Morphological, Rheological, and Mechanical Properties of Polyamide 6/Polypropylene Blends Compatibilized by Electron-Beam Irradiation in the Presence of a Reactive Agent
Abstract
:1. Introduction
2. Results and Discussion
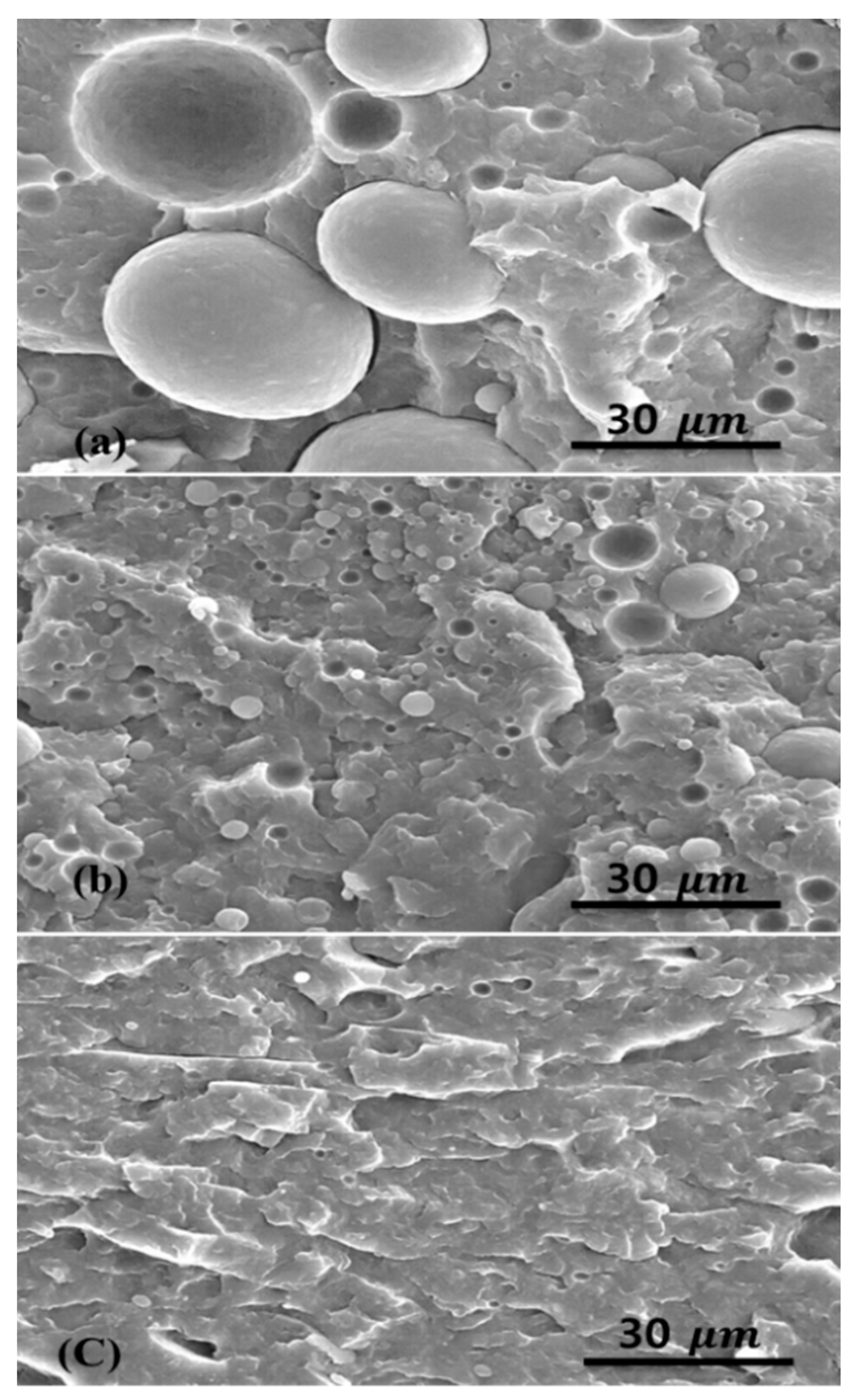
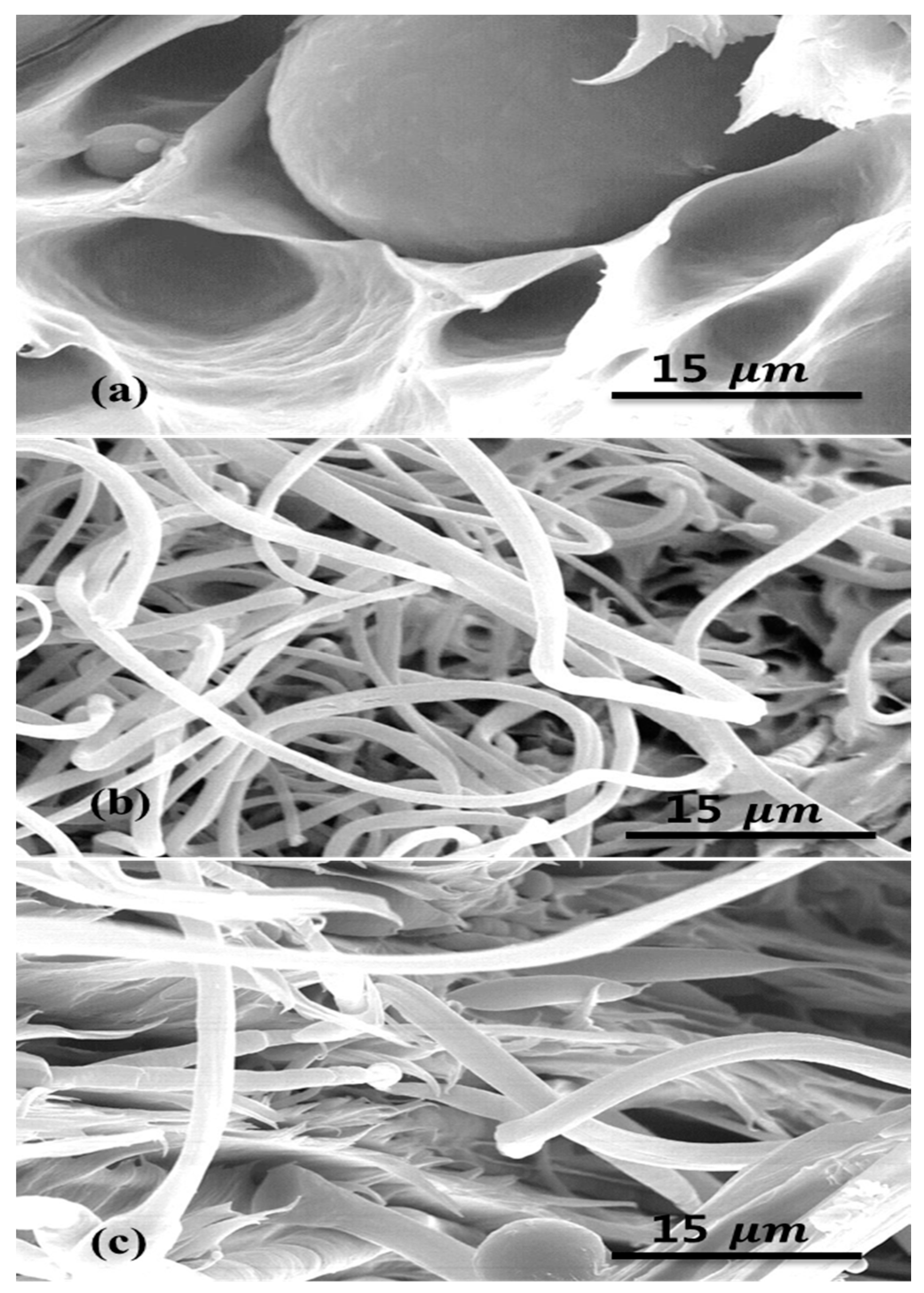
2.1. Morphology
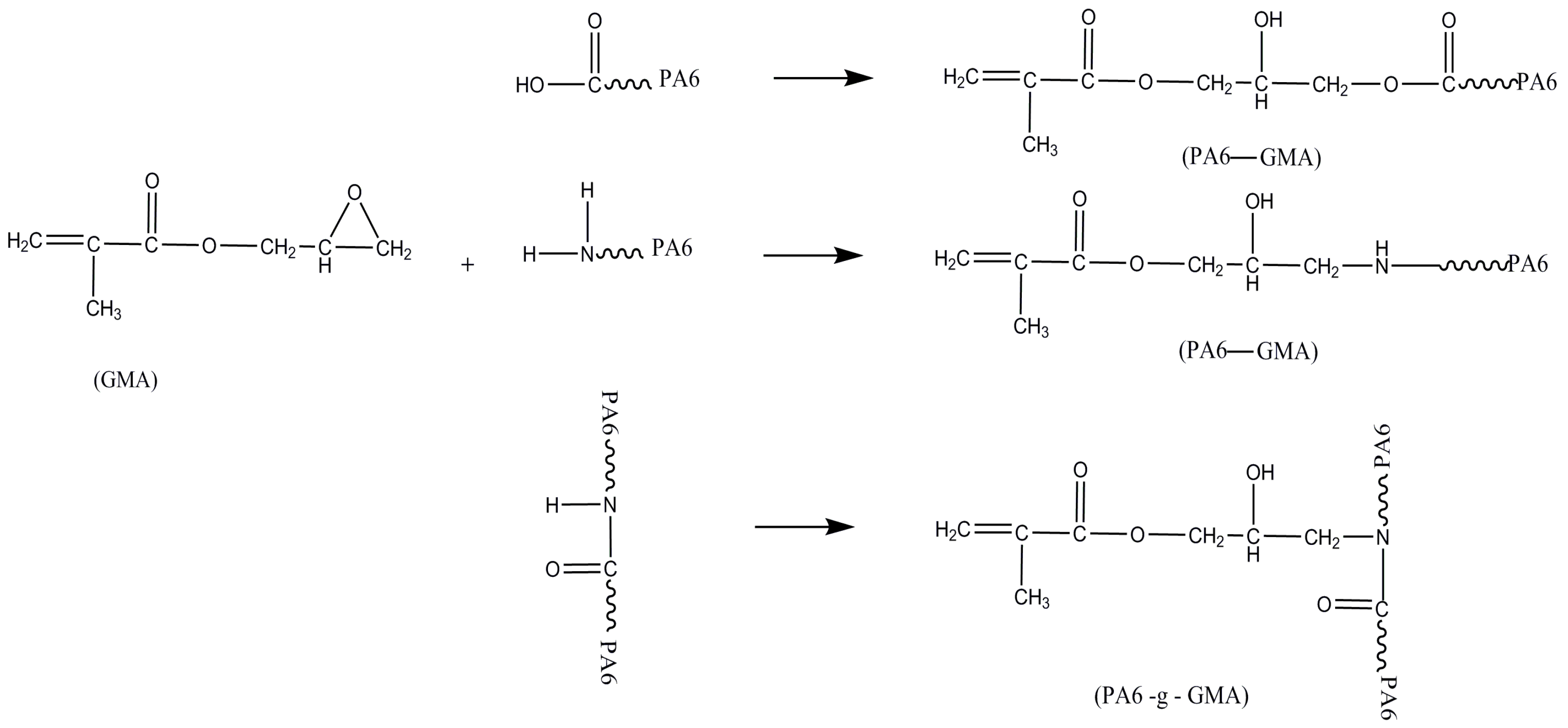
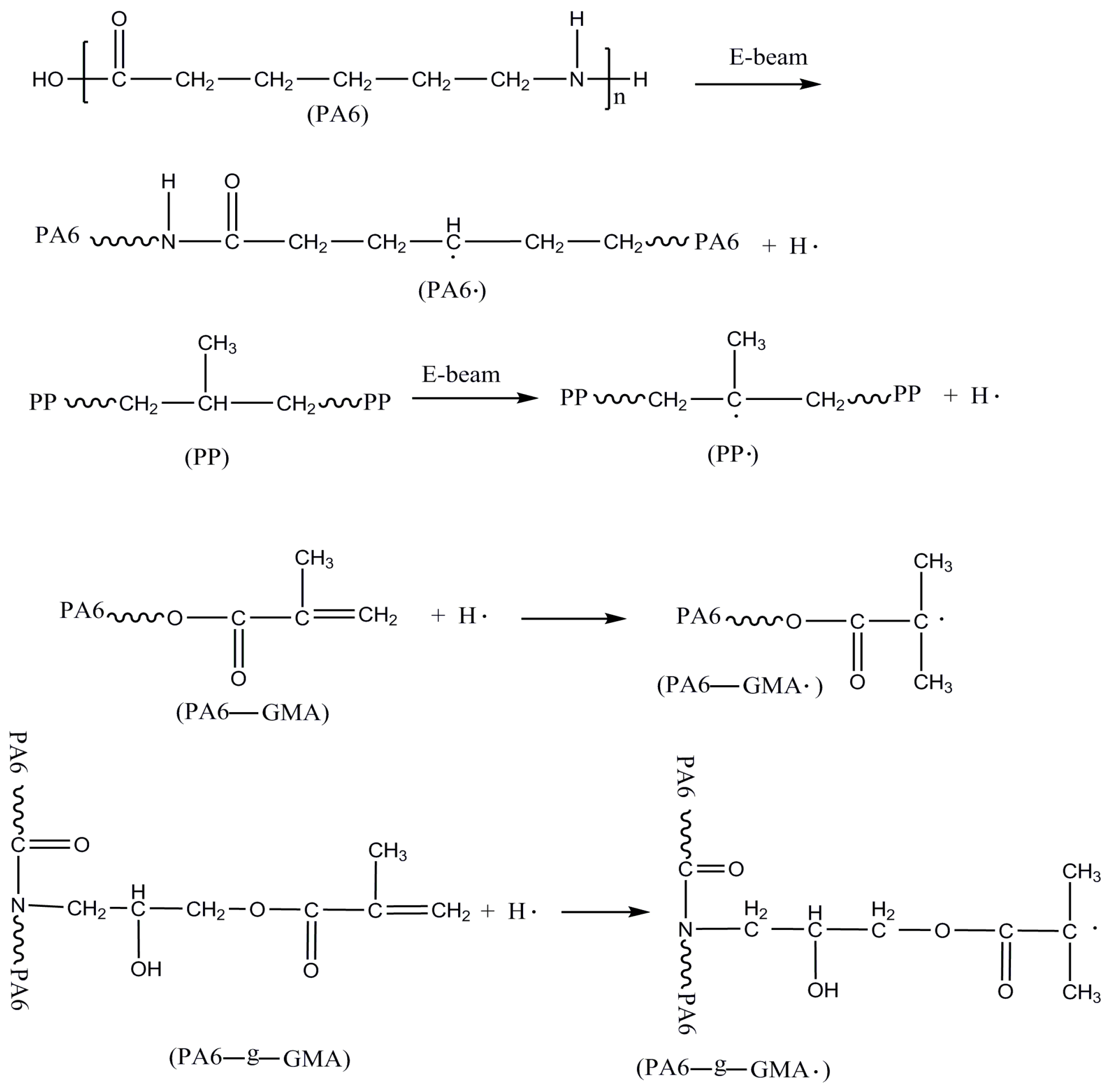
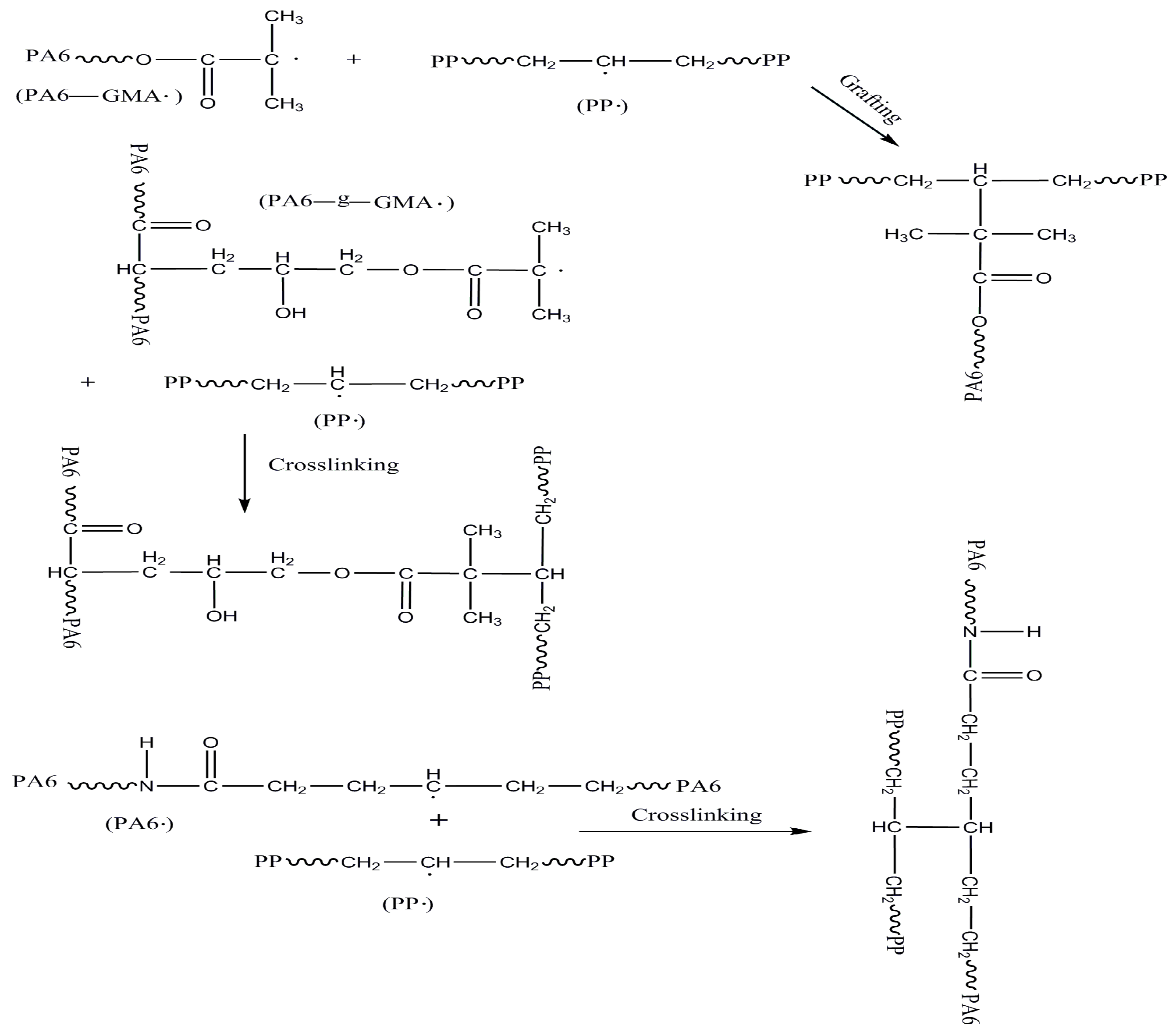
2.2. Mechanisms of PA6/PP Compatibilization
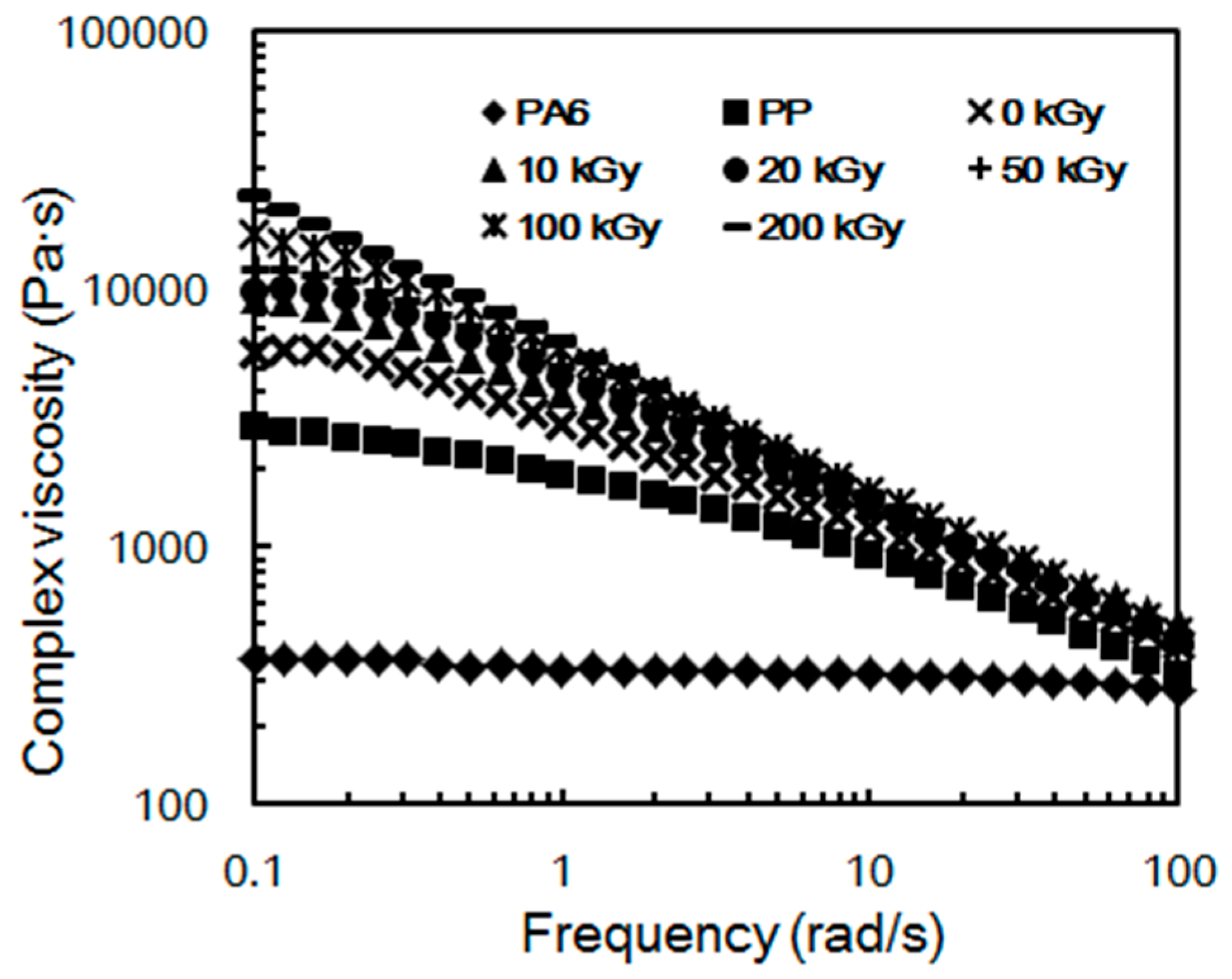
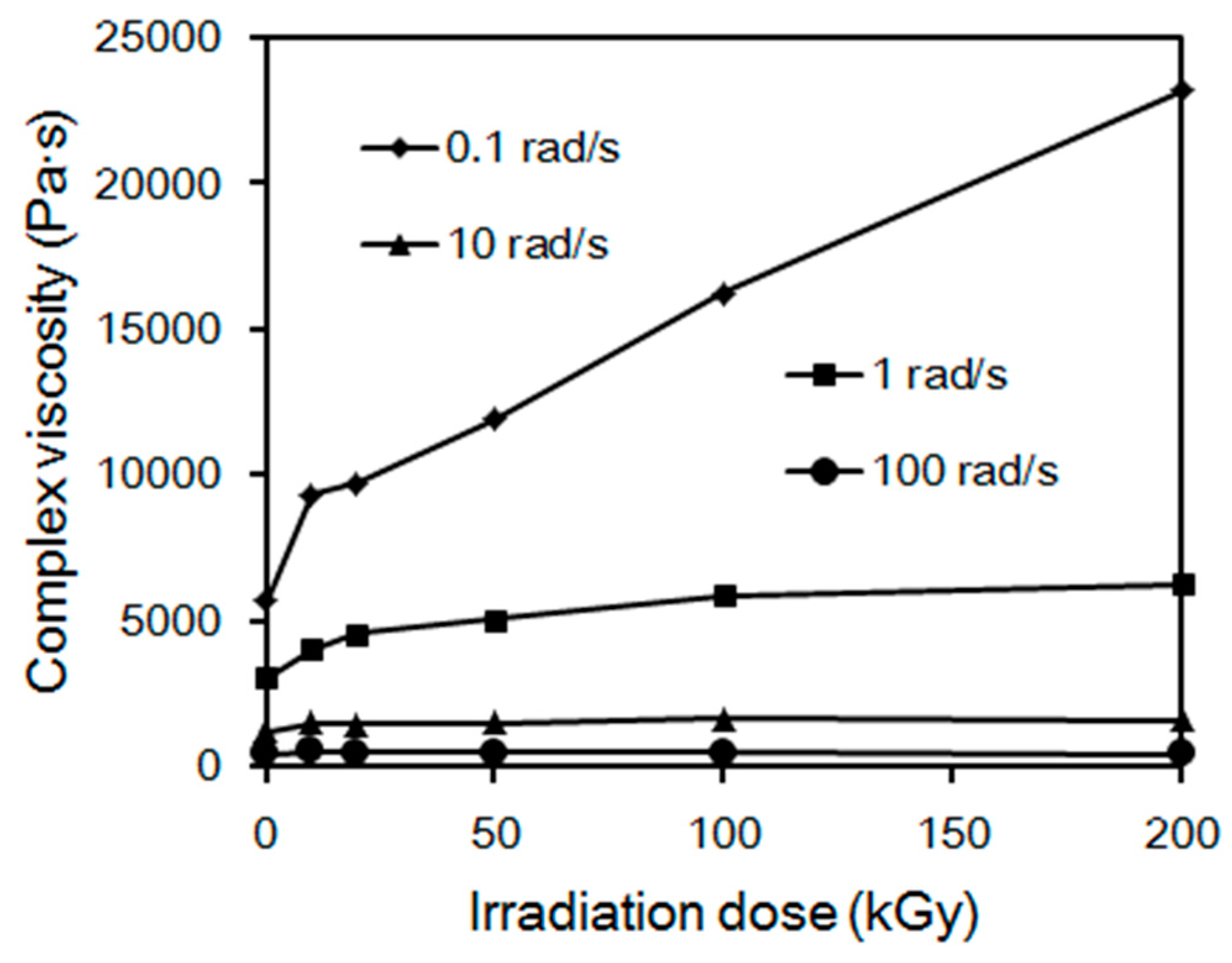
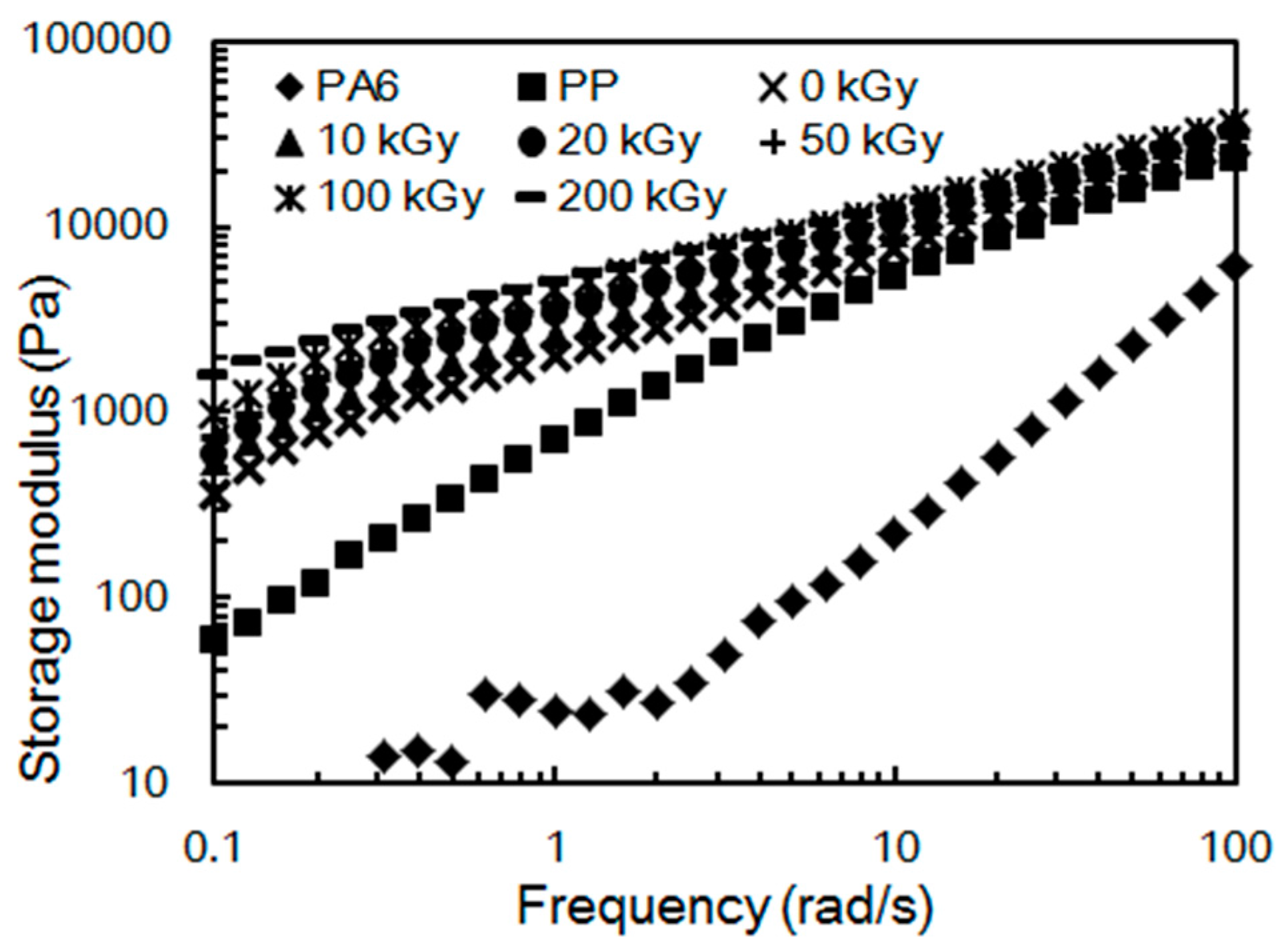
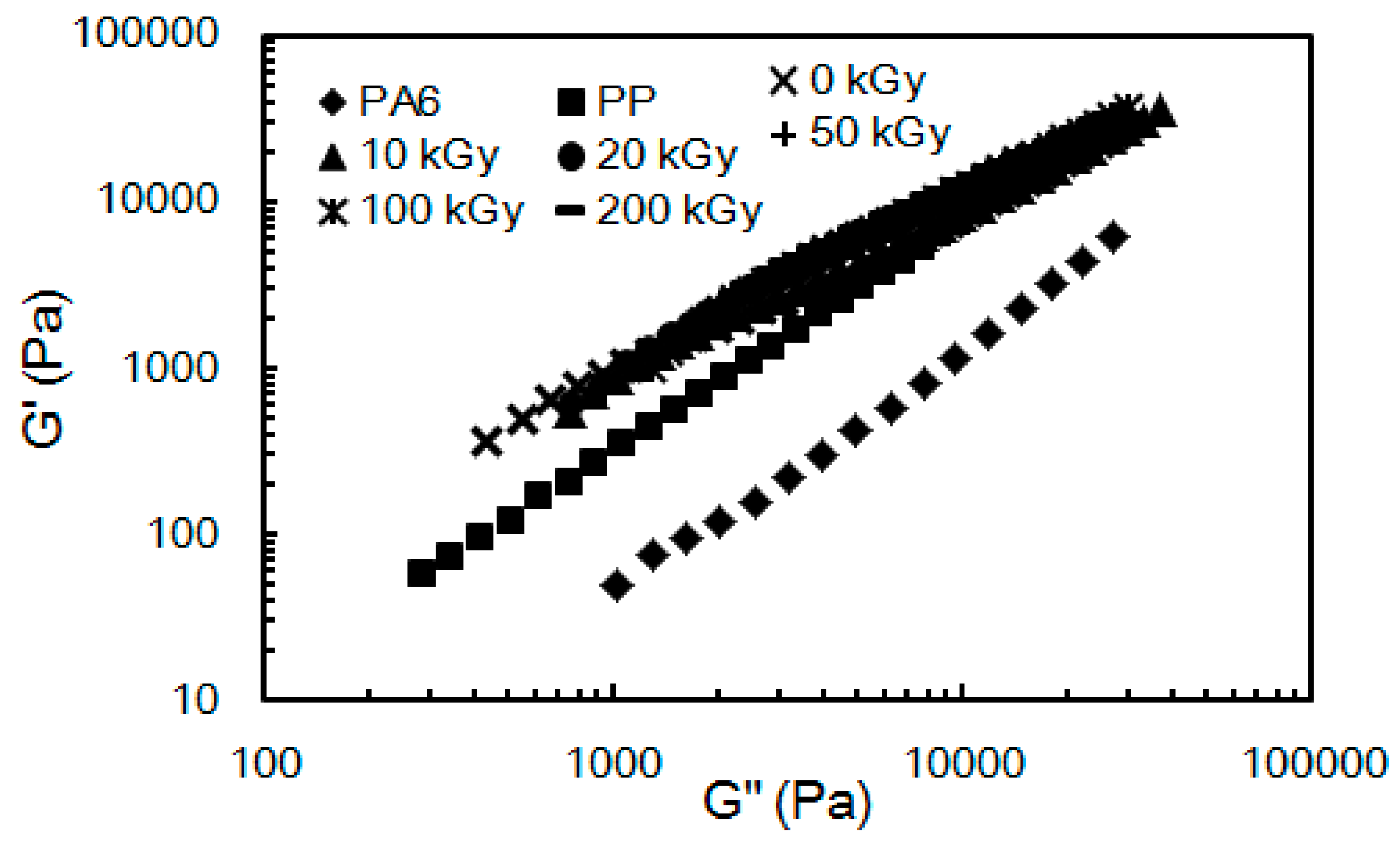
2.3. Rheological Properties
2.4. Mechanical Properties
3. Experimental
3.1. Materials
3.2. Melt Mixing of PA6, PP, and GMA
3.3. Electron-Beam Irradiation
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akkapeddi, M.K. Commercial Polymer Blends. In Polymer Blends Handbook; Utracki, L.A., Ed.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2002; Volume 2, pp. 1023–1115. [Google Scholar]
- Bai, S.L.; Wang, G.T.; Hiver, J.M.; G’Sell, C. Microstructures and mechanical properties of polypropylene/polyamide 6/polyethylene-octene elastomer blends. Polymer 2004, 45, 3063–3071. [Google Scholar] [CrossRef]
- Dadbin, S.; Frounchi, M.; Goudarzi, D. Electron beam induced crosslinking of nylon 6 with and without the presence of TAC. Polym. Degrad. Stab. 2005, 89, 436–441. [Google Scholar] [CrossRef]
- Sacchi, A.; Landro, L.D.; Pegoraro, M.; Severini, F. Morphology of isotactic propylene-polyamide 66 blends and their mechanical properties. Eur. Polym. J. 2004, 40, 1705–1713. [Google Scholar] [CrossRef]
- Piglowski, J.; Gancarz, I.; Walžlak, M.; Kammer, H.W. Crystallization in modified blends of polyamide and polypropylene. Polymer 2000, 41, 6813–6824. [Google Scholar] [CrossRef]
- Tseng, F.P.; Lin, J.J.; Tseng, C.R.; Chang, F.C. Poly(oxypropylene)-amide grafted polypropylene as novel compatibilizer for PP and PA6 blends. Polymer 2001, 42, 713–725. [Google Scholar] [CrossRef]
- Laoutid, F.; Francois, D.; Paint, Y.; Bonnaud, L.; Dubois, P. Using nanosilica to fine-tune morphology and properties of polyamide 6/poly(propylene) blends. Macromol. Mater. Eng. 2013, 298, 328–338. [Google Scholar] [CrossRef]
- Park, J.S.; Lim, Y.M.; Nho, Y.C. Preparation of high density polyethylene/waste polyurethane blends compatibilized with polyethylene-graft-maleic anhydride by radiation. Materials 2015, 8, 1626–1635. [Google Scholar] [CrossRef]
- Singh, A.; Bahari, K. Use of High-energy Radiation in Polymer Blends. In Polymer Blends Handbook; Utracki, L.A., Ed.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2002; Volume 2, pp. 757–859. [Google Scholar]
- Dong, W.; Chen, G.; Zhang, W. Radiation effects on the immiscible polymer blend of nylon1010 and high-impact strength polystyrene (II): Properties and morphology. Radiat. Phys. Chem. 2001, 60, 629–635. [Google Scholar] [CrossRef]
- Adem, E.; Burillo, G.; Borja, M.A.; Carreón, M.P. Radiation compatibilization of polyamide 6/polypropylene blends, enhanced by the presence of compatibilizing agent. Nucl. Instrum. Meth. Phys. Res. B 2005, 236, 295–300. [Google Scholar] [CrossRef]
- Shin, B.Y.; Han, D.H. Morphological and mechanical properties of polyamide 6/linear Low density polyethylene blend compatibilized by electron-beam initiated mediation process. Radiat. Phys. Chem. 2014, 83, 198–207. [Google Scholar] [CrossRef]
- Chen, C.C.; Chueh, J.Y.; Tseng, H.; Huang, H.M.; Lee, S.Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Jeong, B.J.; Xanthos, M. Reactive modification of PBT with applications in low density extrusion foaming. Polym. Eng. Sci. 2007, 47, 244–253. [Google Scholar] [CrossRef]
- Maccosko, C.W.; Jeon, H.K.; Hoye, T.R. Reactions at polymer-polymer interfaces for blend compatibilization. Prog. Polym. Sci. 2005, 30, 939–947. [Google Scholar] [CrossRef]
- Konig, C.; Duin, M.V.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Huang, J.W.; Chang, C.C.; Kang, C.C.; Yeh, M.Y. Crystallization kinetics and nucleation paramerters of Nylon 6 and poly(ethylene-co-glycidyl methacrylate) blend. Thermochim. Acta 2007, 468, 66–74. [Google Scholar] [CrossRef]
- Shin, B.Y.; Kim, J.H. Rheological and mechanical properties of polyamide 6 modified by electron-beam initiated mediation process. Radiat. Phys. Chem. 2015, 112, 88–96. [Google Scholar] [CrossRef]
- Kondyurin, A.; Bilek, M. Ion Beam Treatment of Polymers; ELSEVIER: Oxford, UK, 2008; pp. 29–73. [Google Scholar]
- Bhattacharya, A.; Ray, P. Basic Features and Technique. In Polymer Grafting and Crosslinking; Bhattacharya, A., Rawlins, J.W., Ray, P., Eds.; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 2008; pp. 7–64. [Google Scholar]
- Harrell, E.R.; Nakajima, N. Modified Cole–Cole plot based on viscoelastic properties for characterizing molecular architecture of elastomers. J. Appl. Polym. Sci. 1984, 29, 995–1010. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, B.C.; Kim, S.H. Structural effect of linear and star-shaped poly(lactic acid) on physical properties. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 939–946. [Google Scholar] [CrossRef]
- Scaffaro, R.; La Mantia, F.P.; Botta, L.; Morreale, N.; Dintcheva, N.T.; Mariani, P. Competition between chain scission and branching formation in the processing of high density polyethylene: Effect of processing parameters and of stabilizers. Polym. Eng. Sci. 2009, 49, 1316–1325. [Google Scholar] [CrossRef]
- Lee, H.G.; Sung, Y.-T.; Lee, Y.K.; Kim, W.N. Effect of PP-g-MAH on the mechanical, morphological and rheological properties of polypropylene and poly (acrylonitrile-butadiene-styrene) blends. Macromol. Res. 2009, 17, 417–423. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Rheological, morphological, and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2014, 53, 501–517. [Google Scholar] [CrossRef]
- Woods, R.J.; Pikaev, A.K. Applied Radiation Chemistry: Radiation Processing; John Wiley & Sons, Inc.: New York, NY, USA, 1994; pp. 341–391. [Google Scholar]
- Han, D.; Jang, J.; Kim, B.; Shin, B. Manufacturing and foaming of high melt viscosity of polypropylene by using electron beam radiation technology. Polym. Eng. Sci. 2006, 46, 431–437. [Google Scholar] [CrossRef]
- Ha, M.H.; Kim, M.S.; Kim, B.K.; Kim, W.; Lee, M.C.; Kim, H.D. Effects of the blending sequence in polyolefin ternary blends. J. App. Polym. Sci. 2004, 92, 804–811. [Google Scholar] [CrossRef]


| Sample | Tm1 (°C) | Xc (%) of PA6 in blend | Tm2 (℃) | Xc (%) of PA6 in Blend | Residue (%) |
|---|---|---|---|---|---|
| PA6 | – | – | 216 | 32.0 | – |
| PP | 164 | 33.1 | – | – | – |
| Blend irradiated at 0 kGy | 157 | 31.7 | 216 | 28.5 | 20.1 |
| Blend irradiated at 10 kGy | 156 | 39.5 | 216 | 28.2 | 20.5 |
| Blend irradiated at 20 kGy | 155 | 39.4 | 216 | 24.6 | 20.8 |
| Blend irradiated at 50 kGy | 155 | 26.3 | 217 | 27.8 | 20.3 |
| Blend irradiated at 100 kGy | 153 | 26.4 | 215 | 27.1 | 20.5 |
| Blend irradiated at 200 kGy | 151 | 27.0 | 214 | 27.5 | 21.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.Y.; Ha, M.H.; Han, D.H. Morphological, Rheological, and Mechanical Properties of Polyamide 6/Polypropylene Blends Compatibilized by Electron-Beam Irradiation in the Presence of a Reactive Agent. Materials 2016, 9, 342. https://doi.org/10.3390/ma9050342
Shin BY, Ha MH, Han DH. Morphological, Rheological, and Mechanical Properties of Polyamide 6/Polypropylene Blends Compatibilized by Electron-Beam Irradiation in the Presence of a Reactive Agent. Materials. 2016; 9(5):342. https://doi.org/10.3390/ma9050342
Chicago/Turabian StyleShin, Boo Young, Man Ho Ha, and Do Hung Han. 2016. "Morphological, Rheological, and Mechanical Properties of Polyamide 6/Polypropylene Blends Compatibilized by Electron-Beam Irradiation in the Presence of a Reactive Agent" Materials 9, no. 5: 342. https://doi.org/10.3390/ma9050342
APA StyleShin, B. Y., Ha, M. H., & Han, D. H. (2016). Morphological, Rheological, and Mechanical Properties of Polyamide 6/Polypropylene Blends Compatibilized by Electron-Beam Irradiation in the Presence of a Reactive Agent. Materials, 9(5), 342. https://doi.org/10.3390/ma9050342






