Specific Ion Effects in Cholesterol Monolayers
Abstract
:1. Introduction
2. Results
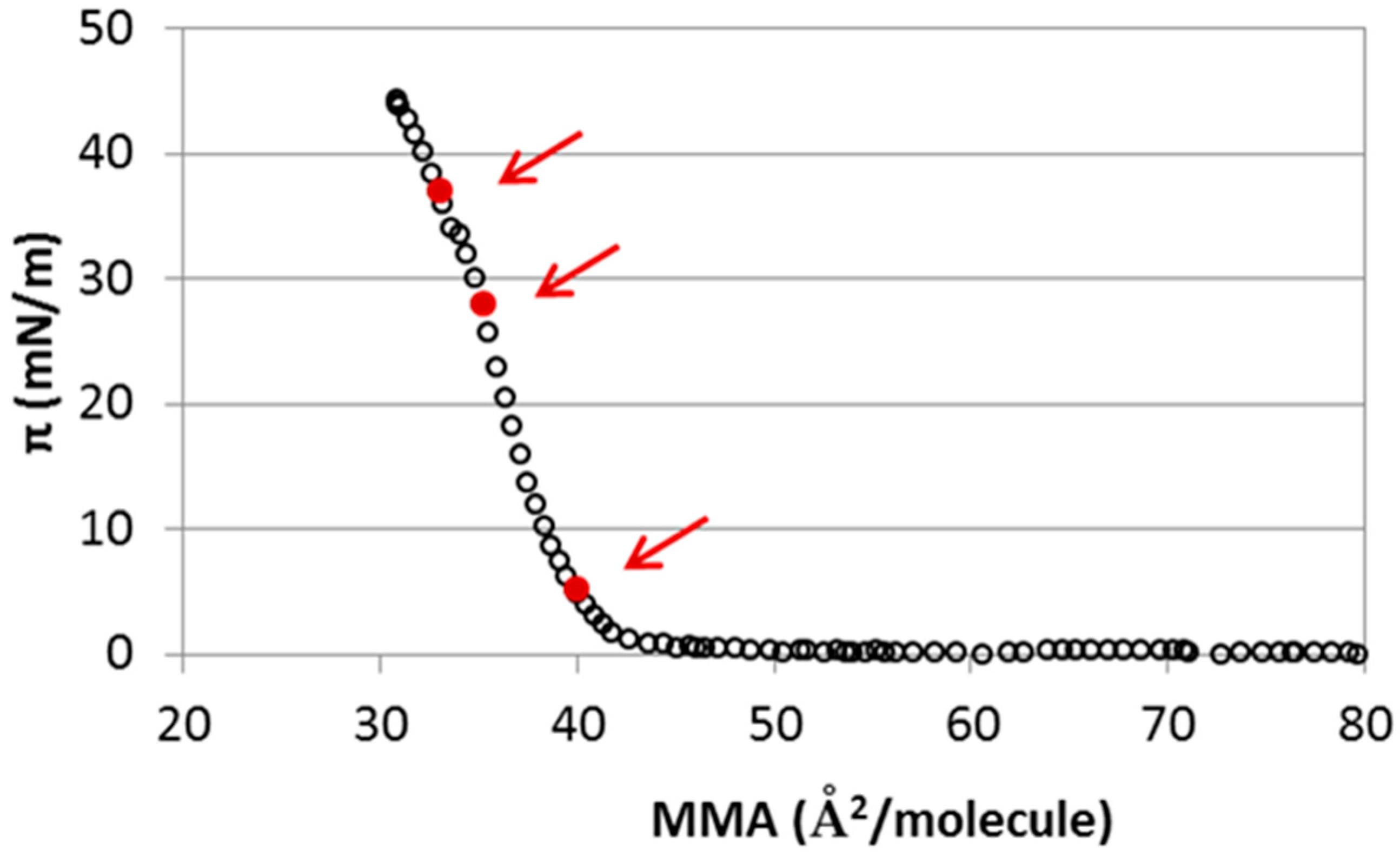
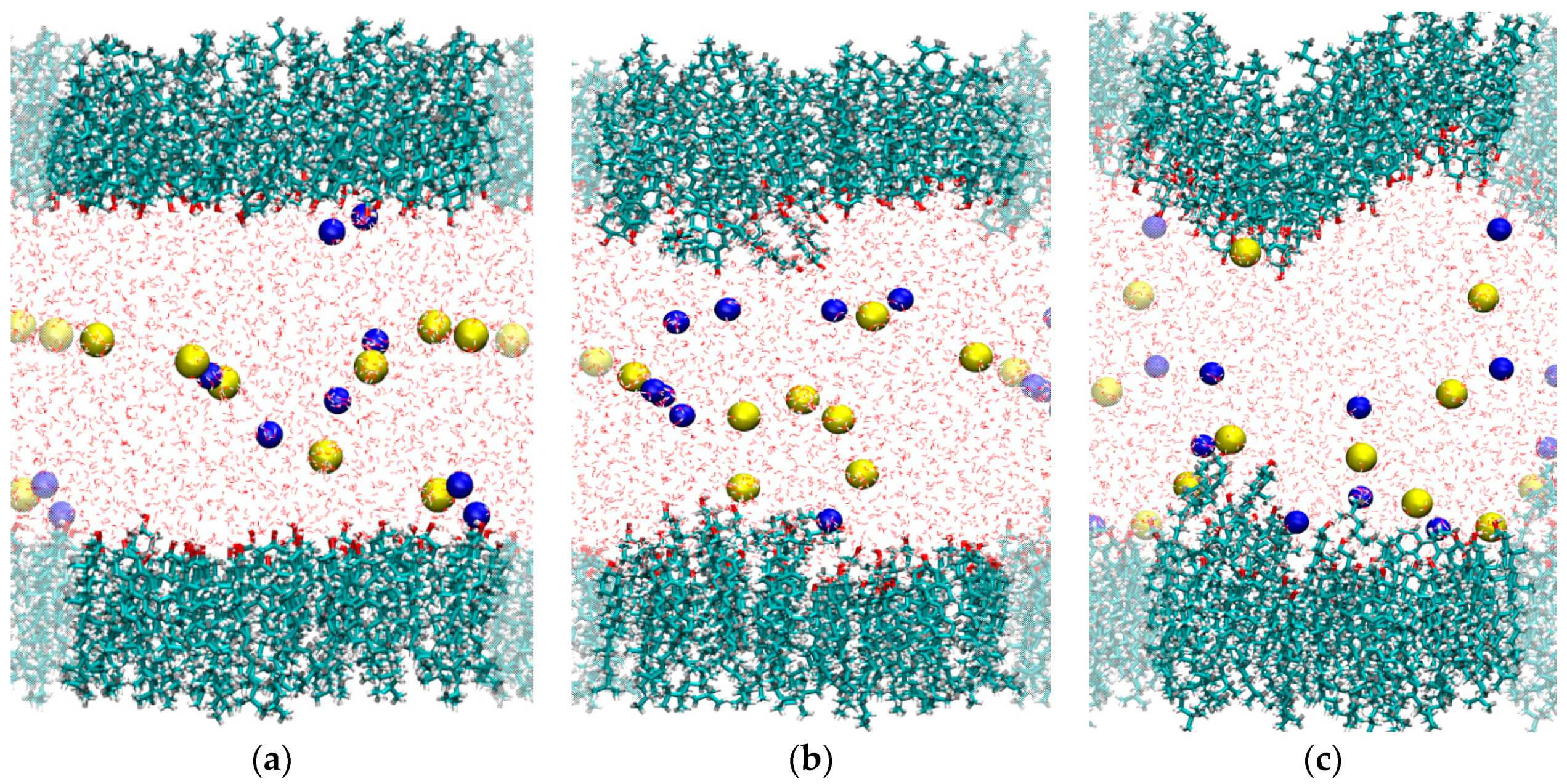
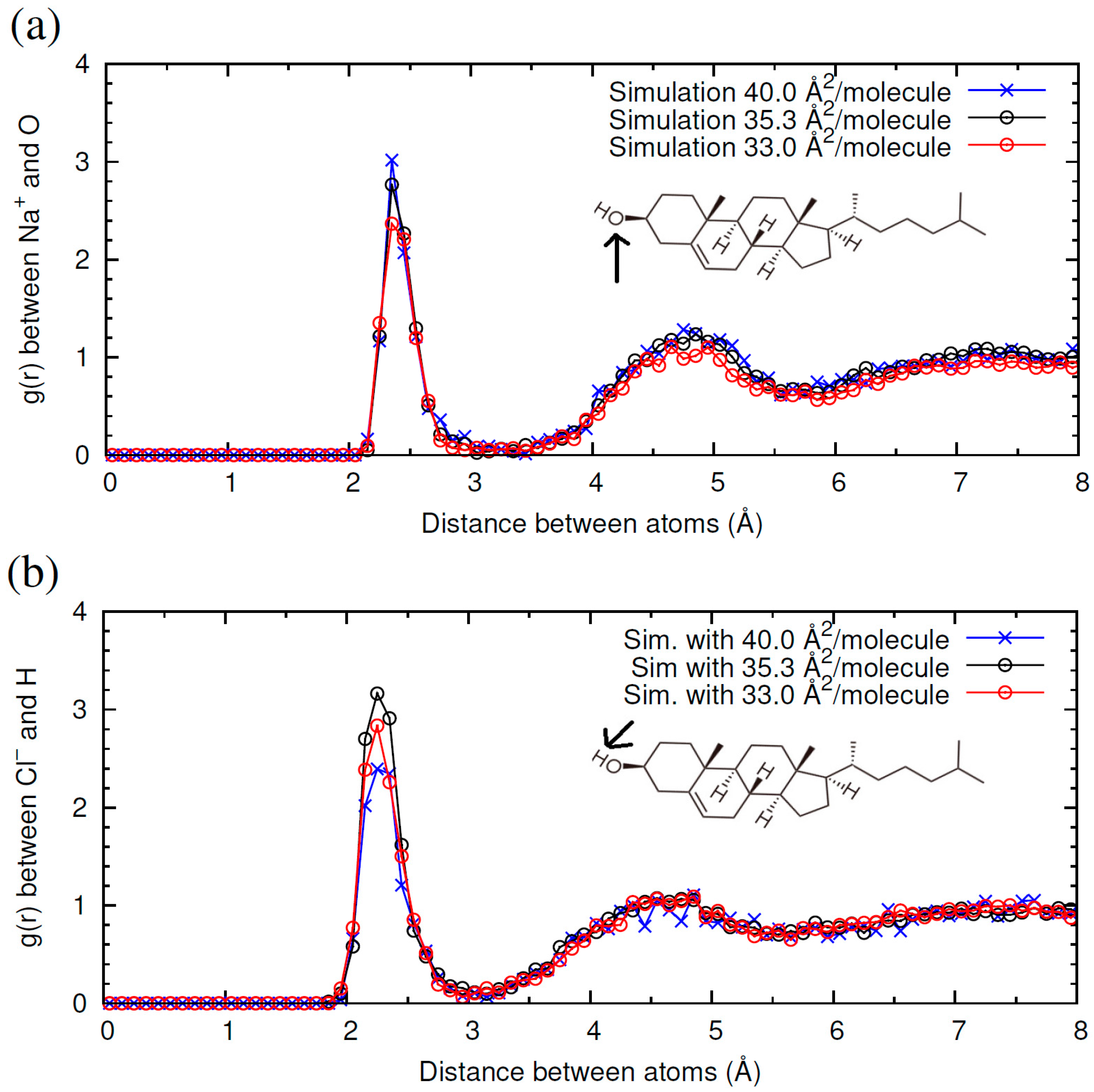
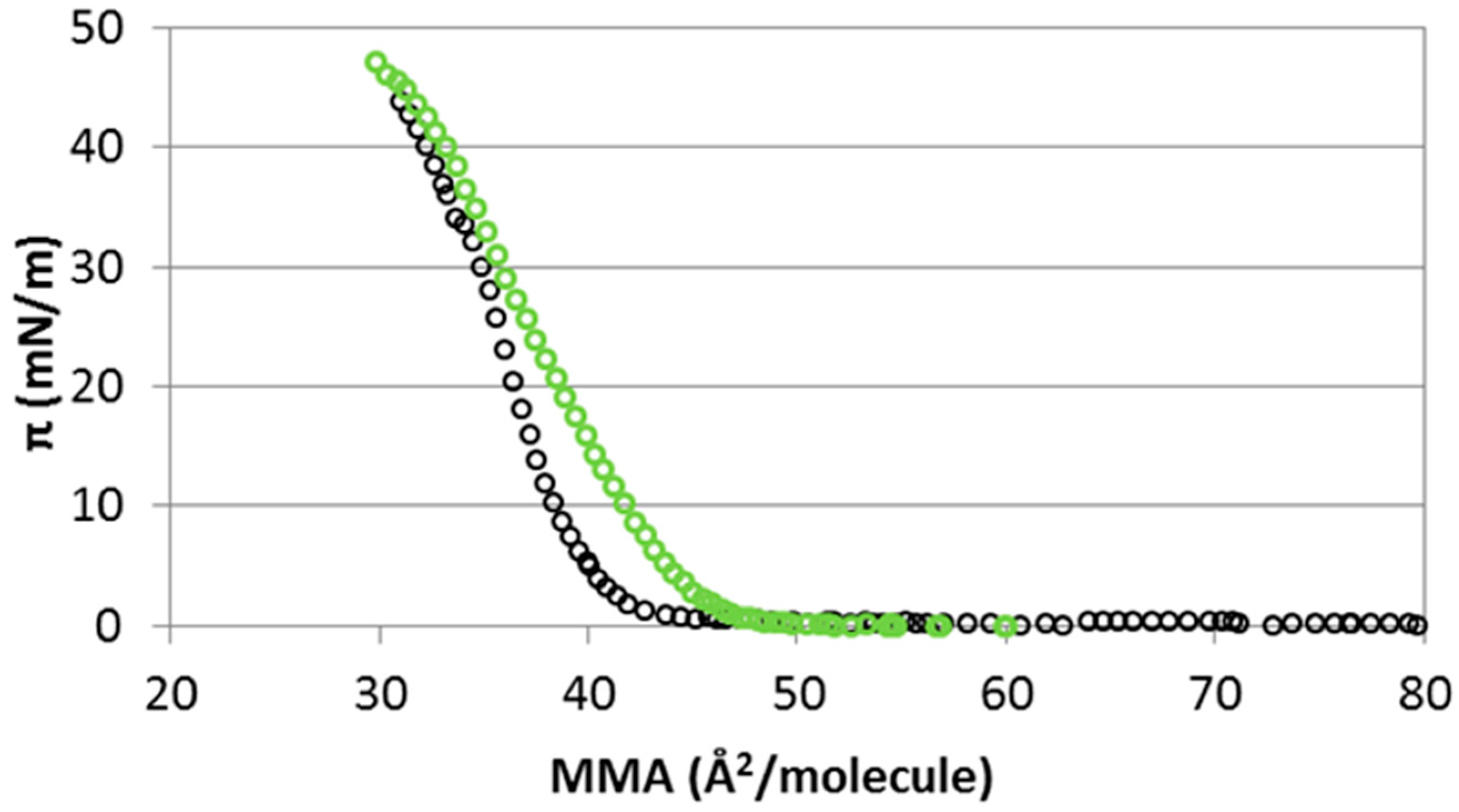
2.1. Interaction of Cholesterol Monolayers with NaCl
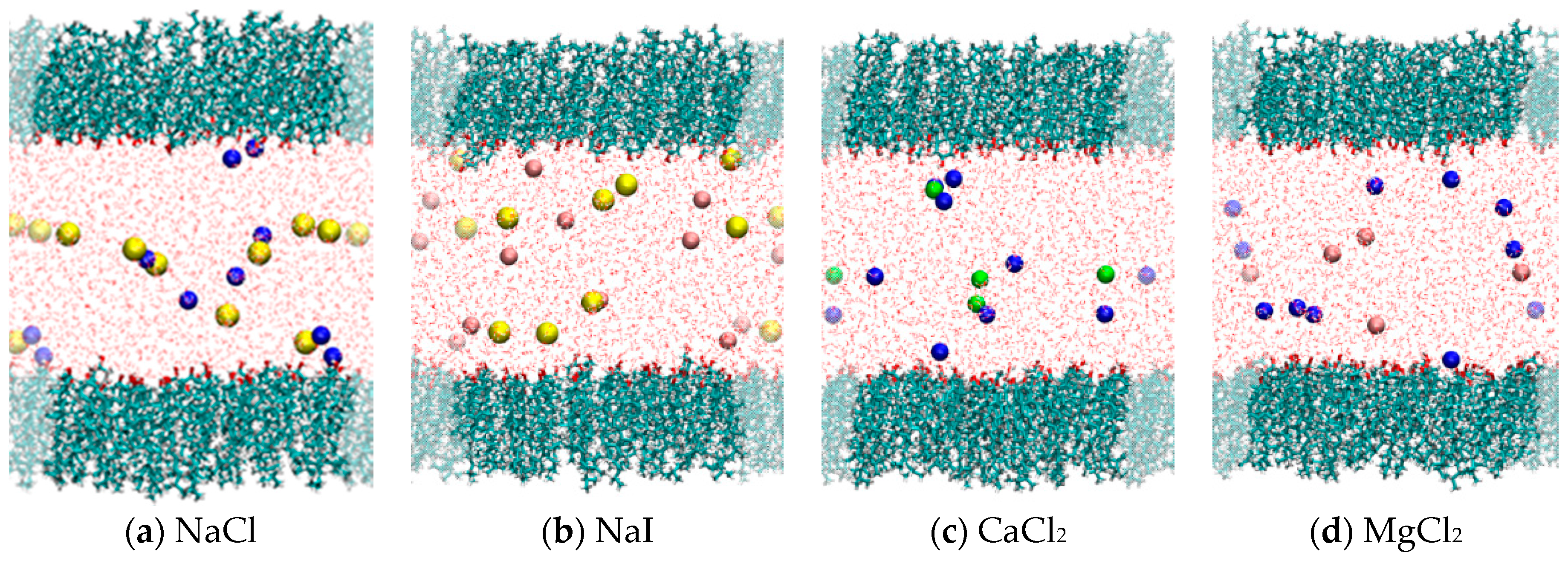
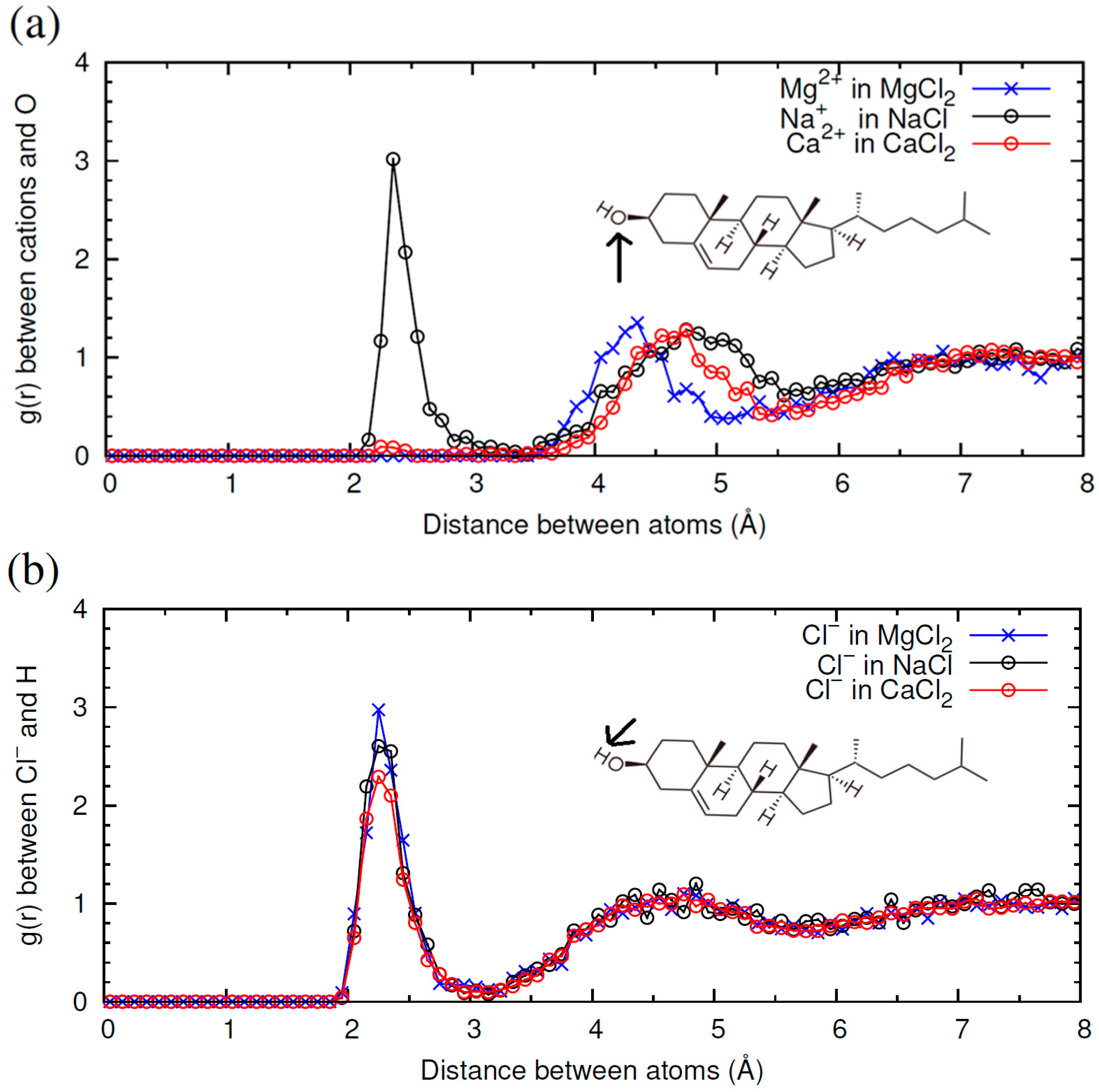
2.2. Comparison of Simulation Results for Different Electrolytes: NaI, NaCl, MgCl2 and CaCl2
2.3. Experimental Results: Interaction of CHOL Monolayers With Subphases with Different Electrolytes: NaCl, NaI, MgCl2 and CaCl2
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental
4.3. Methods for MD Simulations
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CHOL | Cholesterol molecule |
| DPPS | 1,2-Dipalmitoyl-sn-glycero-3-phosphoserine |
| MD | Molecular Dynamics Simulations |
| MMA | area per molecule |
| NVT | Molecular dynamics simulations performed at constant number of particles (N), volume (V) and temperature (T) |
| π | Surface pressure |
References
- Evans, D.F.; Wennerström, H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet; VCH Publishers: New York, NY, USA, 1999. [Google Scholar]
- Kunz, W. Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 2010, 15, 34–39. [Google Scholar] [CrossRef]
- Marcus, Y. Ions in Water and Biophysical Implications: From Chaos to Cosmos; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Martín-Molina, A.; Rodríguez-Beas, C.; Faraudo, J. Effect of calcium and magnesium on phosphatidylserine membranes: Experiments and all-atomic simulations. Biophys. J. 2012, 102, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2015. [Google Scholar]
- Levitan, I.; Barrantes, F.J. Cholesterol Regulation of Ion Channels and Receptors; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Fonollosa, J.; De Pera, M.; Estelrich, J.; De La Maza, A.; Parra, J.L. Influence of cholesterol on liposome fluidity by EPR: Relationship with percutaneous absorption. J. Control. Release 2000, 68, 85–95. [Google Scholar] [CrossRef]
- Cabrera, I.; Abasolo, I.; Corchero, J.L.; Elizondo, E.; Gil, P.R.; Moreno, E.; Faraudo, J.; Sala, S.; Bueno, D.; González-Mira, E.; et al. α-Galactosidase a loaded nanoliposomes with enhanced enzymatic activity and intracellular penetration. Adv. Healthc. Mater. 2016, 5, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Úbeda, M.; Rodríguez-Pulido, A.; Nogales, A.; Martín-Molina, A.; Aicart, E.; Junquera, E. Effect of lipid composition on the structure and theoretical phase diagrams of DC-Chol/DOPE-DNA lipoplexes. Biomacromolecules 2010, 11, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Paiva, D.; Martín-Molina, A.; Cardoso, I.; Quesada-Pérez, M.; do Carmo Pereira, M.; Rocha, S. The effect of a fluorinated cholesterol derivative on the stability and physical properties of cationic DNA vectors. Soft Matter 2013, 9, 401–409. [Google Scholar] [CrossRef]
- Ferrer-Tasies, L.; Moreno-Calvo, E.; Cano-Sarabia, M.; Aguilella-Arzo, M.; Angelova, A.; Lesieur, S.; Ricart, S.; Faraudo, J.; Ventosa, N.; Veciana, J. Quatsomes: Vesicles formed by self-assembly of sterols and quaternary ammonium surfactants. Langmuir 2013, 29, 6519–6528. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef] [PubMed]
- Ege, C.; Ratajczak, M.K.; Majewski, J.; Kjaer, K.; Lee, K.Y.C. Evidence for lipid/cholesterol ordering in model lipid membranes. Biophys. J. 2006, 91, L01–L03. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.T.; Huang, J.; Feigenson, G.W.; Nagle, J.F. Effects of cholesterol and unsaturated DOPC lipid on chain packing of saturated gel-phase DPPC bilayers. Gen. Physiol. Biophys. 2009, 28, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Finegold, L. Cholesterol in Membrane Models; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Jurak, M. Thermodynamic aspects of cholesterol effect on properties of phospholipid monolayers: Langmuir and Langmuir–blodgett monolayer study. J. Phys. Chem. B 2013, 117, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Dynarowicz-Łątka, P.; Hąc-Wydro, K. Interactions between phosphatidylcholines and cholesterol in monolayers at the air/water interface. Colloids Surfaces B 2004, 37, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Leathes, J.B. Croonian lectures on the rôle of fats in vital phenomena. Lancet 1925, 205, 853–856. [Google Scholar] [CrossRef]
- Zulueta Díaz, Y.D.L.M.; Mottola, M.; Vico, R.V.; Wilke, N.; Fanani, M.L. The rheological properties of lipid monolayers modulate the incorporation of ι-ascorbic acid alkyl esters. Langmuir 2016, 32, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Wydro, P. The interactions between cholesterol and phospholipids located in the inner leaflet of humane erythrocytes membrane (DPPE and DPPS) in binary and ternary films—The effect of sodium and calcium ions. Colloids Surfaces B 2011, 82, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Savva, M.; Acheampong, S. The interaction energies of cholesterol and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine in spread mixed monolayers at the air−water interface. J. Phys. Chem. B 2009, 113, 9811–9820. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, K.; Mattila, J.P.; Kinnunen, P.K.J. Interfacial behavior of cholesterol, ergosterol, and lanosterol in mixtures with DPPC and DMPC. Biophys. J. 2008, 95, 2340–2355. [Google Scholar] [CrossRef] [PubMed]
- Miñones, J.; Pais, S.; Miñones, J.; Conde, O.; Dynarowicz-Łatka, P. Interactions between membrane sterols and phospholipids in model mammalian and fungi cellular membranes—A Langmuir monolayer study. Biophys. Chem. 2009, 140, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Korchowiec, B.; Paluch, M.; Corvis, Y.; Rogalska, E. A Langmuir film approach to elucidating interactions in lipid membranes: 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine/cholesterol/metal cation systems. Chem. Phys. Lipids 2006, 144, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Bustos, T.; González-González, G.; Morales-Sanfrutos, J.; Megía-Fernández, A.; Santoyo-González, F.; Osuna, A. Functionalization of immunostimulating complexes (ISCOMs) with lipid vinyl sulfones and their application in immunological techniques and therapy. Int. J. Nanomedicine 2012, 7, 5941–5956. [Google Scholar] [PubMed]
- Grechishnikova, I.V.; Bergström, F.; Johansson, L.B.Å.; Brown, R.E.; Molotkovsky, J.G. New fluorescent cholesterol analogs as membrane probes. Biochim. Biophys. Acta Biomembr. 1999, 1420, 189–202. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Sinn, C.G.; Antonietti, M.; Dimova, R. Binding of calcium to phosphatidylcholine–phosphatidylserine membranes. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 410–419. [Google Scholar] [CrossRef]
- Boström, M.; Williams, D.R.M.; Ninham, B.W. Specific ion effects: The role of co-ions in biology. Europhys. Lett. 2003, 63, 610–615. [Google Scholar] [CrossRef]
- Löfgren, H.; Pascher, I. Molecular arrangements of sphingolipids. The monolayer behaviour of ceramides. Chem. Phys. Lipids 1977, 20, 273–284. [Google Scholar] [CrossRef]
- Pocivavsek, L.; Frey, S.L.; Krishan, K.; Gavrilov, K.; Ruchala, P.; Waring, A.J.; Walther, F.J.; Dennin, M.; Witten, T.A.; Lee, K.Y.C. Lateral stress relaxation and collapse in lipid monolayers. Soft Matter 2008, 4, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Feller, S.E.; MacKerell, A.D. An Improved Empirical Potential Energy Function for Molecular Simulations of Phospholipids. J. Phys. Chem. B 2000, 104, 7510–7515. [Google Scholar] [CrossRef]
- Lim, J.B.; Rogaski, B.; Klauda, J.B. Update of the cholesterol force field parameters in CHARMM. J. Phys. Chem. B 2012, 116, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.M.; Cottin-Bizonne, C.; Ybert, C.; Bocquet, L. Aqueous electrolytes near hydrophobic surfaces: Dynamic effects of ion specificity and hydrodynamic slip. Langmuir 2008, 24, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI membrane builder toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]

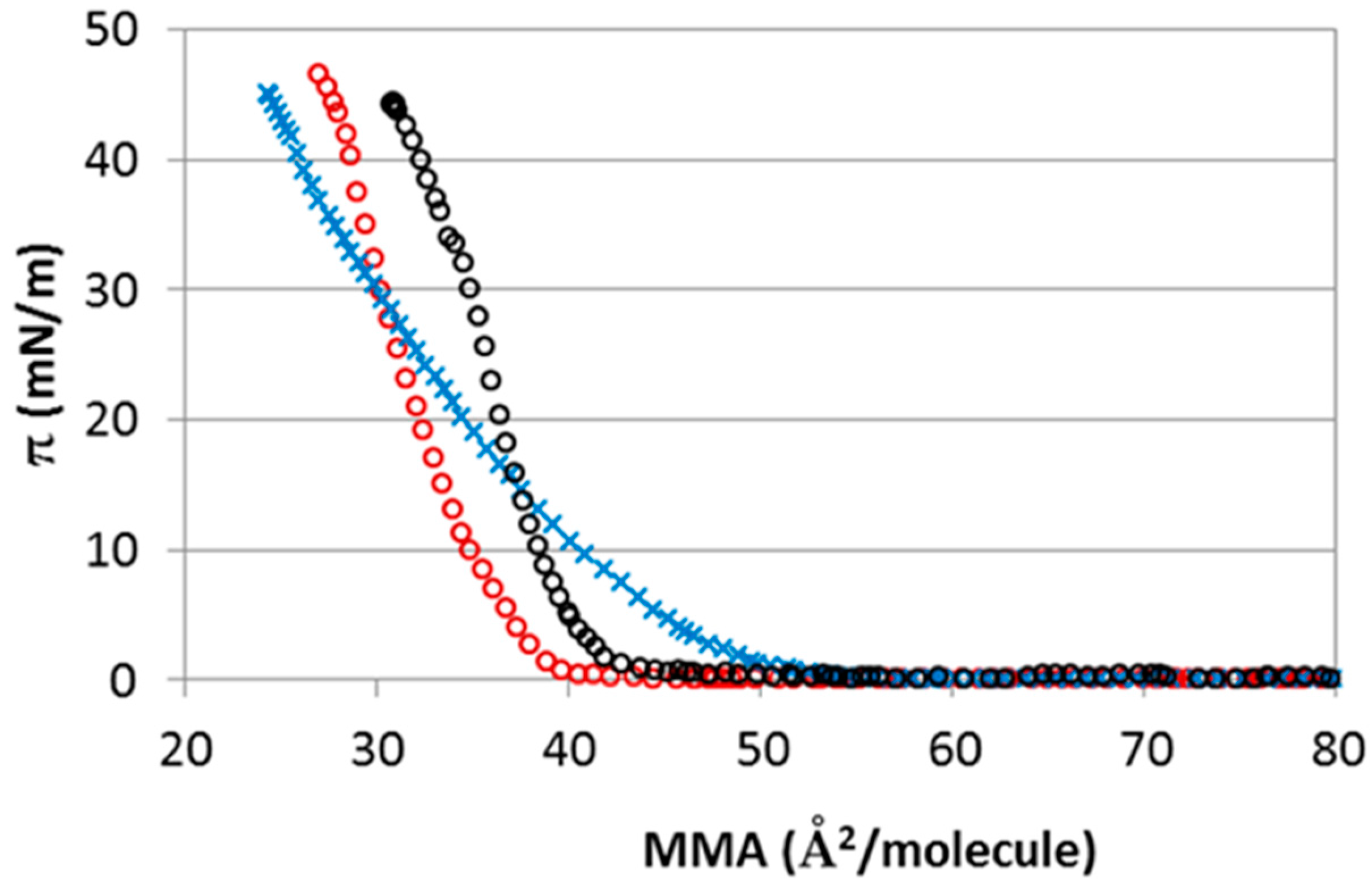
| Salts | Elasticity (mN/m) | A0 (Å2/molecule) | Πcoll (mN/m) |
|---|---|---|---|
| NaCl (15 mM) | 245 ± 5 | 40 ± 2 | 44 ± 2 |
| NaI (15 mM) | 160 ± 5 | 45 ± 2 | 46 ± 2 |
| CaCl2 (5 mM) | 230 ± 4 | 38 ± 2 | 46.0 ± 1.0 |
| MgCl2 (5 mM) | 140 ± 4 | 46 ± 2 | 46.2 ± 1.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Castillo-Santaella, T.; Maldonado-Valderrama, J.; Faraudo, J.; Martín-Molina, A. Specific Ion Effects in Cholesterol Monolayers. Materials 2016, 9, 340. https://doi.org/10.3390/ma9050340
Del Castillo-Santaella T, Maldonado-Valderrama J, Faraudo J, Martín-Molina A. Specific Ion Effects in Cholesterol Monolayers. Materials. 2016; 9(5):340. https://doi.org/10.3390/ma9050340
Chicago/Turabian StyleDel Castillo-Santaella, Teresa, Julia Maldonado-Valderrama, Jordi Faraudo, and Alberto Martín-Molina. 2016. "Specific Ion Effects in Cholesterol Monolayers" Materials 9, no. 5: 340. https://doi.org/10.3390/ma9050340
APA StyleDel Castillo-Santaella, T., Maldonado-Valderrama, J., Faraudo, J., & Martín-Molina, A. (2016). Specific Ion Effects in Cholesterol Monolayers. Materials, 9(5), 340. https://doi.org/10.3390/ma9050340









