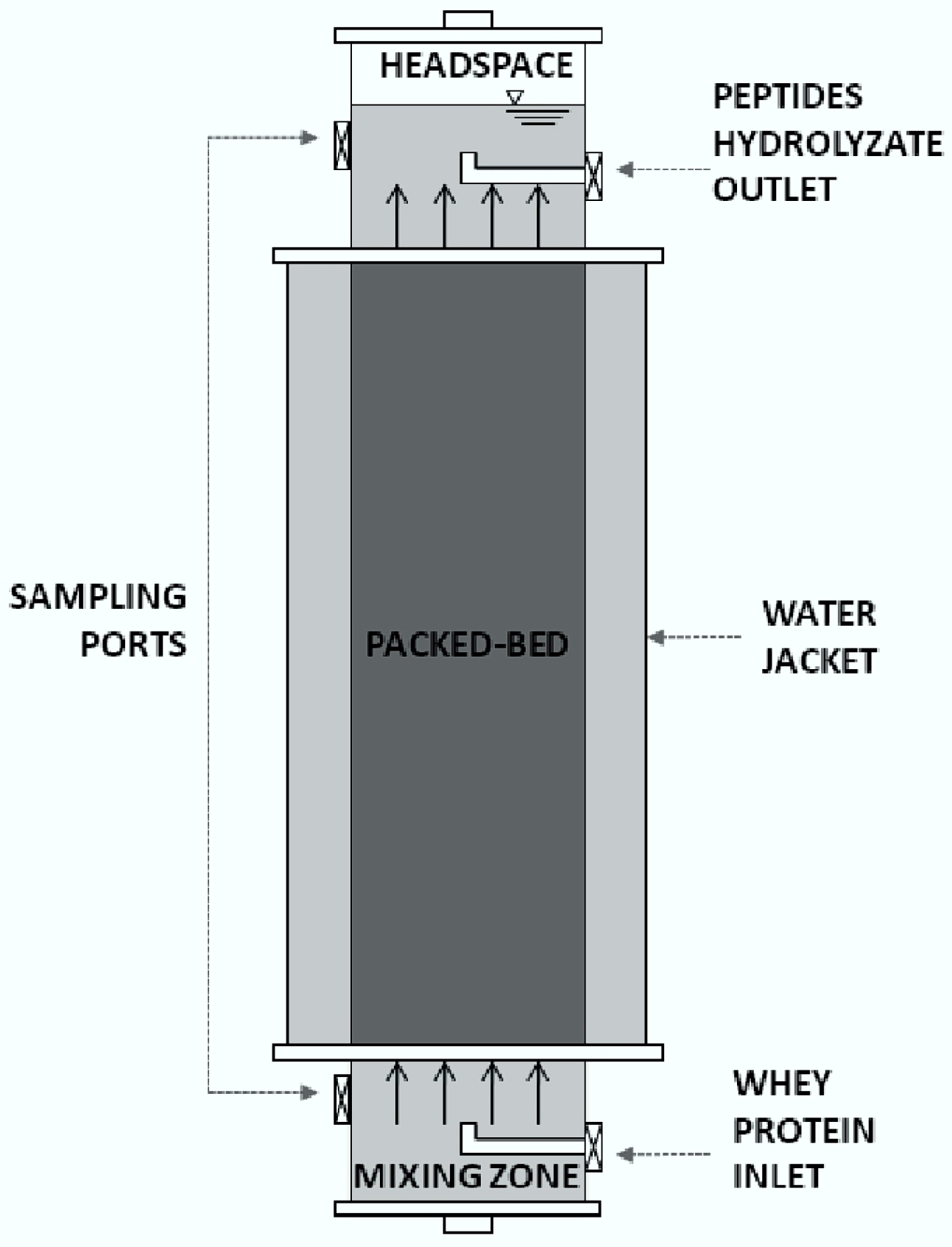
2.5. Continuous Production of Peptides
3. Materials and Methods
3.1. Materials
3.2. Preparation and Physicochemical Characterization of Pretreated Corn Cob Powder
3.3. Activation of Corn Cob and Agarose Supports
3.4. Preparation of the Trypsin Derivatives
3.5. Determination of Batch and Continuous Operation Parameters
3.5.1. Reuse
3.5.2. Whey Protein Hydrolysates Characterization
3.6. Characterization of the Continuous Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| AG | Agarose |
| BApNA | N-α-benzoyl-DL-arginine-p-nitroanilide |
| CCP | Corn Cob Powder |
| CSTR | Continuous Stirred Tank Reactor |
| DMSO | Dimethyl Sulfoxide |
| EDA | 1,2-ethanodiamine |
| FTIR | Fourier Transform Infrared Spectrometry |
| HRT | Hydraulic Retention Time |
| IDA | Iminodiacetic Acid |
| PFR | Plug Flow Reactor |
| SEM | Scanning Electronic Microscopy |
| SLR | Substrate Loading Rate |
| TFA | Trifluoracetic Acid |
| TNBS | 2,4,6-trinitrobenzene Sulfonic Acid |
References
- Brena, B.M.; Batista-Viera, F. Immobilization of Enzymes. In Immobilization of Enzymes and Cells, 2nd ed.; Guisan, J.M., Ed.; Human Press Inc.: Totowa, NJ, USA, 2006; pp. 15–30. [Google Scholar]
- Banerjee, S.; Mudliar, S.; Sen, R.; Giri, B.; Satpute, D.; Chakrabarti, T.; Pandey, R.A. Commercializing lignocellulosic bioethanol: Technology bottlenecks and possible remedies. Biofuel Bioprod. Biorefin. 2010, 4, 77–93. [Google Scholar] [CrossRef]
- Genisheva, Z.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Evaluating the potential of wine-making residues and corn cobs as support materials for cell immobilization for ethanol production. Ind. Crop Prod. 2011, 34, 979–985. [Google Scholar] [CrossRef]
- Ittrat, P.; Chacho, T.; Pholprayoon, J.; Suttiwarayanon, N.; Charoenpanich, J. Application of agriculture waste as a support for lipase immobilization. Biocatal. Agric. Biotechnol. 2014, 3, 77–82. [Google Scholar] [CrossRef]
- Azubuike, C.P.; Okhamafe, A.O. Physicochemical, spectroscopic and thermal properties of microcrystalline cellulose derived from corn cobs. Int. J. Recycl. Org. Waste Agric. 2012, 1, 9. [Google Scholar] [CrossRef]
- Cao, Q.; Xie, K.C.; Lv, Y.K.; Bao, W.R. Process effects on activated carbon with large specific surface area from corn cob. Bioresour. Technol. 2006, 97, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wanitwattanarumlug, B.; Luengnaruemitchai, A.; Wongkasemjit, S. Characterization of Corn Cobs from Microwave and Potassium Hydroxide Pretreatment. Int. Sch. Sci. Res. Innov. 2012, 6, 528–532. [Google Scholar]
- Yan, J.H.; Hou, Y.C.; Ren, S.H.; Niu, M.G.; Wu, W.Z. Two-Step Treatment of Corn Cob in H2O-SO2 System. Ind. Eng. Chem. Res. 2014, 53, 13256–13263. [Google Scholar]
- Alves, C.C.O.; Franca, A.S.; Oliveira, L.S. Removal of phenylalanine from aqueous solutions with thermo-chemically modified corn cobs as adsorbents. LWT Food Sci. Technol. 2013, 51, 1–8. [Google Scholar] [CrossRef]
- Ashour, A.; Amer, M.; Marzouk, A.; Shimizu, K.; Kondo, R.; El-Sharkawy, S. Corncobs as a Potential Source of Functional Chemicals. Molecules 2013, 18, 13823–13830. [Google Scholar] [CrossRef] [PubMed]
- Kaliyan, N.; Morey, R.V. Densification characteristics of corn cobs. Fuel Process Technol. 2010, 91, 559–565. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghaly, A.E.; Bingxi, L. Physical Properties of Corn Residues. Am. J. Biochem. Biotechnol. 2012, 8, 44–53. [Google Scholar]
- Rivas, B.; Moldes, A.B.; Dominguez, J.M.; Parajo, J.C. Lactic acid production from corn cobs by simultaneous saccharification and fermentation: A mathematical interpretation. Enzyme Microb. Technol. 2004, 34, 627–634. [Google Scholar] [CrossRef]
- Sanchez, C.; Egues, I.; Garcia, A.; Llano-Ponte, R.; Labidi, J. Lactic acid production by alkaline hydrothermal treatment of corn cobs. Chem. Eng. J. 2012, 181, 655–660. [Google Scholar] [CrossRef]
- D’Souza, S.F.; Godbole, S.S. Immobilization of invertase on rice husk using polyethylenimine. J. Biochem. Biophys. Methods 2002, 52, 59–62. [Google Scholar] [CrossRef]
- Silva, A.M.; Tavares, A.P.M.; Rocha, C.M.R.; Cristóvão, R.O.; Teixeira, J.A.; Macedo, E.A. Immobilization of commercial laccase on spent grain. Process Biochem. 2012, 47, 1095–1101. [Google Scholar] [CrossRef]
- Brigida, A.I.; Pinheiro, A.D.; Ferreira, A.L.; Goncalves, L.R. Immobilization of Candida antarctica lipase B by adsorption to green coconut fiber. Appl. Biochem. Biotechnol. 2008, 146, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.; Gonçalves, M.P.; Teixeira, J.A. Immobilization of trypsin on spent grains for whey protein hydrolysis. Process Biochem. 2011, 46, 505–511. [Google Scholar] [CrossRef]
- Bezerra, T.M.S.; Bassan, J.C.; Santos, V.T.O.; Ferraz, A.; Monti, R. Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process Biochem. 2015, 50, 417–423. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Guisan, J.M. Immobilization of enzymes as the 21st century begins. In Immobilization of Enzymes and Cells, 2nd ed.; Guisan, J.M., Ed.; Human Press Inc.: Totowa, NJ, USA, 2006; pp. 1–13. [Google Scholar]
- Tavano, O.L. Protein hydrolysis using proteases: An important tool for food biotechnology. J. Mol. Catal. B Enzym. 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fuentes, M.; Betancor, L.; Grazu, V.; Lopez-Gallego, F.; Pessela, C.C.B.; Hidalgo, A.; Fernández-Lorente, G.; Fernández-Lafuente, R.; et al. Glyoxyl agarose: A fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Massolini, G.; Calleri, E. Immobilized trypsin systems coupled on-line to separation methods: Recent developments and analytical applications. J. Sep. Sci. 2005, 28, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Woodard, S.L.; Mayor, J.M.; Bailey, M.R.; Barker, D.K.; Love, R.T.; Lane, J.R.; Delaney, D.E.; McComas-Wagner, J.M.; Mallubhotla, H.D.; Hood, E.E.; et al. Maize (Zea mays)-derived bovine trypsin: Characterization of the first large-scale, commercial protein product from transgenic plants. Biotechnol. Appl. Biochem. 2003, 38, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.E.; Hmidet, N.; Ghorbel-Bellaaj, O.; Fakhfakh-Zouari, N.; Bougatef, A.; Nasri, M. Solvent-Stable Digestive Alkaline Proteinases from Striped Seabream (Lithognathus mormyrus) Viscera: Characteristics, Application in the Deproteinization of Shrimp Waste, and Evaluation in Laundry Commercial Detergents. Appl. Biochem. Biotechnol. 2011, 164, 1096–1110. [Google Scholar]
- Ktari, N.; Ben Khaled, H.; Nasri, R.; Jellouli, K.; Ghorbel, S.; Nasri, M. Trypsin from zebra blenny (Salaria basilisca) viscera: Purification, characterisation and potential application as a detergent additive. Food Chem. 2012, 130, 467–474. [Google Scholar] [CrossRef]
- Nagpal, R.; Behare, P.; Rana, R.; Kumar, A.; Kumar, M.; Arora, S.; Morotta, F.; Jain, S.; Yadav, H. Bioactive peptides derived from milk proteins and their health beneficial potentials: An update. Food Funct. 2011, 2, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Leksrisompong, P.P.; Miracle, R.E.; Drake, M. Characterization of flavor of whey protein hydrolysates. J. Agric. Food Chem. 2010, 58, 6318–6327. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K. Therapeutic applications of whey protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar] [PubMed]
- Petersen, B.L.; Ward, L.S.; Bastian, E.D.; Jenkins, A.L.; Campbell, J.; Vuksan, V. A whey protein supplement decreases post-prandial glycemia. Nutr. J. 2009, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gimenez, P.; Salom, J.B.; Marcos, J.F.; Valles, S.; Martinez-Maqueda, D.; Recio, I.; Torregrosa, G.; Alborch, E.; Manzanares, P. Antihypertensive effect of a bovine lactoferrin pepsin hydrolysate: Identification of novel active peptides. Food Chem. 2012, 131, 266–273. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Ragauskas, A. Pretreatment and Lignocellulosic Chemistry. Bioenergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Wu, J.Q.; Yuan, T.Q.; Sun, R.C. Preparation and Characterization of Lignocellulosic Oil Sorbent by Hydrothermal Treatment of Populus Fiber. Materials 2014, 7, 6733–6747. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.C.; Misra, M. Characterization of natural fiber surfaces and natural fiber composites. Compos. A Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Chambers, J.L.; Stroud, R.M. Difference Fourier Refinement of the Structure of DIP-Trypsin at 1.5 A with a Minicomputer Technique. Acta Crystallogr. Sect. B 1977, 33, 1824–1837. [Google Scholar] [CrossRef]
- George, J.; Sreekala, M.S.; Thomas, S. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001, 41, 1471–1485. [Google Scholar] [CrossRef]
- Marques, D.; Pessela, B.C.; Betancor, L.; Monti, R.; Carrascosa, A.V.; Rocha-Martin, J.; Guisán, J.M.; Fernandez-Lorente, G. Protein Hydrolysis by Immobilized and Stabilized Trypsin. Biotechnol. Prog. 2011, 27, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Immobilized Enzymes. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Elsevier: Waterloo, ON, Canada, 2011; pp. 461–476. [Google Scholar]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Sashidhar, R.B.; Capoor, A.K.; Ramana, D. Quantitation of Epsilon-Amino Group Using Amino-Acids as Reference-Standards by Trinitrobenzene Sulfonic-Acid—A Simple Spectrophotometric Method for the Estimation of Hapten to Carrier Protein Ratio. J. Immunol. Methods 1994, 167, 121–127. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, X.H. Enzyme bioreactors. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Elsevier: Waterloo, ON, Canada, 2011; pp. 319–329. [Google Scholar]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Mota, M.V.; Tavares, P.; Pereira, A.; Gonçalves, M.P.; Torres, D.; Rocha, C.; Teixeira, J.A. Preparation of ingredients containing an ACE-inhibitory peptide by tryptic hydrolysis of whey protein concentrates. Int. Dairy J. 2007, 17, 481–487. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Ferraz, A.; Baeza, J.; Rodriguez, J.; Freer, J. Estimating the chemical composition of biodegraded pine and eucalyptus wood by DRIFT spectroscopy and multivariate analysis. Bioresour. Technol. 2000, 74, 201–212. [Google Scholar] [CrossRef]
- Santi, C.J.; Milagres, A.M.F.; Ferraz, A.; Carvalho, W. The effects of lignin removal and drying on the porosity and enzymatic hydrolysis of sugarcane bagasse. Cell. Chem. Technol. 2013, 20, 3165–3177. [Google Scholar]
- Stone, J.E.; Scallan, M.A. Structural model for the cell wall of water-swollen wood pulp fibres based on their accessibility to macromolecules. Cell. Chem. Technol. 1968, 2, 343–358. [Google Scholar]
- Guisan, J.M. Aldehyde-Agarose Gels as Activated Supports for Immobilization-Stabilization of Enzymes. Enzyme Microb. Technol. 1988, 10, 375–382. [Google Scholar] [CrossRef]
- Nevell, T.P. Methods in Carbohydrate Chemistry; Whistler, B., Ed.; Academic Press: New York, NY, USA, 1963; pp. 210–225. [Google Scholar]
- Fernandez-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Santana, C.; Soler, G.; Bastida, A.; Guisán, J.M. Preparation of activated supports containing low pK amino groups. A new tool for protein immobilization via the carboxyl coupling method. Enzyme Microb. Technol. 1993, 15, 546–550. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Alonso-Morales, N.; Dellamora, G.; Mateo, C.; Fernandez-Lafuente, R.; Guisán, J.M. Glutaraldehyde in protein immobilization. In Immobilization of Enzymes and Cells, 2nd ed.; Guisan, J.M., Ed.; Human Press Inc.: Totowa, NJ, USA, 2006; pp. 57–64. [Google Scholar]
- Mateo, C.; Bolivar, J.M.; Godoy, C.A.; Rocha-Martin, J.; Pessela, B.C.; Curiel, J.A.; Muñoz, R.; Guisán, J.M.; Fernández-Lorente, G. Improvement of Enzyme Properties with a Two-Step Immobilizaton Process on Novel Heterofunctional Supports. Biomacromolecules 2010, 11, 3112–3117. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Esposito, T.S.; Marcuschi, M.; Ribeiro, K.; Cavalli, R.O.; Oliveira, V.; Bezerra, R.S. Purification and partial characterisation of a trypsin from the processing waste of the silver mojarra (Diapterus rhombeus). Food Chem. 2011, 129, 777–782. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.E.; Chen, L.L. The Lowry Modification of the Folin Reagent for Determination of Proteinase Activity. Anal. Biochem. 1965, 10, 175–177. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, A.C.; Draghetta, W.; Del Lamma, S.N.; Camargo, A.C.; Greene, L.J. A convenient manual trinitrobenzenesulfonic acid method for monitoring amino acids and peptides in chromatographic column effluents. Anal. Biochem. 1979, 96, 317–321. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2000; p. 668. [Google Scholar]

| Sample | Treatment Yield (%) | Chemical Composition a | Mass Balance b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Lignin | Glucan | Xilan | Arabinosyl Groups | Acetyl Groups | Extratives c | Total Lignin | Glucan | Xilan | Arabinosyl Groups | Acetyl Groups | Extratives c | ||
| Untreated | 100 | 12.73 ± 0.03 | 28.02 ± 0.05 | 24.16 ± 0.16 | 7.15 ± 0.02 | 2.32 ± 0.02 | 6.12 | 12.73 ± 0.03 | 28.02 ± 0.05 | 24.16 ± 0.16 | 7.15 ± 0.02 | 2.32 ± 0.02 | 6.12 |
| Steam/Alkali | 52.6 ± 0.02 | 8.01 ± 0.18 | 47.62 ± 0.87 | 22.08 ± 0.20 | 7.43 ± 0.01 | 0.03 ± 0.03 | 0.97 | 4.21 ± 0.02 | 25.04 ± 0.03 | 11.61 ± 0.08 | 3.91 ± 0.01 | 0.01 ± 0.01 | 0.51 |
| Derivatives | IY a (%) | EA b (%) | OpH c | OT d (°C) | Half-Lifetime (h) | SF e |
|---|---|---|---|---|---|---|
| Trypsin-glyoxyl-AG * | 82.56 | 74.3 | 9.0 | 50 | 212 | 1156 |
| Trypsin-glyoxyl-CCP ** | 85.34 | 75.4 | 9.0 | 60 | 200 | 1090 |
| Trypsin-glutaraldehyde-AG | 81.93 | 79.7 | 9.0 | 45 | 0.8 | 4.36 |
| Trypsin-glutaraldehyde-CCP | 94.61 | 74.2 | 8.5 | 45 | 0.97 | 5.27 |
| Trypsin-IDA-glyoxyl-AG | 84.70 | 73.1 | 9.0 | 55 | 110 | 600 |
| Trypsin-IDA-glyoxyl-CCP | 83.33 | 75 | 9.0 | 55 | 162 | 883 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassan, J.C.; De Souza Bezerra, T.M.; Peixoto, G.; Da Cruz, C.Z.P.; Galán, J.P.M.; Vaz, A.B.d.S.; Garrido, S.S.; Filice, M.; Monti, R. Immobilization of Trypsin in Lignocellulosic Waste Material to Produce Peptides with Bioactive Potential from Whey Protein. Materials 2016, 9, 357. https://doi.org/10.3390/ma9050357
Bassan JC, De Souza Bezerra TM, Peixoto G, Da Cruz CZP, Galán JPM, Vaz ABdS, Garrido SS, Filice M, Monti R. Immobilization of Trypsin in Lignocellulosic Waste Material to Produce Peptides with Bioactive Potential from Whey Protein. Materials. 2016; 9(5):357. https://doi.org/10.3390/ma9050357
Chicago/Turabian StyleBassan, Juliana Cristina, Thaís Milena De Souza Bezerra, Guilherme Peixoto, Clariana Zanutto Paulino Da Cruz, Julián Paul Martínez Galán, Aline Buda dos Santos Vaz, Saulo Santesso Garrido, Marco Filice, and Rubens Monti. 2016. "Immobilization of Trypsin in Lignocellulosic Waste Material to Produce Peptides with Bioactive Potential from Whey Protein" Materials 9, no. 5: 357. https://doi.org/10.3390/ma9050357
APA StyleBassan, J. C., De Souza Bezerra, T. M., Peixoto, G., Da Cruz, C. Z. P., Galán, J. P. M., Vaz, A. B. d. S., Garrido, S. S., Filice, M., & Monti, R. (2016). Immobilization of Trypsin in Lignocellulosic Waste Material to Produce Peptides with Bioactive Potential from Whey Protein. Materials, 9(5), 357. https://doi.org/10.3390/ma9050357







