New Cu-Free Ti-Based Composites with Residual Amorphous Matrix
Abstract
:1. Introduction
2. Results
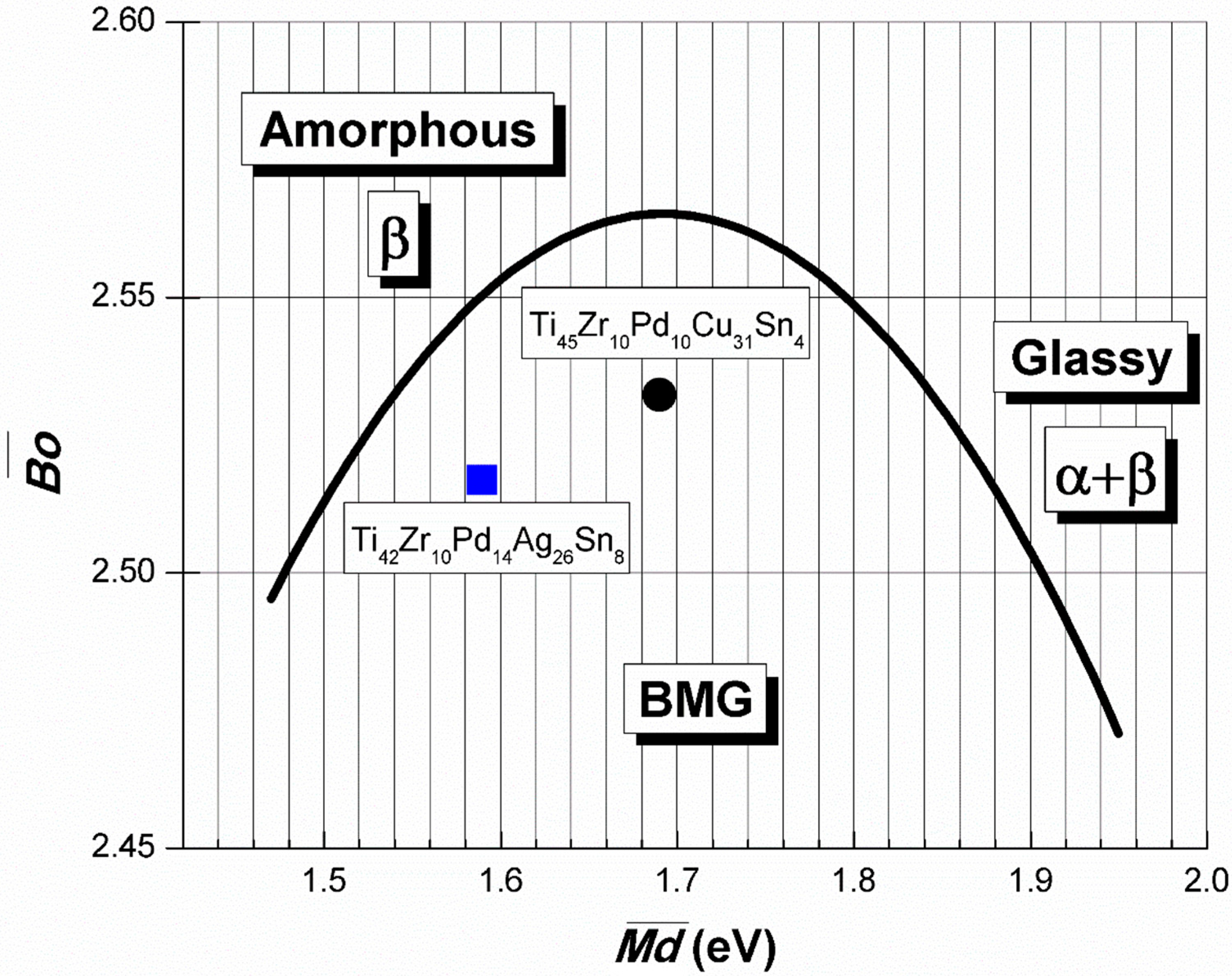
2.1. Thermodynamic Considerations Regarding the Master Alloy
2.2. Structural Characterization
2.3. XRD Studies and Thermal Characterization
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DSC | differential scanning calorimetry |
| XRD | X-ray diffraction |
| OM | optical microscopy |
| SEM | scanning electron microscopy |
| HR-TEM | high-resolution transmission electron microscopy |
| SAED | selected area diffraction pattern |
| BSE | back-scattering electrons |
| EDX/EDAX | energy-dispersive X-ray analysis |
| ICDD PDF4+ | International Center for Diffraction Data Powder Diffraction File |
Appendix A. Theoretical Design of New Alloy Composition
| Element | Bo | Md (eV) |
|---|---|---|
| Ti | 2.790 | 2.447 |
| Zr | 3.086 | 2.934 |
| Pd | 2.208 | 0.387 |
| Cu | 2.114 | 0.567 |
| Ag | 2.094 | 0.196 |
| Sn | 2.283 | 2.100 |
| Nb | 3.099 | 2.424 |
| Alloy | Ti (% at.) | Zr (% at.) | Pd (% at.) | Cu (% at.) | Ag (% at.) | Sn (% at.) | Bo | Md (eV) |
|---|---|---|---|---|---|---|---|---|
| Ti45Zr10Pd10Cu31Sn4 | 45 | 10 | 10 | 31 | – | 4 | 2.532 | 1.689 |
| Ti42Zr10Pd14Ag26Sn8 | 42 | 10 | 14 | – | 26 | 8 | 2.517 | 1.589 |
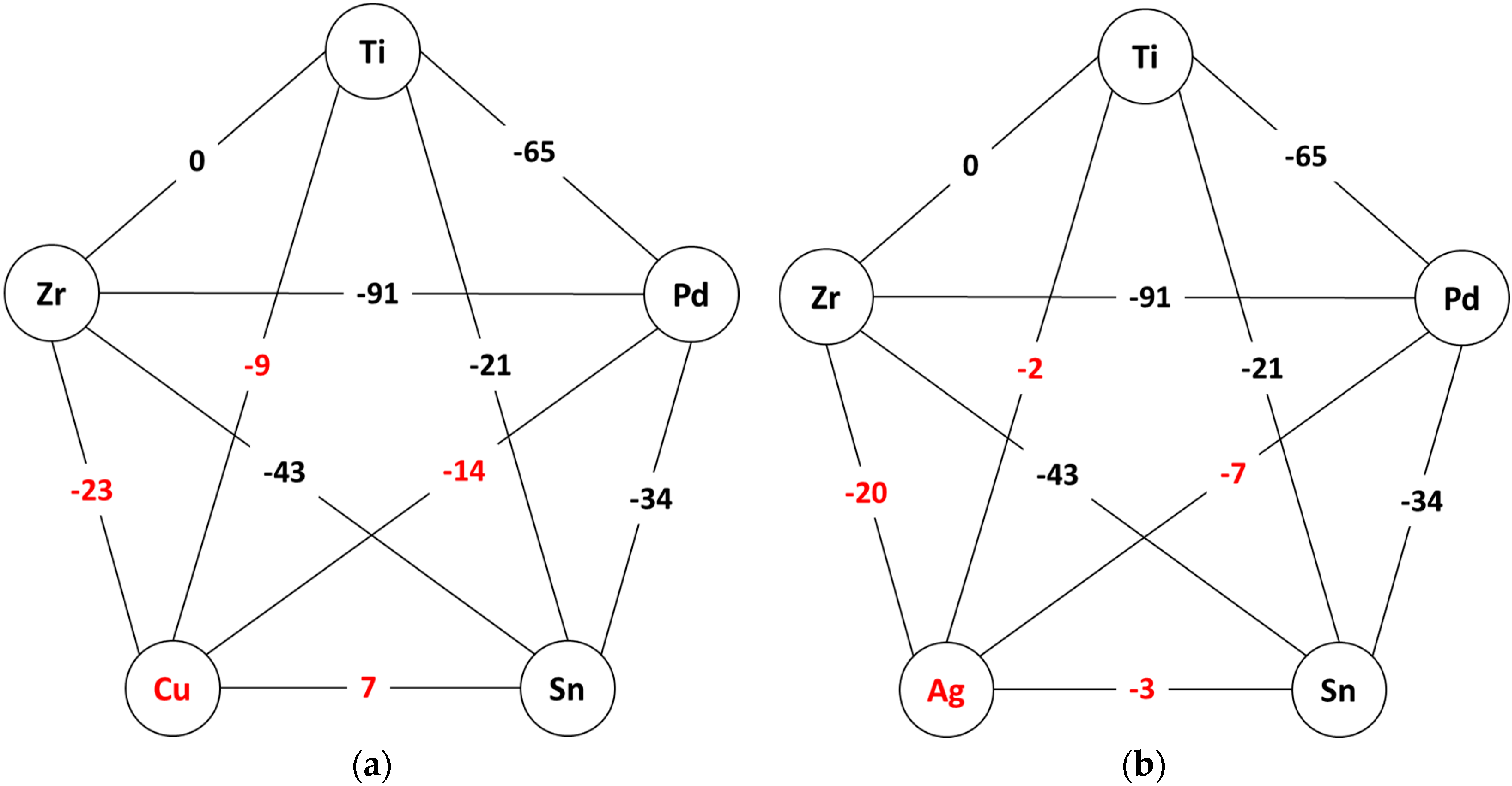
Appendix B. Biological Effect of Copper and Silver
References
- Abdel-Hady, M.G.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mat. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mat. Sci. Eng. R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Niinomi, M. Metals for Biomedical Devices; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mat. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.-H.; Wena, C. A new look at biomedical Ti-based shape memory alloys. Acta. Biomat. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.; Gebert, A.; Ghinea, A.C.; Gostin, P.F.; Abdi, S.; Mickel, C.; Eckert, J. Designing biocompatible Ti-based metallic glasses for implant applications. Mat. Sci. Eng. C 2013, 33, 875–833. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Recent Titanium R&D for Biomedical Applications in Japan. J. Min. Met. Mat. Soc. 1999, 51, 32–34. [Google Scholar]
- Greer, A.L. Metallic glasses... on the threshold. Mat. Today 2009, 12, 14–23. [Google Scholar] [CrossRef]
- Axinte, E. Metallic glasses from “alchemy” to pure science: Present and future of design, processing and applications of glassy metals. Mat. Des. 2012, 35, 518–556. [Google Scholar] [CrossRef]
- Wang, W.; Dong, C.; Shek, C. Bulk metallic glasses. Mat. Sci. Eng. R 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Eckert, J.; Das, J.; Pauly, S.; Duhamel, C. Mechanical properties of bulk metallic glasses and composites. J. Mater. Res. 2007, 22, 285–301. [Google Scholar] [CrossRef]
- Schroers, J.; Kumar, G.; Hodges, T.M.; Chan, S.; Kyriakides, T.R. Bulk Metallic Glasses for Biomedical Applications. JOM 2009, 61, 21–29. [Google Scholar] [CrossRef]
- Nicoara, M.; Raduta, A.; Parthiban, R.; Locovei, C.; Eckert, J.; Stoica, M. Low Young’s modulus Ti-based porous bulk glassy alloy without cytotoxic elements. Acta. Biomat. 2016, 36, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Klement, W.; Willens, R.H.; Duwez, P. Non-crystalline structure in solidifed gold-silicon alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid. Acta. Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Peker, A.; Johnson, W. Beryllium Bearing Amorphous Metallic Alloys Formed. US Patent 5,288,344, 22 February 1994. [Google Scholar]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta. Mat. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Zhang, T.; Inoue, A. Ti-based amorphous alloys with a large supercooled liquid region. Mat. Sci. Eng. 2001, A304–A306, 771–774. [Google Scholar] [CrossRef]
- Zhang, T.; Inoue, A. Thermal and Mechanical Properties of Ti-Ni-Cu-Sn Amorphous Alloys with a Wide Supercooled Region before Crystallization. Mat. Trans. JIM 1998, 39, 1001–1006. [Google Scholar] [CrossRef]
- Guo, F.; Wang, J.; Poon, J.S.; Shiflet, G.J. Ductile titanium-based glassy alloy ingots. Appl. Phys. Lett. 2005, 86. [Google Scholar] [CrossRef]
- Tang, M.Q.; Zhang, H.F.; Zhu, Z.W.; Fu, H.M.; Wang, A.M.; Li, H.; Hu, Z.Q. TiZr-base Bulk Metallic Glass with over 50 mm in Diameter. J. Mater. Sci. Technol. 2010, 26, 481–486. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wang, X.M.; Qin, F.X.; Yoshimura, M.; Inoue, A. New TiZrCuPd Quaternary Bulk Glassy Alloys with Potential of Biomedical Applications. Mat. Trans. 2007, 48, 2445–2448. [Google Scholar] [CrossRef]
- Wang, H.; Park, E.S.; Oak, J.J.; Setyawan, A.D.; Zhu, S.L.; Wada, T.; Wang, X.M.; Takeuchi, A.; Kato, H. Effect of cobalt microalloying on the glass forming ability of Ti-Cu-Pd-Zr metallic-glass. J. Non Cryst. Sol. 2013, 379, 155–160. [Google Scholar] [CrossRef]
- Suo, Z.; Qiu, K.; Li, Q.; Ren, Y.; Hu, Z. Effect of Nb on glass forming ability and plasticity of (Ti-Cu)-based bulk. Mat. Sci. Eng. A 2010, 527, 2486–2491. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Inoue, A. Glass-forming ability and mechanical properties of Ti-based bulk. Intermetallics 2008, 16, 1031–1035. [Google Scholar] [CrossRef]
- Oak, J.-J.; Louzguine-Luzgin, D.V.; Inoue, A. Fabrication of Ni-free Ti-based bulk-metallic glassy alloy having potential for application as biomaterial, and investigation of its mechanical properties, corrosion, and crystallization behavior. J. Mat. Res. 2007, 22, 1346–1353. [Google Scholar] [CrossRef]
- Zheng, N.; Qu, R.T.; Pauly, S.; Calin, M.; Gemming, T.; Zhang, Z.F.; Eckert, J. Design of ductile bulk metallic glasses by adding “soft” atoms. Appl. Phys. Lett. 2012, 100, 1–4. [Google Scholar] [CrossRef]
- Qin, F.X.; Wang, X.M.; Inoue, A. Effects of Ta on Microstructure and Mechanical Property of Ti-Zr-Cu-Pd-Ta Alloys. Mat. Trans. 2007, 48, 2390–2394. [Google Scholar] [CrossRef]
- Oak, J.-J.; Kimura, H.; Inoue, A. Effects of Additional Elements on Structure, Mechanical Strength and Chemical Properties of Ni-free Ti-based Bulk Metallic Glasses for Biomaterials. Adv. Mat. Res. 2007, 26–28, 785–788. [Google Scholar] [CrossRef]
- Qin, F.; Wang, X.; Xie, G.; Inoue, A. Distinct plastic strain of Ni-free Ti-Zr-Cu-Pd-Nb bulk metallic glasses with potential for biomedical applications. Intermetallics 2008, 16, 1026–1030. [Google Scholar] [CrossRef]
- Oak, J.-J.; Hwang, G.-W.; Park, Y.-H.; Kimura, H.; Yoon, S.-Y.; Inoue, A. Characterization of Surface Properties, Osteobalst Cell Culture in Vitro and Processing with Flow-Viscosity of Ni-Free Ti-Based Bulk Metallic Glass for Biomaterials. J. Biomech. Sci. Eng. 2009, 4, 384–391. [Google Scholar] [CrossRef]
- Abdel-Hady, M.M.; Hinoshita, K.; Morinaga, M. General approach to phase stability and elastic properties of beta-type Ti-alloys using electronic parameters. Scr. Mat. 2006, 55, 477–480. [Google Scholar] [CrossRef]
- Oak, J.-J.; Louzguine-Luzgin, D.V.; Inoue, A. Synthetic relationship between titanium and alloying elements in designing Ni-free Ti-based bulk metallic glass alloys. Appl. Phys. Lett. 2007, 91, 1–4. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mat. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Villars, P.; Okamoto, H.; Cenzual, K. ASM Alloy Phase Diagrams Database. Available online: http://www.asminternational.org (accessed on 28 April 2016).
- Schlede, E.; Aberer, W.; Fuchs, T.; Gerner, I.; Lessmann, H.; Maurer, T.; Rossbacher, R.; Stropp, G.; Wagner, E.; Kayser, D. Chemical substances and contact allergy—244 substances ranked according to allergenic potency. Toxicology 2003, 193, 219–259. [Google Scholar] [CrossRef]
- Hornez, J.; Lefevre, A.; Joly, D.; Hildebrand, H. Multiple parameter cytotoxicity index on dental alloys and pure metals. Biomol. Eng. 2002, 19, 103–117. [Google Scholar] [CrossRef]
- Craig, R.; Hanks, C. Cytotoxicity of Experimental Casting Alloys Evaluated by Cell Culture Tests. J. Dent. Res. 1990, 69, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.; Lockwood, P.; Schedle, A. Effect of silver, copper, mercury, and nickel ions on cellular proliferation during extended, low-dose exposures. J. Biomed. Mat. Res. 2000, 52, 360–364. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Tseng, H.-J.; Lin, Y.-C. The biocompatibility and antibacterial properties of waterborne polyurethane-silver nanocomposites. Biomaterials 2010, 31, 6796–6808. [Google Scholar] [CrossRef] [PubMed]
- Elshahawy, W.M.; Watanabe, I.; Kramer, P. In vitro cytotoxicity evaluation of elemental ions released from different prosthodontic materials. Dent. Mat. 2009, 25, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mat. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Stohs, S.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Choi, O.; Yu, C.-P.; Fernandez, G.E.; Hua, Z. Interactions of nanosilver with Escherichia coli cells in planktonic and biofilm cultures. Water Res. 2010, 44, 6095–6103. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B 2010, 79, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Secinti, K.D.; Özalp, H.; Attar, A.; Sargon, M.F. Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. J. Clin. Neurosci. 2011, 18, 391–395. [Google Scholar] [CrossRef] [PubMed]
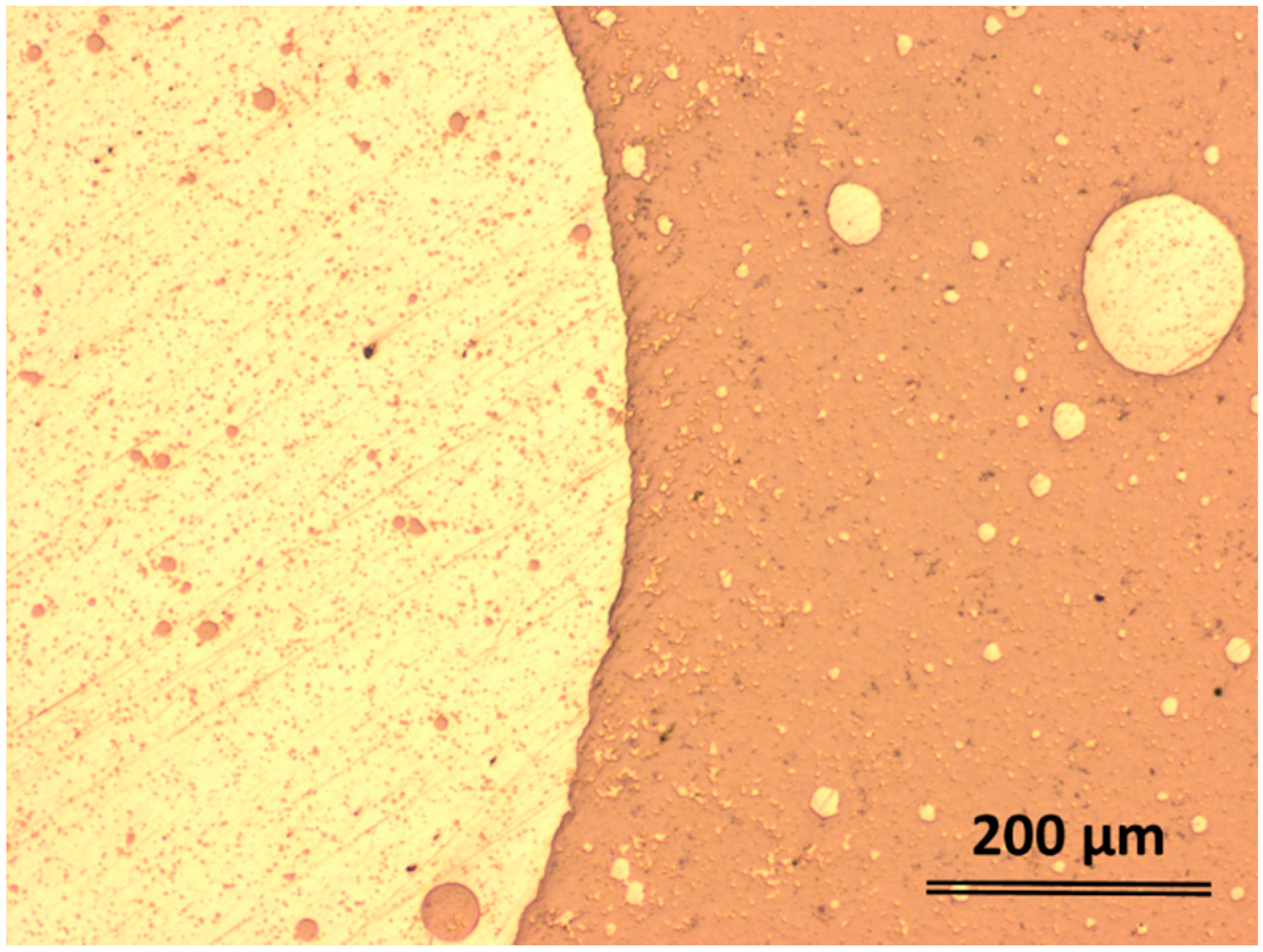
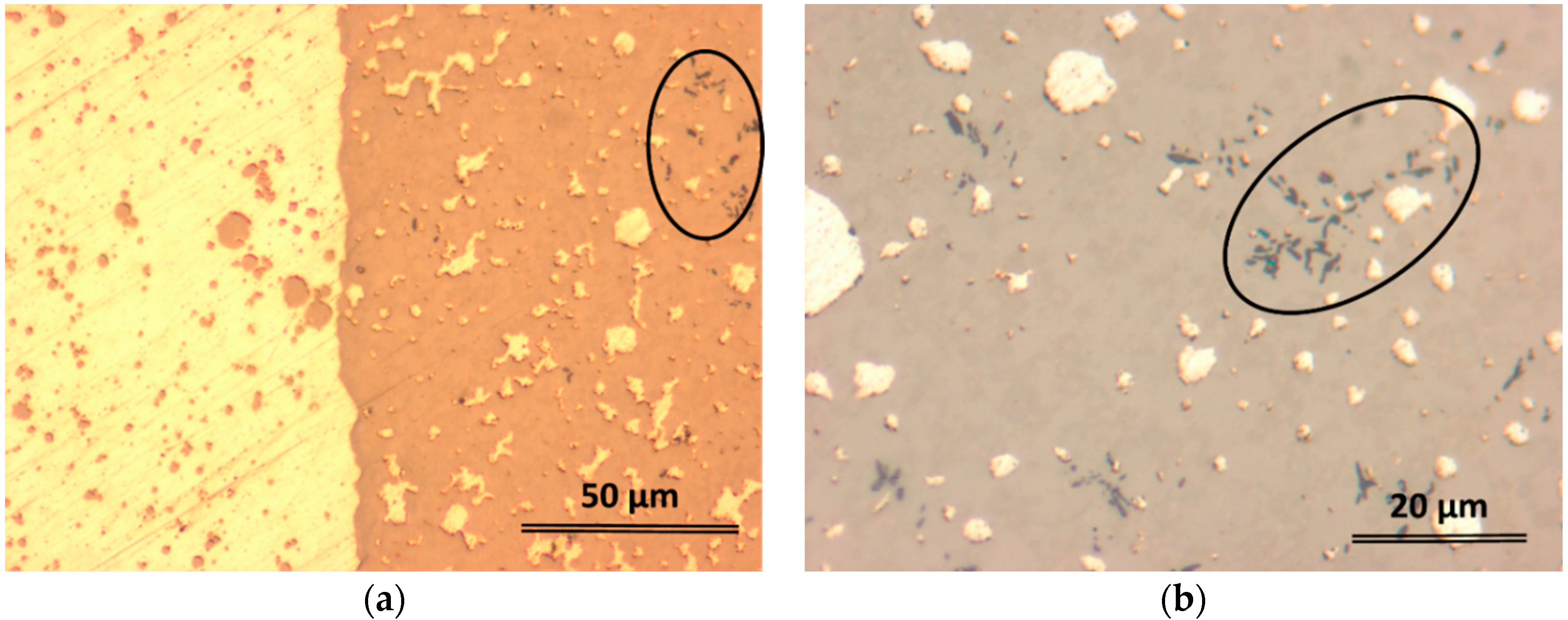
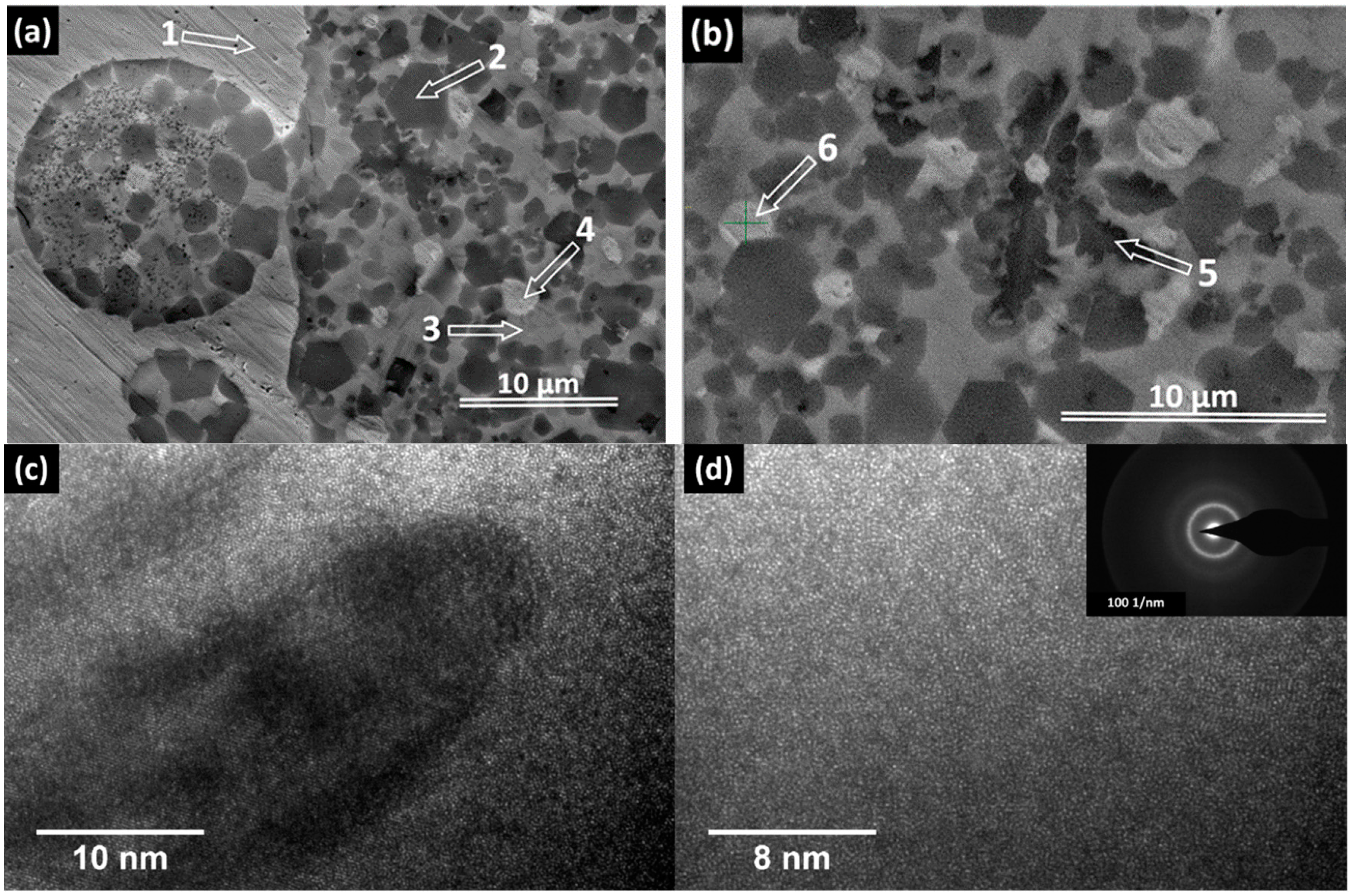
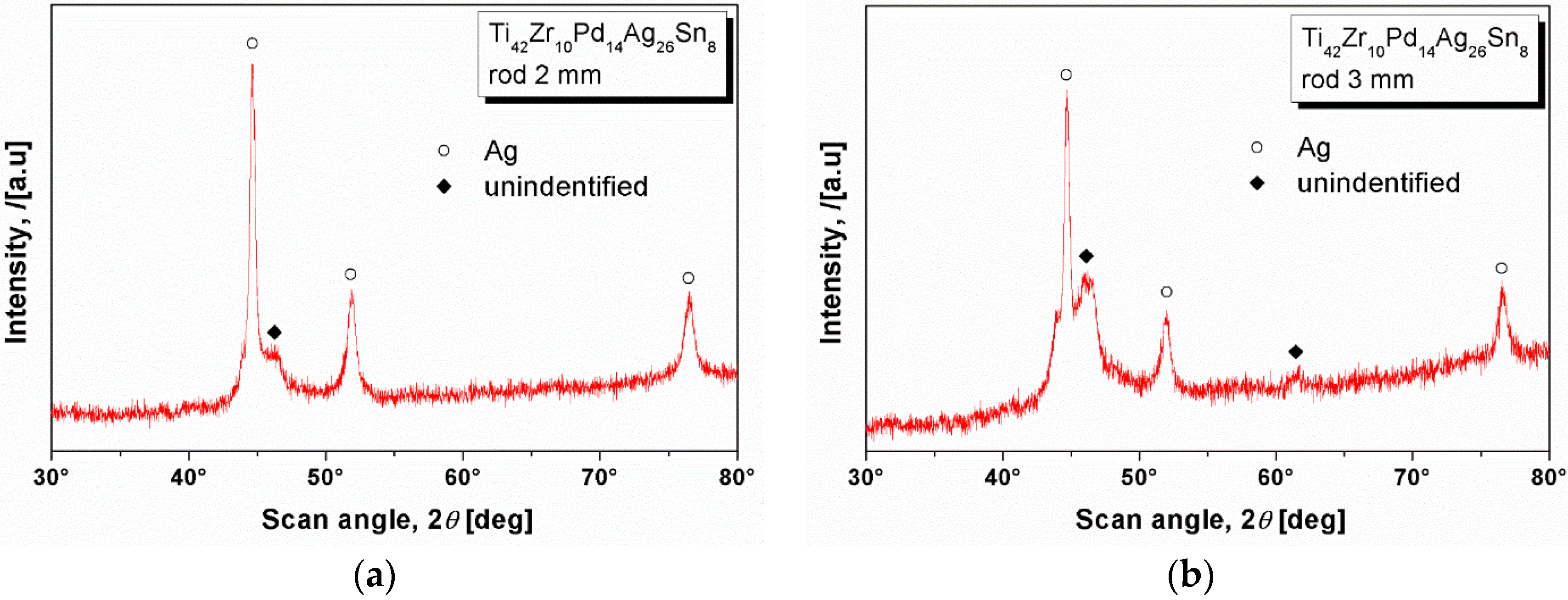
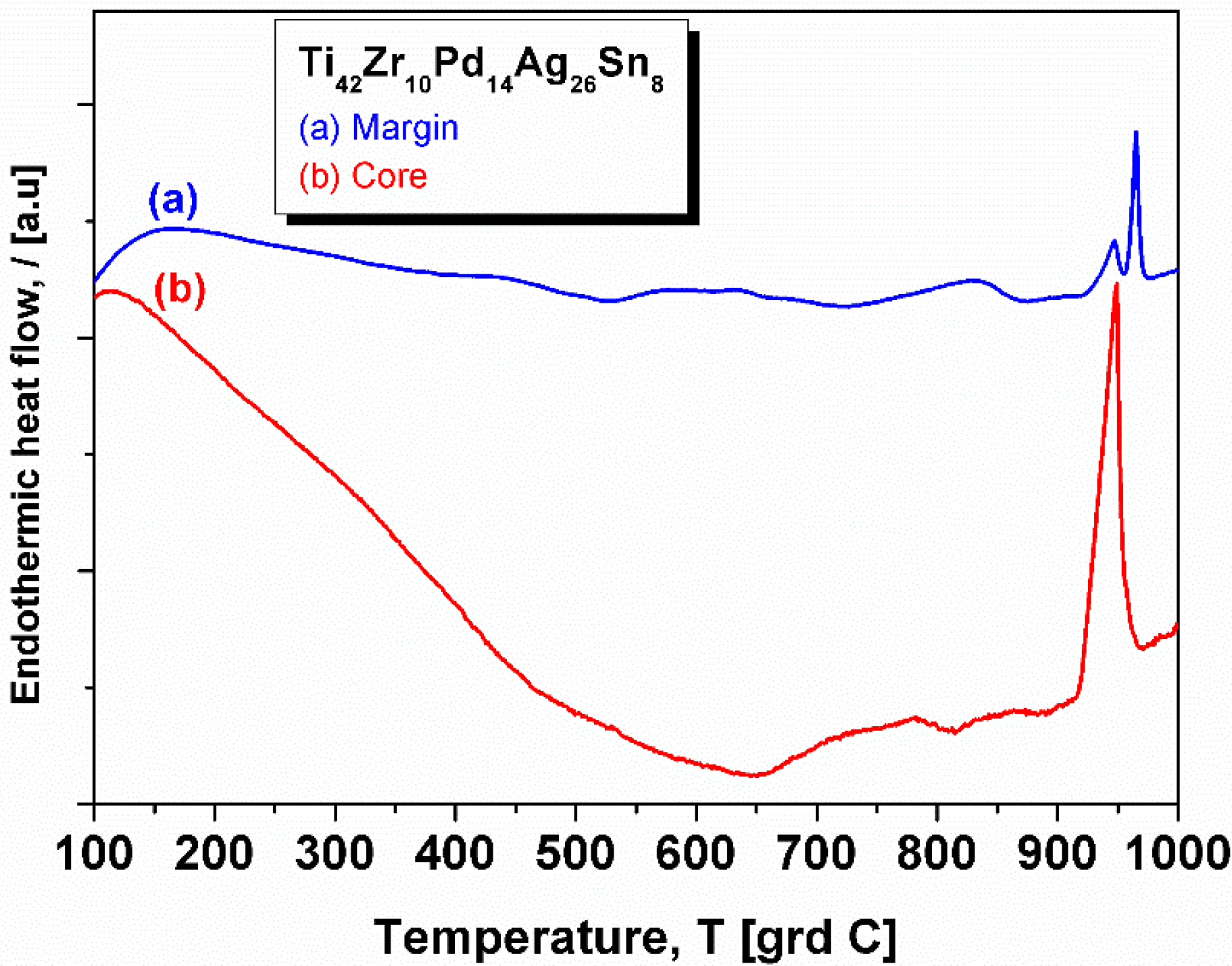
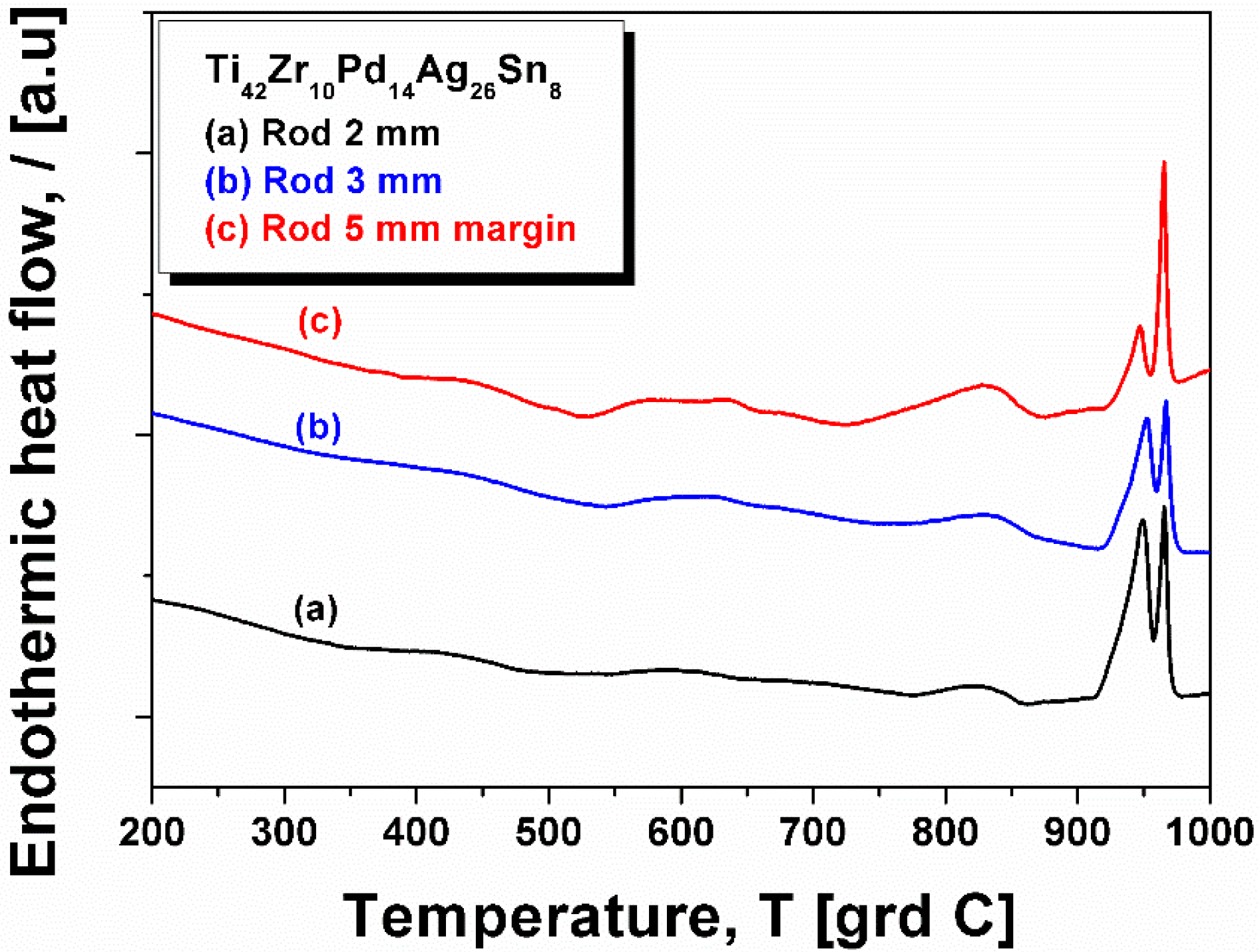
| Element | Zone 2 | Zone 3 | Zone 4 | Zone 5 | Zone 6 |
|---|---|---|---|---|---|
| Pd | 13.60 | 24.14 | 0.96 | 10.89 | 4.32 |
| Ag | 3.97 | 7.00 | 75.06 | 2.28 | 53.51 |
| Sn | 7.54 | 6.88 | 1.66 | 6.37 | 3.60 |
| Ti | 52.37 | 29.26 | 0.85 | 39.93 | 14.34 |
| Zr | 22.51 | 32.72 | 21.47 | 40.54 | 24.23 |
| Total | 100 | 100 | 100 | 100 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoara, M.; Locovei, C.; Șerban, V.A.; Parthiban, R.; Calin, M.; Stoica, M. New Cu-Free Ti-Based Composites with Residual Amorphous Matrix. Materials 2016, 9, 331. https://doi.org/10.3390/ma9050331
Nicoara M, Locovei C, Șerban VA, Parthiban R, Calin M, Stoica M. New Cu-Free Ti-Based Composites with Residual Amorphous Matrix. Materials. 2016; 9(5):331. https://doi.org/10.3390/ma9050331
Chicago/Turabian StyleNicoara, Mircea, Cosmin Locovei, Viorel Aurel Șerban, R. Parthiban, Mariana Calin, and Mihai Stoica. 2016. "New Cu-Free Ti-Based Composites with Residual Amorphous Matrix" Materials 9, no. 5: 331. https://doi.org/10.3390/ma9050331
APA StyleNicoara, M., Locovei, C., Șerban, V. A., Parthiban, R., Calin, M., & Stoica, M. (2016). New Cu-Free Ti-Based Composites with Residual Amorphous Matrix. Materials, 9(5), 331. https://doi.org/10.3390/ma9050331





