An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Solid-State 29Si MAS NMR Spectroscopy
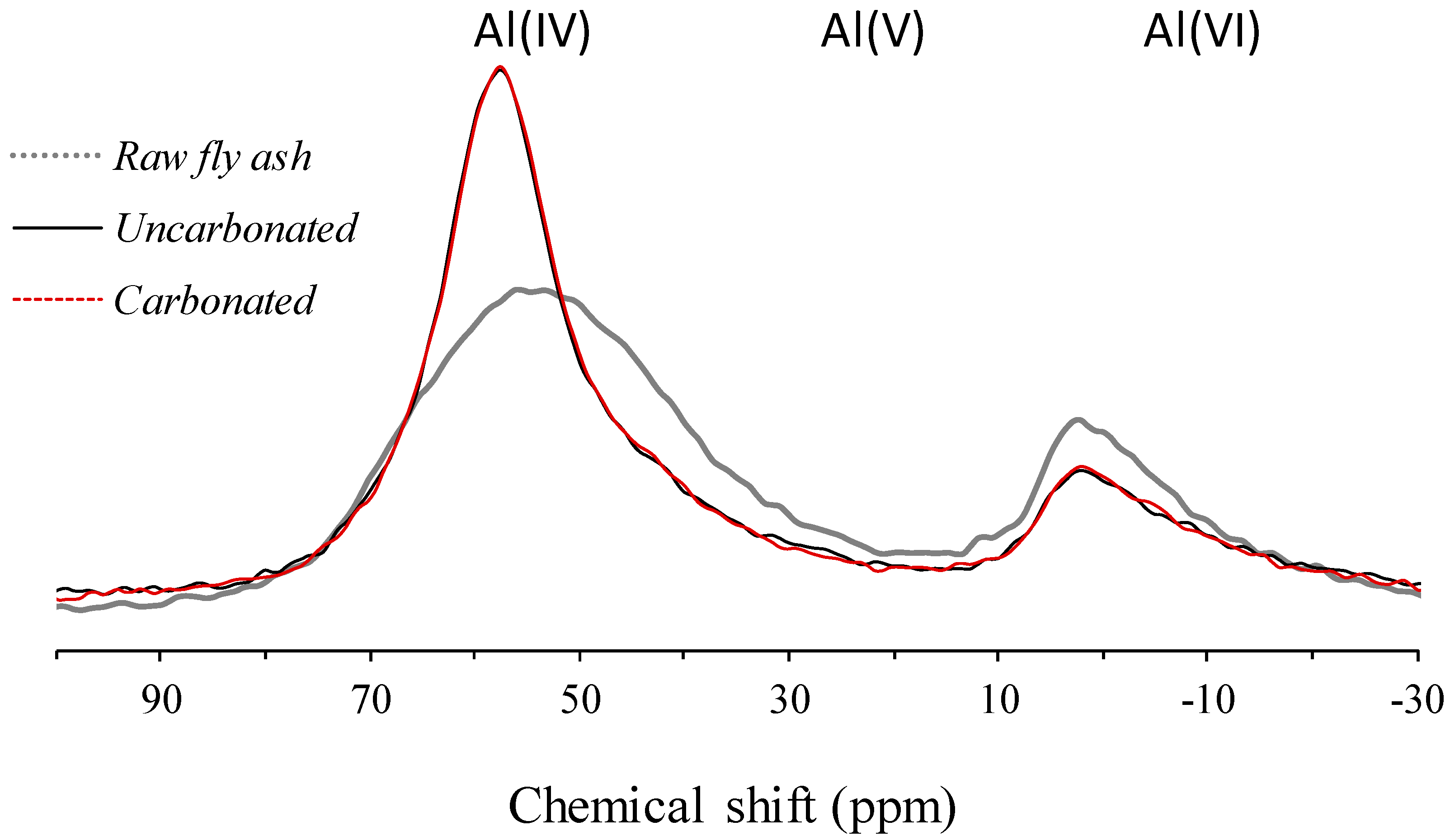
3.2. Solid-State 27Al MAS NMR Spectroscopy
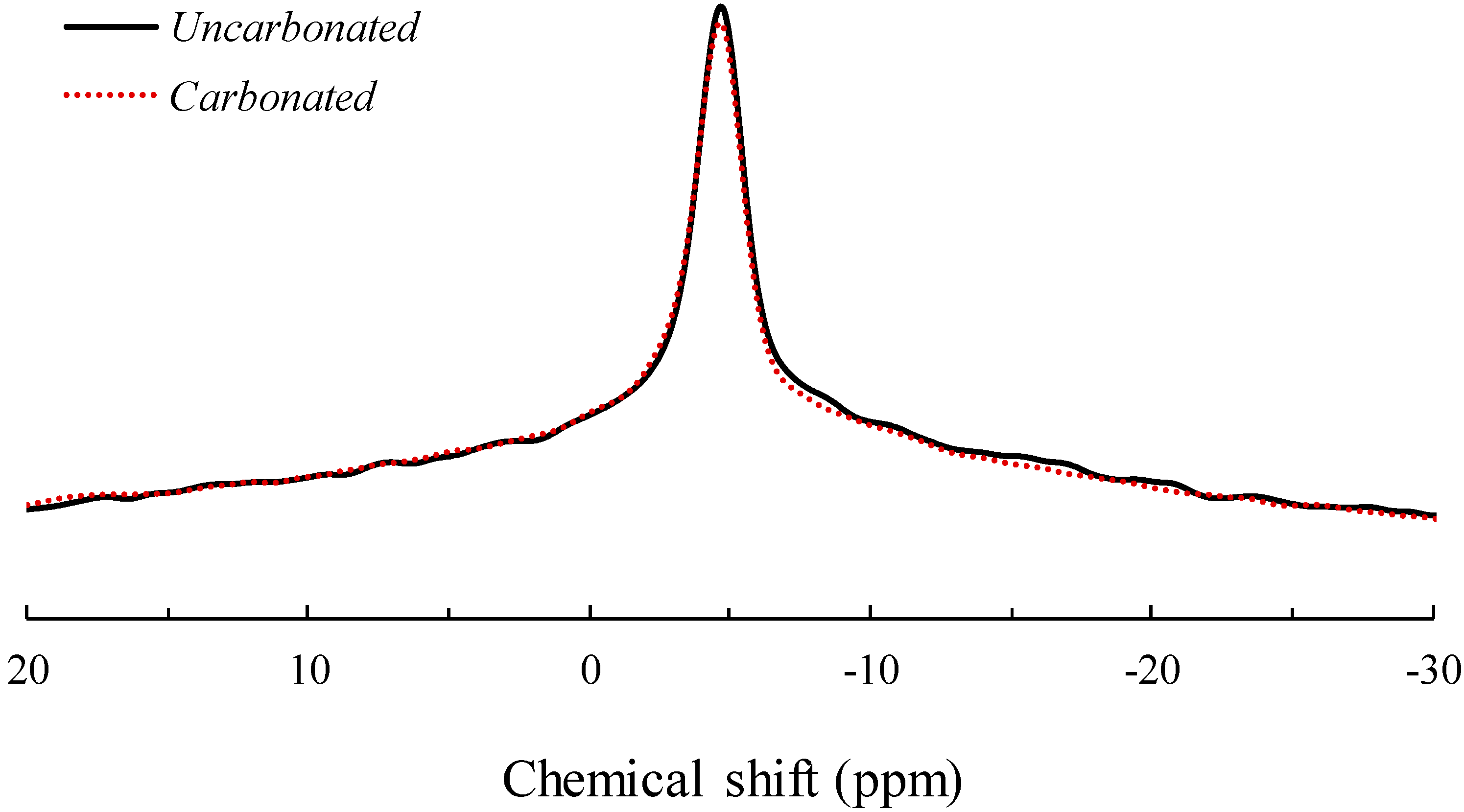
3.3. Solid-State 23Na MAS NMR Spectroscopy
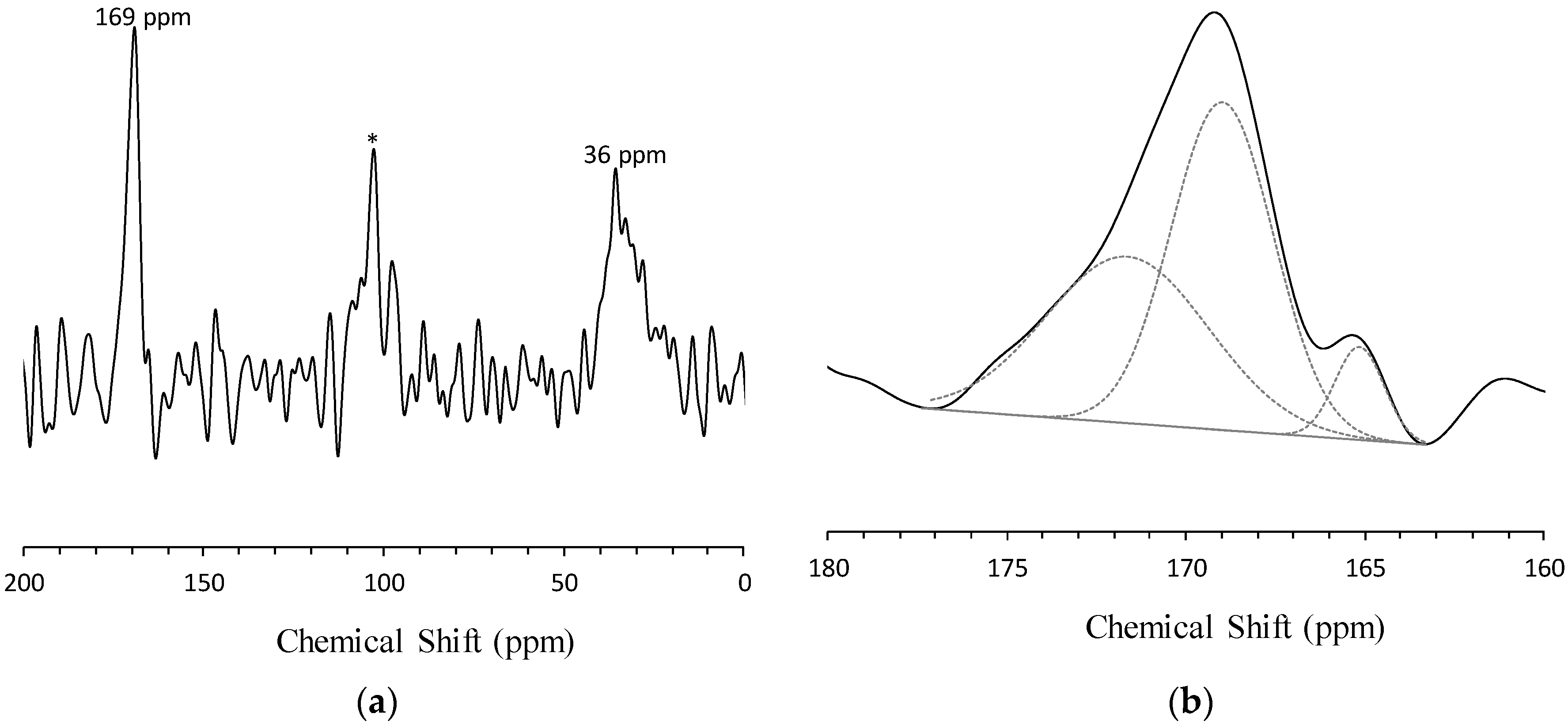
3.4. Solid-State 13C MAS NMR Spectroscopy
3.5. Si-Environment Seeded with CO32−
4. Discussion and Conclusions
- The dissolved silanol groups (monomeric silicates) in AFA exposed to a CO2-rich environment rapidly forms Si with a higher degree of polymerization, as shown in Table 2, which shows that the carbonated AFA had fewer residual silanol groups.
- Exposure to a CO2-rich environment lowered the pH level in the pore solution, as evidenced in previous studies [9]; therefore, the gel forming in AFA was promoted.
- These two factors may contribute to the formation of the binding gel phase in carbonated AFA and are responsible for the strength enhanced in the AFA exposed to a CO2-rich environment, observed in a recent study by the authors [10].
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bernal, S.A.; Provis, J.L.; Walkley, B.; Nicolas, S.R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; Deventer, J.S. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Cioffi, R.; Tarallo, O. Synthesis and characterization of novel epoxy geopolymer hybrid composites. Materials 2013, 6, 3943–3962. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Palomo, A.; Alonso, S.; Fernandez-Jiménez, A.; Sobrados, I.; Sanz, J. Alkaline activation of fly ashes: NMR study of the reaction products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Effect of fly ash characteristics on delayed high-strength development of geopolymers. Constr. Build. Mater. 2016, 102, 260–269. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, N.K.; Lee, H.K. Fresh and hardened properties of alkali-activated fly ash/slag pastes with superplasticizers. Constr. Build. Mater. 2014, 50, 169–176. [Google Scholar] [CrossRef]
- Fernández-Jimenez, A.; Torre, A.G.; Palomo, A.; López-Olmo, G.; Alonso, M.M.; Aranda, M.A.G. Quantitative determination of phases in the alkali activation of fly ash. Part I. Potential ash reactivity. Fuel 2006, 85, 625–634. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of alkali-activated materials: Progress and perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Brice, D.G.; Kilcullen, A.; Duxson, P.; Deventer, J.S. Accelerated carbonation testing of alkali-activated binders significantly underestimates service life: The role of pore solution chemistry. Cem. Concr. Res. 2012, 42, 1317–1326. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, J.G.; Kim, G.M.; Lee, H.K. Strength development of alkali-activated fly ash exposed to carbon dioxide-rich environment at early age. J. Korean Ceram. Soc. 2016, 83, 18–23. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. 29Si NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir 2005, 21, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.; Jun, Y.; Jeong, Y.; Oh, J.E. Microstructural and strength improvements through the use of Na2CO3 in a cementless Ca(OH)2-activated Class F fly ash system. Cem. Concr. Res. 2015, 67, 215–225. [Google Scholar] [CrossRef]
- Widgeon, S.J.; Sen, S.; Mera, G.; Ionescu, E.; Riedel, R.; Navrotsky, A. 29Si and 13C solid-state NMR spectroscopic study of nanometer-scale structure and mass fractal characteristics of amorphous polymer derived silicon oxycarbide ceramics. Chem. Mater. 2010, 22, 6221–6228. [Google Scholar] [CrossRef]
- Engelhardth, G.; Michel, D. High Resolution Solid State NMR of Silicates and Zeolites; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Mobasher, N.; Bernal, S.A.; Provis, J.L. Structural evolution of an alkali sulfate activated slag cement. J. Nucl. Mater. 2015, 468, 97–104. [Google Scholar] [CrossRef]
- Merwin, L.H.; Sebald, A.; Rager, H.; Schneider, H. 29Si and 27Al MAS NMR spectroscopy of mullite. Phys. Chem. Miner. 1991, 18, 47–52. [Google Scholar] [CrossRef]
- Xue, X.; Stebbins, J.F. 23Na NMR chemical shifts and local Na coordination environments in silicate crystals, melts and glasses. Phys. Chem. Miner. 1993, 20, 297–307. [Google Scholar] [CrossRef]
- Kohn, S.C.; Brooker, R.A.; Dupree, R. 13C MAS NMR: A method for studying CO2 speciation in glasses. Geochim. Cosmochim. Acta 1991, 55, 3879–3884. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jimenez, A.; Palomo, A.; Sobrados, I.; Sanz, J. Effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Part II: 29Si MAS-NMR survey. Microporous Mesoporous Mater. 2008, 109, 525–534. [Google Scholar] [CrossRef]
- Chloup-Bondant, M.; Evrard, O. Tricalcium aluminate and silicate hydration. Effect of limestone and calcium sulfate. In Nuclear Magnetic Resonance Spectroscopy of Cement-Based Materials; Colmbet, P., Grimmer, A.R., Zanni, H., Sozzani, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Suzuki, K.; Nishikawa, T.; Ito, S. Formation and carbonation of C-S-H in water. Cem. Concr. Res. 1985, 15, 213–224. [Google Scholar] [CrossRef]
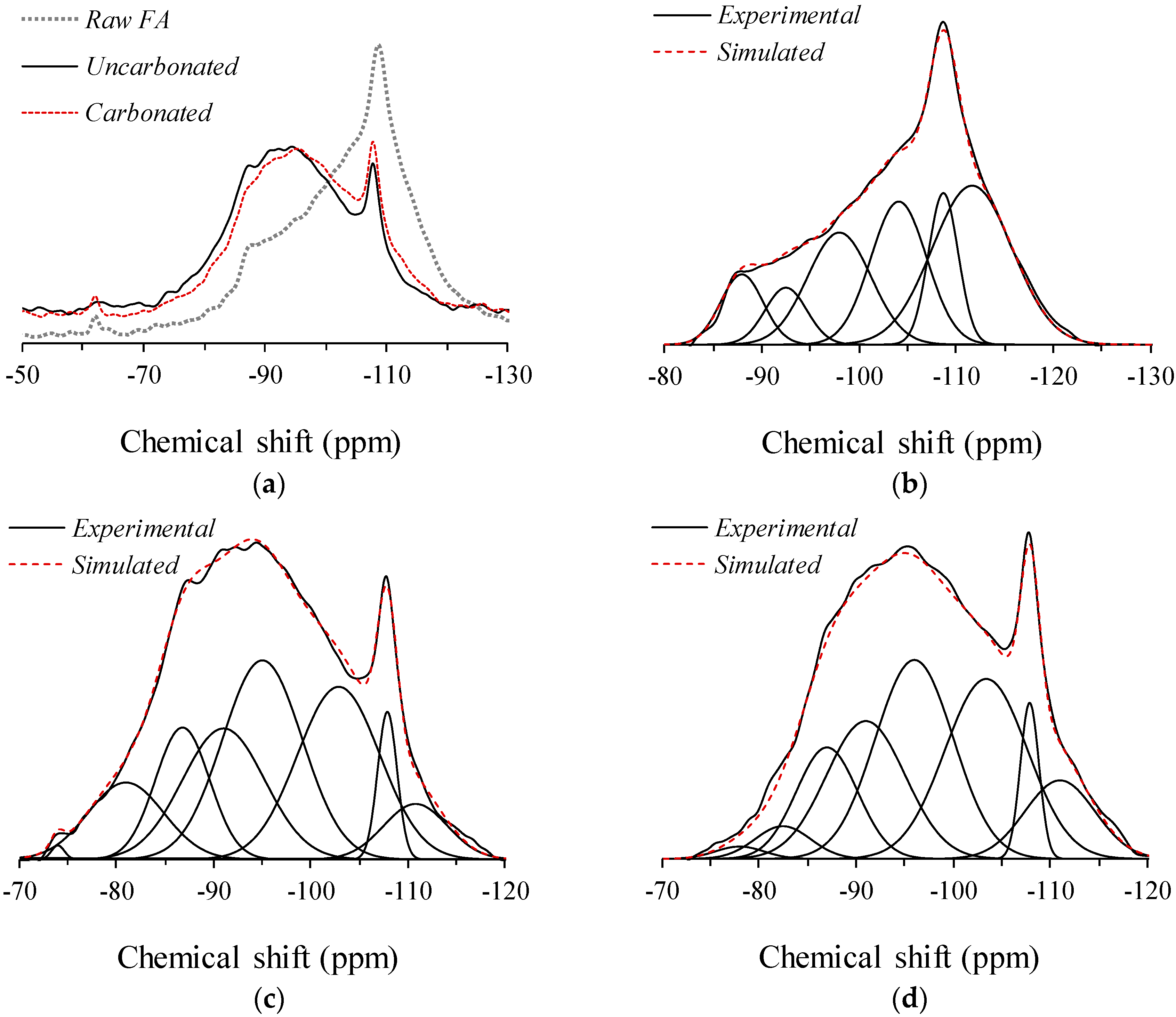
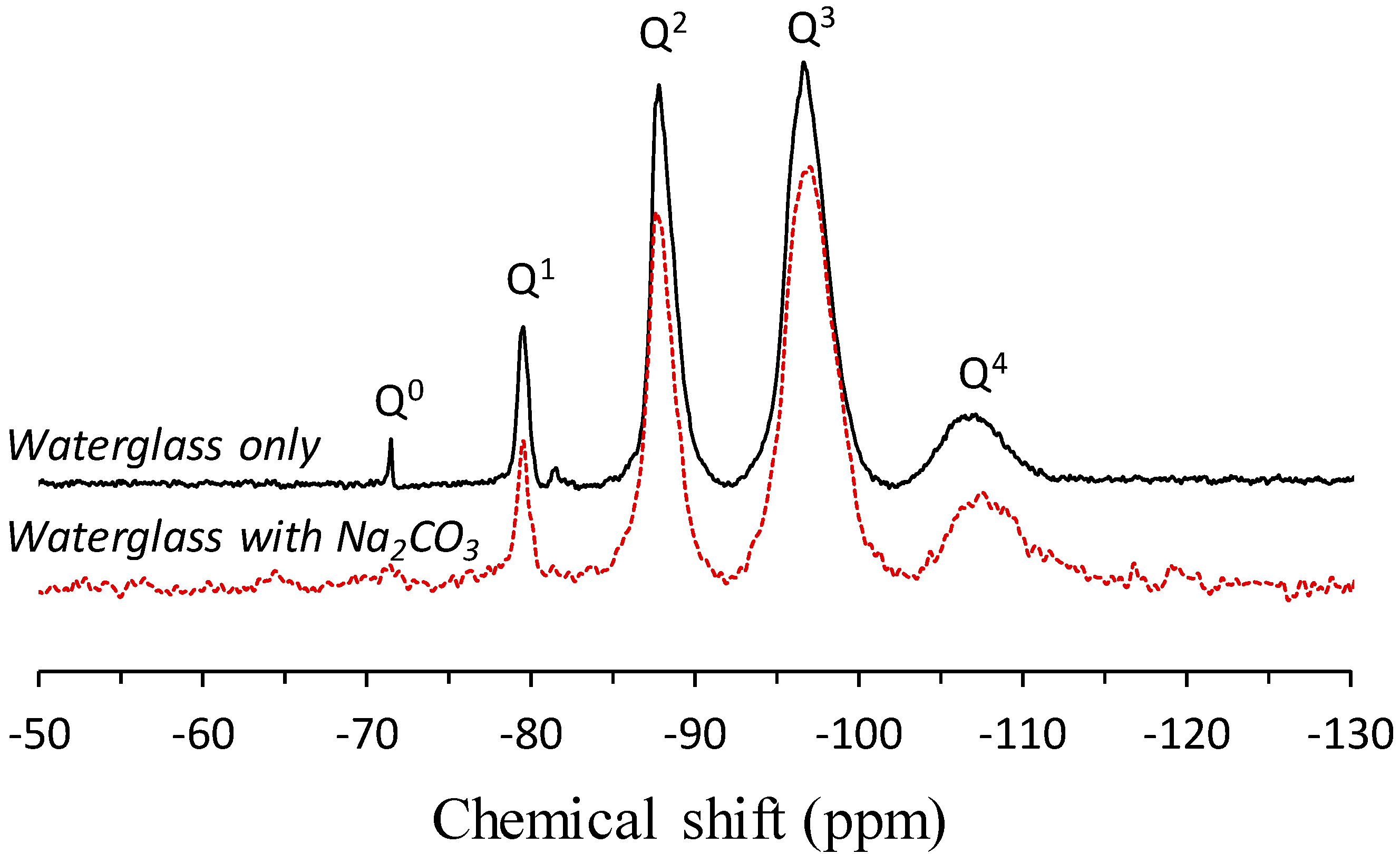
| Site Type | Silanol Groups | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | ||
|---|---|---|---|---|---|---|---|---|
| Chemical Shift (ppm) | −75 | −82 | −87 | −92 | −96 | −103 | −108 | −111 |
| Raw Fly Ash | – | – | 7.9% | 6.3% | 18.9% | 21.2% | 12.0% | 33.7% |
| Uncarbonated | 0.3% | 9.6% | 11.8% | 17.9% | 26.2% | 23.6% | 4.8% | 5.9% |
| Carbonated | 1.0% | 3.1% | 11.6% | 18.5% | 26.4% | 25.2% | 4.9% | 9.3% |
| Site Type | Aluminosilicate Gel 1 | Si/Al 2 | Unreacted Fly Ash 3 | Residual Silanol Groups 4 |
|---|---|---|---|---|
| Uncarbonated | 79.5% | 1.79 | 10.7% | 9.9% |
| Carbonated | 81.6% | 1.81 | 14.2% | 4.1% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-M.; Jang, J.-G.; Chae, S.-A.; Lee, H.-K. An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment. Materials 2016, 9, 308. https://doi.org/10.3390/ma9050308
Park S-M, Jang J-G, Chae S-A, Lee H-K. An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment. Materials. 2016; 9(5):308. https://doi.org/10.3390/ma9050308
Chicago/Turabian StylePark, Sol-Moi, Jeong-Gook Jang, Seen-Ae Chae, and Haeng-Ki Lee. 2016. "An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment" Materials 9, no. 5: 308. https://doi.org/10.3390/ma9050308
APA StylePark, S.-M., Jang, J.-G., Chae, S.-A., & Lee, H.-K. (2016). An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment. Materials, 9(5), 308. https://doi.org/10.3390/ma9050308






