Composite Hydrogels for Bone Regeneration
Abstract
:1. The Bone
1.1. Bone Anatomy
1.2. Chemical Composition
1.3. Histology of the Bone
- MSCs are multipotent stromal cells that can differentiate into diverse cell types such as myocytes and adipocytes but also osteoblast progenitors, osteoblasts, bone-lining cells, chondrocytes and osteocytes. Osteoblasts are the most prevalent cell type in the bone and their function is to secrete matrix components, such as collagen I, in response to mechanical stimuli, and to promote the mineralisation of the bone matrix. Osteocytes, derived from osteoblasts, have mechanosensor and modulator (promotion of nerve growth) activities. Bone-lining cells are instead able to release enzymes to remove the layer of osteoids that covers mineralised matrix, allowing osteoclasts to attach and begin resorption.
- HSCs are stem cells that give rise to blood cells such as monocyte, macrophages or platelets, but also preosteoclasts and osteoclasts. Osteoclasts are responsible for bone resorption; by the secretion of protons, they can lower the pH and so solubilize the mineral phase. These cells are responsible for an intricate balance between formation, maintenance and destruction of bone tissue. This equilibrium is maintained by mechanical factors and the action of cytokines and hormones, such as calcitonin and parathyroid hormone (PTH), which can control the levels of calcium and phosphate in the blood. Calcitonin is a thyroid hormone that reduces blood calcium levels by inhibiting osteoclasts and decreasing Ca resorption in the kidneys. Conversely, PTH is a hormone that increases blood calcium levels, acting upon the PTH1 receptor in bone and kidney, and the PTH2 receptor in the central nervous system, pancreas, testis, and placenta [6].
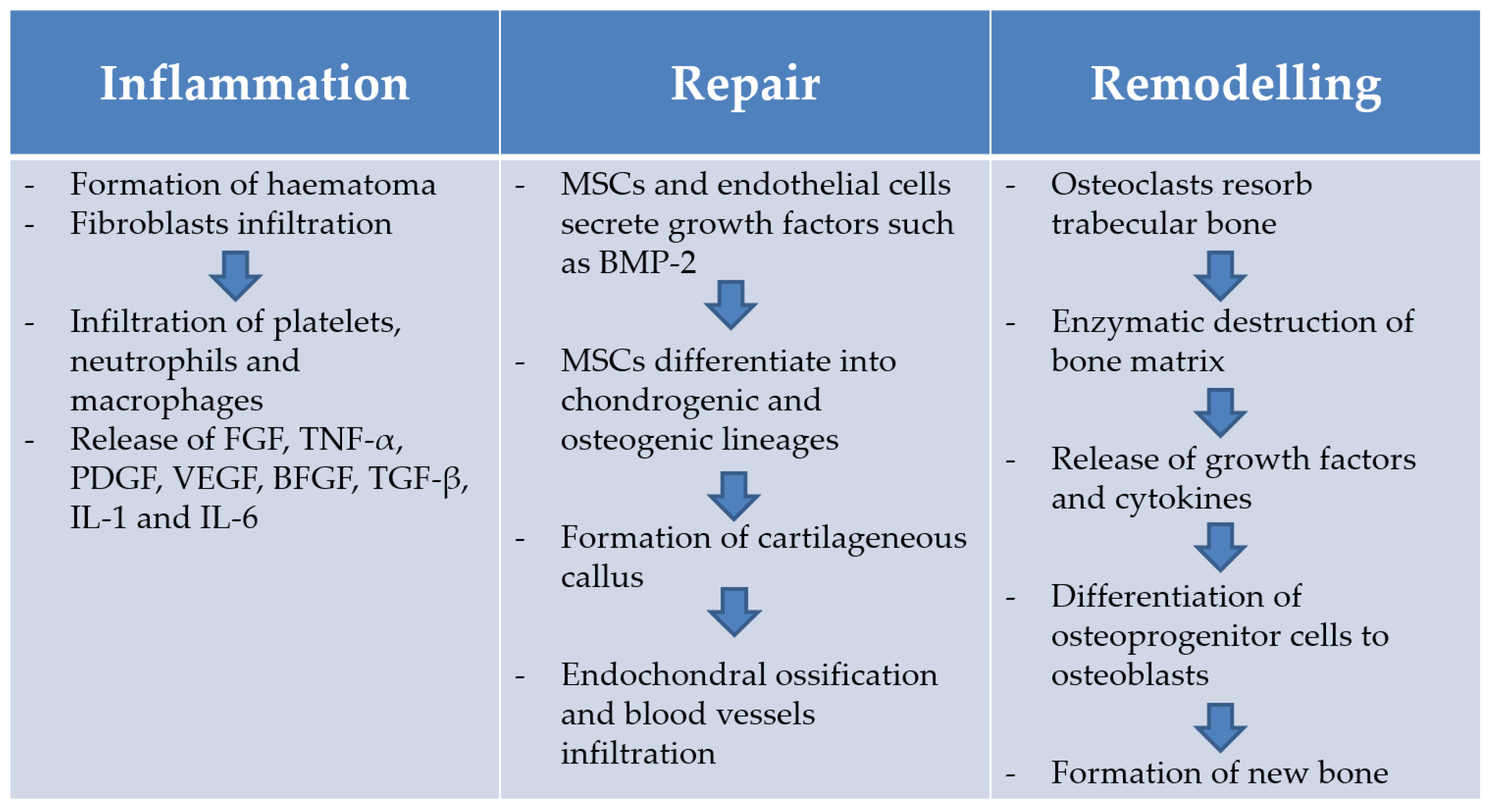
1.4. Bone Healing
2. Bone Tissue Engineering
2.1. The Need for Effective Bone Repair Strategies: Economic, Social and Clinical Aspects
2.2. Limitations of the Current Treatments
2.3. Requirements for Successful Development of Bone Tissue Engineering Scaffolds
- Osteoconduction refers to the growth of new tissue on the external and internal (pores) surfaces of the implant. This is greatly dependent on the physical form and chemical composition of the material. Factors such as hydrophilicity, porosity, biocompatibility and biodegradability of the material will affect its osteoconductive properties. For example, porosity (optimal pore size 200–350 µm) is crucial for allowing neovascularisation and diffusion of nutrients and gases required for the formation of the new bone [35]. Mechanical competence of the material is also important to provide an osteoconductive scaffold. This review will look at different approaches that have been studied in an attempt to improve the mechanical performance of hydrogel scaffolds.
- Osteogenicity is the property of those scaffolds that contain osteoprogenitor cells and favour their adhesion and proliferation [36]. This review will look at the different types of stem cells that can be used in bone regeneration and strategies for their inclusion in hydrogel scaffolds.
- Osteoinduction is the capacity of attracting immature cells to a healing site and stimulating these cells to develop into bone-forming cells. Materials that are osteoinductive are able to induce bone formation in ectopic sites [34]. This review will consider the complexity of controlled delivery of drugs and growth factors from bone regeneration scaffolds.
3. Hydrogels as Scaffolds and Delivery Platforms
3.1. Osteoconductive Composite Hydrogels: Strategies to Improve Hydrogels Mechanical Competence
3.1.1. Hydroxyapatite
3.1.2. Bioactive Glass
3.1.3. Carbon Nanotubes (CNTs) and Other Carbon Materials
3.2. Osteoinductive Composite Hydrogels: Controlled Delivery of Drugs and Growth Factors
3.2.1. Bisphosphonates
3.2.2. Statins
3.2.3. Growth Factors
3.3. Osteogenic Composite Hydrogels
3.3.1. Mesenchymal Stem Cells
3.3.2. Adipose Derived Stem Cells
3.3.3. Stem Cells from Human Exfoliated Deciduous Teeth
3.3.4. Embryonic Stem Cells
4. Concluding Considerations
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ASCs | adipose-derived stem cells |
| BFGF | basic fibroblast growth factor |
| BMP | bone morphogenic protein |
| BPs | bisphosphonates |
| BTE | bone tissue engineering |
| C60 | fullerenes |
| CaP | calcium phosphate |
| CDA | calcium deficient apatite |
| CNTs | carbon nanotubes |
| Cs | chitosan |
| ECM | extracellular matrix |
| ESC | embryonic stem cells |
| f-CNT | functionalized-carbon nanotubes |
| FGF | fibroblast growth factors |
| GG | gellan gum |
| GNPs | gold nanoparticle |
| HA | hyaluronic acid |
| Hap | hydroxyapatite |
| HMG-CoA | 3-hydroxy-2-methylglutaryl-CoA |
| hMSCs | human mesenchymal stem cells |
| HSC | hematopoietic stem cells |
| IGF | insulin like growth factor |
| MSC | mesenchymal stem cells |
| MSNPs | molybdenum di-sulfite nanoplatelets |
| MWNTs | multi-wall nanotubes |
| nHap | nanohydroxyapatite |
| PDGF | platelet-derived growth factors |
| PEG | polyethylene glycol |
| PLGA | poly(lactide-co-glycolide) |
| PPF | poly(propylene fumarate) |
| PTH | parathyroid hormone |
| SGH | self-supporting graphene hydrogels |
| SHEDs | stem cells from human exfoliated deciduous teeth |
| SIM | simvastatin |
| SWNTs | single-wall nanotubes |
| TCP | tricalcium phosphate |
| TE | tissue engineering |
| TGF-β | transforming growth factor |
| TNF-α | tumour necrosis factor-α |
| US-tubes | ultra-short single-wall nanotubes |
| VEGF | vascular endothelial growth factor |
| Zol | zoledronate |
References
- Skeikh, Z.E.A. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Muniz Renno, A.C.; Matsumoto, M.; Araki Ribeiro, D. Bone Regeneration: Growth Factors, Augmentation Procedures and Tissue Engineering Applications; Legard, V., Schulter, R., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 93–118. [Google Scholar]
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2002; pp. 3–26. [Google Scholar]
- Scanlon, V.C.; Sanders, T. Essentials of Anatomy and Physiology, 6th ed.; F.A. Davis Company: Philadelphia, PA, USA, 2011. [Google Scholar]
- Buckwalter, J.A.; Cooper, R.R. Bone structure and function. Instr. Course Lect. 1987, 36, 27–48. [Google Scholar] [PubMed]
- Lanyon, L.E. Osteocytes, strain detection, bone modeling and remodeling. Calcif. Tissue Int. 1993, 53, S102–S107. [Google Scholar] [CrossRef] [PubMed]
- Geris, L.; Vander Sloten, J.; Van Oosterwyck, H. In silico biology of bone modelling and remodelling: Regeneration. Philos Trans. R. Lond. A 2009, 367, 2031–2053. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed]
- Braddock, M.; Houstton, P.; Campbell, C.; Ashcroft, P. Born again bone: Tissue engineering for bone repair. Physiology 2001, 10, 208–213. [Google Scholar]
- Marzona, L.; Pavolini, B. Play and players in bone fracture healing match. Clin. Cases Miner. Bone Metab. 2009, 6, 159–162. [Google Scholar] [PubMed]
- Giannotti, S.; Bottai, V.; Dell’Osso, G.; Pini, E.; De Paola, G.; Bugelli, G.; Guido, G. Current medical treatment strategies concerning fracture healing. Clin. Cases Miner. Bone Metab. 2013, 10, 116–120. [Google Scholar] [PubMed]
- Baldini, N.; Cenni, E.; Ciappetti, G.; Granchi, D.; Savarino, L. Bone repair and regeneration. In Bone Repair Biomaterials; Planell, J.A., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2009. [Google Scholar]
- Pietschmann, P.; Gruber, R.; Peterlik, M. Pathophysiology and aging of bone. In Radiology of Osteoporosis; Grampp, S., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 2003. [Google Scholar]
- Office of the Surgeon General (US). The Frequency of Bone Disease. In Bone Health and Osteoporosis: A Report of the Surgeon General; Office of the Surgeon General (US): Rockville, MD, USA, April 2004. Available online: http://www.ncbi.nlm.nih.gov/books/NBK45515/ (accessed on 30 March 2016). [Google Scholar]
- Shadjou, N.; Hasanzadeh, M. Bone tissue engineering using silica-based mesoporous nanobiomaterials: Recent progress. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 401–409. [Google Scholar] [CrossRef] [PubMed]
- National Osteoporosis Society. Life with Osteoporosis: The Untold Story; National Osteoporosis Society: Camerton, UK, 2014. [Google Scholar]
- National Osteoporosis Society. 25th Anniversary Report—A Fragile Future; National Osteoporosis Society: Camerton, UK, 2011. [Google Scholar]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, T.J.; Calori, G.M.; Schmidmaier, G. Autograft versus BMPs for the treatment of non-unions: What is the evidence? Injury 2013, 44 (Suppl. S1), S40–S42. [Google Scholar] [CrossRef]
- Dreifke, M.; Ebraheim, N.; Jayasuriya, A. Investigation of potential injectable polymeric biomaterials for bone regeneration. J. Biomed. Mater. Res. A 2013, 101, 2436–2447. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Basha, Y.R.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Gauthier, O.; Sourice, S.; Pilet, P.; Rethore, G.; Khairoun, K.; Bouler, J.-M.; Tancret, F.; Weiss, P. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: Syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2016, 31, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yang, F.; Wolke, J.G.; Li, Y.; Jansen, J.A. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010, 6, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Zimmermann, G.; Hernigou, P. Cellular therapies for the treatment of non-union: The past, present and future. Injury 2013, 44 (Suppl. S1), S46–S49. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nature Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Dhert, W.J.A.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater. 2011, 7, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016. Available online: http://www.sciencedirect.com/science/article/pii/S0141813016301155 (accessed on 30 March 2016).
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [PubMed]
- Seyednejad, H.; Gawlitta, D.; Kuiper, R.V.; de Bruin, A.; van Nostrum, C.F.; Vermonden, T.; Dhert, W.J.A.; Hennink, W.E. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials 2012, 33, 4309–4318. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Gottardi, R.; Fedorchak, M.V.; Little, S.R. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J. Control. Release 2015, 219, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Deligkaris, K.; Tadele, T.S.; Olthuis, W.; van den Berg, A. Hydrogel-based devices for biomedical applications. Sens. Actuators B Chem. 2010, 147, 765–774. [Google Scholar] [CrossRef]
- Park, J.B. The use of hydrogels in bone-tissue engineering. Med. Oral Patol. Oral 2011, 16, e115–e118. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu Pryia, M.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Byrne, D.P.; Lacroix, D.; Planell, J.A.; Kelly, D.J.; Prendergast, P.J. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: Application of mechanobiological models in tissue engineering. Biomaterials 2007, 28, 5544–5554. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Alblas, J.; de Wijn, J.R.; Hennink, W.E.; Verbout, A.J.; Dhert, W.J. Hydrogels as extracellular matrices for skeletal tissue engineering: State-of-the-art and novel application in organ printing. Tissue Eng. 2007, 13, 1905–1925. [Google Scholar] [CrossRef] [PubMed]
- Ulubayram, K.; Aksu, E.; Gurhan, S.I.; Serbetci, K.; Hasirci, N. Cytotoxicity evaluation of gelatin sponges prepared with different cross-linking agents. J. Biomater. Sci. Polym. E 2002, 13, 1203–1219. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- D’Este, M.; Eglin, D. Hydrogels in calciumphophate moldable and injectable bone substitutes: Sticky excipients or advanced 3D carriers? Acta Biomater. 2013, 9, 5421–5430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mi, W.; Wang, H.; Su, Y.; He, C. Nano-hydroxyapatite/polyacrylamide composite hydrogels with high mechanical strengths and cell adhesion properties. Colloids Surf. B 2014, 123, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds based bone tissue engineering: The role of chitosan. Tissue Eng. B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; de Moraes, M.A.; Beppu, M.M.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Development of silk fibroin/nanohydroxyapatite composite hydrogels for bone tissue engineering. Eur. Polym. J. 2015, 67, 66–77. [Google Scholar] [CrossRef]
- Watanabe, J.; Kashii, M.; Hirao, M.; Oka, K.; Sugamoto, K.; Yoshikawa, H.; Akashi, M. Quick-forming hydroxyapatite/agarose gel composites induce bone regeneration. J. Biomed. Mater. Res. A 2007, 83, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, Y.; Tong, H.; Shen, X.; Chen, L.; Ran, J. A detailed study of homogeneous agarose/hydroxyapatite nanocomposites for load-bearing bone tissue. Int. J. Biol. Macromol. 2016, 82, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Amirian, J.; Min, Y.K.; Lee, B.T. HAp granules encapsulated oxidized alginate-gelatin-biphasic calcium phosphate hydrogel for bone regeneration. Int. J. Biol. Macromol. 2015, 81, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Peng, N.; He, M.; Teramoto, Y.; Nishio, Y.; Zhang, L. Fabrication and properties of chitin/hydroxyapatite hybrid hydrogels as scaffold nano-materials. Carbohydr. Polym. 2013, 91, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Arun Kumar, R.; Sivashanmugam, A.; Deepthi, S.; Iseki, S.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Injectable chitin-poly(ε-caprolactone)/nano hydroxyapatite composite microgels prepared by simple regeneration technique for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 9399–9409. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Saffarian Tousi, N.; Velten, M.F.; Bishop, T.J.; Leong, K.K.; Barkhordar, N.S.; Marshall, G.W.; Loomer, P.M.; Aswath, P.B.; Varanasi, V.G. Combinatorial effect of Si4+, Ca2+, and Mg2+ released from bioactive glasses on osteoblast osteocalcin expression and biomineralization. Mater. Sci. Eng. C 2013, 33, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.H.; Doostmohammadi, A. Bioactive glass nanopowder and bioglass coating for biocompatibility improvement of metallic implant. J. Mater. Process. Technol. 2009, 209, 1385–1391. [Google Scholar] [CrossRef]
- Hench, L.L. The story of bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xiao, Y. Evaluation of the in vitro bioactivity of bioceramics. Bone Tissue Regener. Insights 2009, 2, 25–29. [Google Scholar]
- Smith, A.M.; Shelton, R.M.; Perrie, Y.; Harris, J.J. An initial evaluation of gellan gum as a material for tissue engineering applications. J. Biomater. Appl. 2007, 22, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified gellan gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Gantar, A.; da Silva, L.P.; Oliveira, J.M.; Marques, A.P.; Correlo, V.M.; Novak, S.; Reis, R.L. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Mater. Sci. Eng. C 2014, 43, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Killion, J.A.; Kehoe, S.; Geever, L.M.; Devine, D.M.; Sheehan, E.; Boyd, D.; Higginbotham, C.L. Hydrogel/bioactive glass composites for bone regeneration applications: Synthesis and characterisation. Mater. Sci. Eng. C 2013, 33, 4203–4212. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, J.; Jallot, E.; Lao, J. Gelatin-bioactive glass composites scaffolds with controlled macroporosity. Chem. Eng. J. 2014, 256, 9–13. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Liu, J.-H.; Huang, X.-J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Roldo, M.; Fatouros, D.G. Biomedical applications of carbon nanotubes. Annu. Rep. Sect. C 2013, 109, 10–35. [Google Scholar] [CrossRef]
- Tonelli, F.M.; Santos, A.K.; Gomes, K.N.; Lorençon, E.; Guatimosim, S.; Ladeira, L.O.; Resende, R.R. Carbon nanotube interaction with extracellular matrix proteins producing scaffolds for tissue engineering. Int. J. Nanomed. 2012, 7, 4511–4529. [Google Scholar]
- Seo, S.-J.; Kim, J.-J.; Kim, J.-H.; Lee, J.-Y.; Shin, U.S.; Lee, E.-J.; Kim, H.-W. Enhanced mechanical properties and bone bioactivity of chitosan/silica membrane by functionalized-carbon nanotube incorporation. Compos. Sci. Technol. 2014, 96, 31–37. [Google Scholar] [CrossRef]
- Yasmeen, S.; Lo, M.K.; Bajracharya, S.; Roldo, M. Injectable scaffolds for bone regeneration. Langmuir 2014, 30, 12977–12985. [Google Scholar] [CrossRef] [PubMed]
- Sitharaman, B.; Shi, X.; Tran, L.A.; Spicer, P.P.; Rusakova, I.; Wilson, L.J.; Mikos, A.G. Injectable in situ cross-linkable nanocomposites of biodegradable polymers and carbon nanostructures for bone tissue engineering. J. Biomater. Sci. Polym. E 2007, 18, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Lin, L.; Kasper, F.K.; Qin, Y.X.; Mikos, A.G.; Sitharaman, B. Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules 2013, 14, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Wagoner Johnson, A.J.; Herschler, B.A. A review of the mechanical behavior of cap and cap/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.S.; Vollrath, M.; Kaplan, D.L. Effects of clodronate and alendronate on osteoclast and osteoblast co-cultures on silk-hydroxyapatite films. Acta Biomater. 2014, 10, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Grasser, W.; Endo, N.; Akins, R.; Simmons, H.; Thompson, D.D.; Golub, E.; Rodan, G.A. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Investig. 1991, 88, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Mori, K.; Orita, M.; Takeuchi, M. Computational insights into binding of bisphosphates to farnesyl pyrophosphate synthase. Curr. Med. Chem. 2011, 18, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Kettenberger, U.; Luginbuehl, V.; Procter, P.; Pioletti, D.P. In vitro and in vivo investigation of bisphosphonate-loaded hydroxyapatite particles for peri-implant bone augmentation. J. Tissue Eng. Regener. Med. 2015, 37, 3428–3429. [Google Scholar]
- Vieira, H.P.; Leite, I.A.; Araújo Sampaio, T.M.; de Paula, J.D.A.; do Nascimento Andrade, A.; de Abreu, L.C.; Valenti, V.E.; Goulart, F.C.; Adami, F. Bisphosphonates adherence for treatment of osteoporosis. Int. Arch. Med. 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.; Croucher, P.I.; Rogers, M.J. Bisphosphonates: Pharmacology, mechanisms of action and clinical uses. Osteoporos. Int. 1999, 9 (Suppl. S2), S66–S80. [Google Scholar] [CrossRef] [PubMed]
- Verron, E.; Gauthier, O.; Janvier, P.; Pilet, P.; Lesoeur, J.; Bujoli, B.; Guicheux, J.; Bouler, J.M. In vivo bone augmentation in an osteoporotic environment using bisphosphonate-loaded calcium deficient apatite. Biomaterials 2010, 31, 7776–7784. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Sirico, F.; Ferrante, A.; Gimigliano, R.; Gimigliano, F. Risedronate’s efficacy: From randomized clinical trials to real clinical practice. Clin. Cases Miner. Bone Metab. 2010, 7, 19–22. [Google Scholar] [PubMed]
- Patrick, A.R.; Brookhart, M.A.; Losina, E.; Schousboe, J.T.; Cadarette, S.M.; Mogun, H.; Solomon, D.H. The complex relation between bisphosphonate adherence and fracture reduction. J. Clin. Endocr. Metab. 2010, 95, 3251–3259. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Bilezikian, J.P.; Dempster, D.W.; Lewiecki, E.M.; Miller, P.D.; Neer, R.M.; Recker, R.R.; Shane, E.; Shoback, D.; Potts, J.T. Benefits and risks of bisphosphonate therapy for osteoporosis. J. Clin. Endocr. Metab. 2012, 97, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Posadowska, U.; Parizek, M.; Filova, E.; Wlodarczyk-Biegun, M.; Kamperman, M.; Bacakova, L.; Pamula, E. Injectable nanoparticle-loaded hydrogel system for local delivery of sodium alendronate. Int. J. Pharm. 2015, 485, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Elavarasu, S.; Suthanthiran, T.K.; Naveen, D. Statins: A new era in local drug delivery. J. Pharm. Bioallied Sci. 2012, 4, S248–S251. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Piepgrass, W.T.; Lin, Y.-L.; Thomas, M.V.; Puleo, D.A. Localized intermittent delivery of simvastatin hydroxyacid stimulates bone formation in rats. J. Periodontol. 2008, 79, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Hirata, S.; Osawa, K.; Terashita, M.; Kitamura, C.; Fukushima, H. The current and future therapies of bone regeneration to repair bone defects. Int. J. Dent. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Lee, M.D.; Lee, S.K.; Kim, H.M.; Fitzpatrick, L.A. HMG-CoA reductase inhibitors increase BMD in type 2 diabetes mellitus patients. J. Clin. Endocr. Metab. 2000, 85, 1137–1142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bradley, J.D.; Cleverly, D.G.; Burns, A.M.; Helm, N.B.; Schmid, M.J.; Marx, D.B.; Cullen, D.M.; Reinhardt, R.A. Cyclooxygenase-2 inhibitor reduces simvastatin-induced bone morphogenetic protein-2 and bone formation in vivo. J. Periodontal Res. 2007, 42, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.S.W.; Nuttelman, C.R.; Collins, S.D.; Anseth, K.S. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hmsc differentiation and function for bone regeneration. Biomaterials 2006, 27, 6102–6110. [Google Scholar] [CrossRef] [PubMed]
- Kheirallah, M.; Almeshaly, H. Simvastatin, dosage and delivery system for supporting bone regeneration, an update review. J. Oral Maxillofac. Sur. Med. Pathol. 2015. Available online: http://www.sciencedirect.com/science/article/pii/S2212555815002306 (accessed on 30 March 2016).
- Bifulco, M. Debate on adverse effects of statins. Eur. J. Intern. Med. 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.; Dallongeville, J.; Sabouret, P.; Bruckert, E. Discontinuation of statin therapy due to muscular side effects: A survey in real life. Nutr. Metab. Cardiovasc. 2013, 23, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Guo, Z.; Ma, Q.; Chen, Z.; Liu, Z.; Jia, H.; Dang, G. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem. Biophys. Res. Commun. 2003, 308, 458–462. [Google Scholar] [CrossRef]
- Yan, Q.; Xiao, L.Q.; Tan, L.; Sun, W.; Wu, T.; Chen, L.W.; Mei, Y.; Shi, B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: In vitro and in vivo characteristics. J. Biomed. Mater. Res. A 2015, 103, 3580–3589. [Google Scholar] [CrossRef] [PubMed]
- Tanigo, T.; Takaoka, R.; Tabata, Y. Sustained release of water-insoluble simvastatin from biodegradable hydrogel augments bone regeneration. J. Control. Release 2010, 143, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Sukul, M.; Min, Y.-K.; Lee, S.-Y.; Lee, B.-T. Osteogenic potential of simvastatin loaded gelatin-nanofibrillar cellulose-β tricalcium phosphate hydrogel scaffold in critical-sized rat calvarial defect. Eur. Polym. J. 2015, 73, 308–323. [Google Scholar] [CrossRef]
- Lee, S.S.; Hsu, E.L.; Mendoza, M.; Ghodasra, J.; Nickoli, M.S.; Ashtekar, A.; Polavarapu, M.; Babu, J.; Riaz, R.M.; Nicolas, J.D.; et al. Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv. Healthc. Mater. 2015, 4, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; Mehta, M.; Duda, G.N.; Heilshorn, S.C.; Mooney, D.J. Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules 2014, 15, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, G.; Rai, B.; Lim, Z.X.H.; Hui, J.H.; Stein, G.S.; van Wijnen, A.J.; Nurcombe, V.; Prestwich, G.D.; Cool, S.M. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 2012, 33, 6113–6122. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.I.; Ahn, K.M.; Jeon, S.H.; Lee, S.Y.; Lee, J.H.; Tae, G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J. Control. Release 2007, 121, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-B.; Choi, H.; Koh, J.-T.; Song, S.-C. Sustained BMP-2 delivery and injectable bone regeneration using thermosensitive polymeric nanoparticle hydrogel bearing dual interactions with BMP-2. J. Control. Release 2015, 209, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Dyondi, D.; Webster, T.J.; Banerjee, R. A nanoparticulate injectable hydrogel as a tissue engineering scaffold for multiple growth factor delivery for bone regeneration. Int. J. Nanomed. 2013, 8, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barrena, E.; Rosset, P.; Müller, I.; Giordano, R.; Bunu, C.; Layrolle, P.; Konttinen, Y.T.; Luyten, F.P. Bone regeneration: Stem cell therapies and clinical studies in orthopaedics and traumatology. J. Cell Mol. Med. 2011, 15, 1266–1286. [Google Scholar] [CrossRef] [PubMed]
- Zur Nieden, N.I.; Turgman, C.C.; Lang, X.; Larsen, J.M.; Granelli, J.; Hwang, Y.-J.; Lyubovitsky, J.G. Fluorescent hydrogels for embryoid body formation and osteogenic differentiation of embryonic stem cells. ACS Appl. Mater. Interfaces 2015, 7, 10599–10605. [Google Scholar] [CrossRef] [PubMed]
- Seebach, E.; Freischmidt, H.; Holschbach, J.; Fellenberg, J.; Richter, W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014, 10, 4730–4741. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D.; Marecak, D.M.; Marecek, J.F.; Vercruysse, K.P.; Ziebell, M.R. Controlled chemical modification of hyaluronic acid: Synthesis, applications, and biodegradation of hydrazide derivatives. J. Control. Release 1998, 53, 93–103. [Google Scholar] [CrossRef]
- Park, Y.D.; Tirelli, N.; Hubbell, J.A. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials 2003, 24, 893–900. [Google Scholar] [CrossRef]
- Gao, N.; Lü, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Feng, C.; Liu, M. Injectable shell-crosslinked F127 micelle/hydrogel composites with ph and redox sensitivity for combined release of anticancer drugs. Chem. Eng. J. 2016, 287, 20–29. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, U.-J.; Vunjak-Novakovic, G.; Min, B.-H.; Kaplan, D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials 2005, 26, 4442–4452. [Google Scholar] [CrossRef] [PubMed]
- Levi, B.; Longaker, M.T. Coincise review: Adipose derived stromal cells for skeletal regenerative medicine. Stem Cells 2011, 29, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Heymer, A.; Berner, A.; Eulert, J.; Nöth, U. Fabrication of polycaprolactone collagen hydrogel constructs seeded with mesenchymal stem cells for bone regeneration. Biomed. Mater. 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Nuttelman, C.R.; Tripodi, M.C.; Anseth, K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in peg hydrogels. J. Biomed. Mater. Res. A 2004, 68A, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and peg hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, I.S.; Cho, T.H.; Lee, K.B.; Hwang, S.J.; Tae, G.; Noh, I.; Lee, S.H.; Park, Y.; Sun, K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials 2007, 28, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Manzotti, S.; Bevilacqua, C.; Orciani, M.; Di Primio, R.; Mattioli-Belmonte, M. Adult mesenchymal stem cells for bone and cartilage engineering: Effect of scaffold materials. Eur. J. Histochem. 2008, 52, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.; Yaszemski, M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A 2010, 94A, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Steck, E.; Fischer, J.; Lorenz, H.; Gotterbarm, T.; Jung, M.; Richter, W. Mesenchymal stem cell differentiation in an experimental cartilage defect: Restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009, 18, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Salamon, A.; van Vlierberghe, S.; van Nieuwenhove, I.; Baudisch, F.; Graulus, G.-J.; Benecke, V.; Alberti, K.; Neumann, H.-G.; Rychly, J.; Martins, J.; et al. Gelatin-based hydrogels promote chondrogenic differentiation of human adipose tissue-derived mesenchymal stem cells in vitro. Materials 2014, 7, 1342–1359. [Google Scholar] [CrossRef]
- Barba, M.; Cicione, C.; Bernardini, C.; Michetti, F.; Lattanzi, W. Adipose-derived mesenchymal cells for bone regereneration: State of the art. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Banyard, D.A.; Salibian, A.A.; Widgerow, A.D.; Evans, G.R.D. Implications for human adipose-derived stem cells in plastic surgery. J. Cell Mol. Med. 2015, 19, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Büchler, C. Concise review: Adipose tissue-derived stromal cells—Basic and clinical implications for novel cell-based therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle-hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, J.; Yi, C.; Yang, M. The effects of gold nanoparticles on the proliferation, differentiation, and mineralization function of MC3T3-E1 cells in vitro. Chin. Sci. Bull. 2010, 55, 1013–1019. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. Appmaterinterfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.W.; Prasai, D.; Rath, R.; Balikov, D.A.; Bae, H.; Bolotin, K.I.; Sung, H.-J. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2013, 5, 4171–4176. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, L.; Cheng, C.; Wu, Y.; Ma, Z.-F.; Li, D. Ordered gelation of chemically converted graphene for next-generation electroconductive hydrogel films. Angew. Chem. Int. Ed. 2011, 50, 7325–7328. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.Q.; Lu, J.Y.; Cao, C.H.; Luo, D.; Fu, Y.X.; He, Y.S.; Zou, D.R. Induction of osteogenic differentiation of human adipose-derived stem cells by a novel self-supporting graphene hydrogel film and the possible underlying mechanism. ACS Appl. Mater. Interfaces 2015, 7, 20245–20254. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-T.; Chou, W.-L.; Chou, C.-M. Osteoblastic differentiation of stem cells from human exfoliated deciduous teeth induced by thermosensitive hydrogels with strontium phosphate. Mater. Sci. Eng. C 2015, 52, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Sun, Z.; Wang, X.; Yang, H.; Shi, S.; Wang, S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010, 19, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Su, W.T.; Wu, P.S.; Huang, T.Y. Osteogenic differentiation of stem cells from human exfoliated deciduous teeth on poly(epsilon-caprolactone) nanofibers containing strontium phosphate. Mater. Sci. Eng. C 2015, 46, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Luo, E.; Feng, G.; Zhu, S.S.; Li, J.H.; Hu, J. Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos. Int. 2010, 21, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Fong, C.-Y.; Chan, W.-K.; Wong, P.-C.; Bongso, A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002, 20, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chung, E.H.; Rodriguez, R.T.; Firpo, M.T.; Healy, K.E. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J. Biomed. Mater. Res. A 2006, 79, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, T.E.; Levenstein, M.E.; Jones, J.M.; Berggren, W.T.; Mitchen, E.R.; Frane, J.L.; Crandall, L.J.; Daigh, C.A.; Conard, K.R.; Piekarczyk, M.S.; et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006, 24, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, T.P.; Langer, R.; Ferreira, L.S. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat. Methods 2011, 8, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Narayanan, K.; Lim, S.-X.; Gao, S.; Leong, M.F.; Wan, A.C.A. A 3D microfibrous scaffold for long-term human pluripotent stem cell self-renewal under chemically defined conditions. Biomaterials 2012, 33, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Gerecht, S.; Burdick, J.A.; Ferreira, L.S.; Townsend, S.A.; Langer, R.; Vunjak-Novakovic, G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11298–11303. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Leung, M.; Hopper, R.; Ellenbogen, R.; Zhang, M. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials 2010, 31, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Varghese, S.; Elisseeff, J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
| Scaffold Composite Components Organic Inorganic | Ratio | Compressive Strength (MPa) | Compression Modulus (KPa) | Elastic Modulus (MPa) | Reference | |
|---|---|---|---|---|---|---|
| Poly(acrylamide) | nHA | 85:15 | 35.8 | - | - | [52] |
| Silk fibroin | nHA | 85:15 | - | 109.8 | - | [55] |
| Agarose | nHA | 65:35 | 390 | - | 1104.4 | [57] |
| Oxidized alginate-gelatin-BCP | Spherical HA | 65:35 | - | 2.45 dry 0.05 wet | - | [58] |
| Chitin | nHA | 75:25 | 0.3 | - | 0.3 | [60] |
| Gellam gum | Bioglass | 50:50 | - | - | 1.2 | [71] |
| PEG | Bioglass | 80:20 | 2.5 | - | 8 | [72] |
| Chitosan/silica | f-CNTs | 98:2 | - | - | 552 | [77] |
| PPF | Nano Carbon | 0.2 | - | 2061 | - | [80] |
| Cortical bone | - | 130–180 | - | 12,000–18,000 | [82] | |
| Trabecular bone | - | 4–12 | - | 100–500 | ||
| Growth Factor | Mechanism of Action | Limitations |
|---|---|---|
| BMP-2 (Bone morphogenic protein) | Induces osteoblasts proliferation and mesenchymal cells (MSCs) differentiation Induces VEGF-A secretion therefore has a role in angiogenesis 1 | Needs to be delivered in a controlled manner Variable outcomes have been seen in humans Limited capacity to initiate vascular proliferation |
| VEGF (Vascular endothelial growth factor) | Induces endothelial cells mitogenesis Attracts MSCs and induces their differentiation | Delivered alone they lead to the inability to produce organized bone regeneration |
| PDGF (Platelet derived growth factor) | Attracts cells that stabilise growing vasculature Recruits MSCs Upregulates VEGF production | |
| FGF (Fibrobast growth factor) | Involved in the formation of new capillaries | |
| IGF (insulin like growth factor) | Involved in adult neo angiogenesis |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite Hydrogels for Bone Regeneration. Materials 2016, 9, 267. https://doi.org/10.3390/ma9040267
Tozzi G, De Mori A, Oliveira A, Roldo M. Composite Hydrogels for Bone Regeneration. Materials. 2016; 9(4):267. https://doi.org/10.3390/ma9040267
Chicago/Turabian StyleTozzi, Gianluca, Arianna De Mori, Antero Oliveira, and Marta Roldo. 2016. "Composite Hydrogels for Bone Regeneration" Materials 9, no. 4: 267. https://doi.org/10.3390/ma9040267
APA StyleTozzi, G., De Mori, A., Oliveira, A., & Roldo, M. (2016). Composite Hydrogels for Bone Regeneration. Materials, 9(4), 267. https://doi.org/10.3390/ma9040267







