The Influence of Crosslink Density on the Failure Behavior in Amorphous Polymers by Molecular Dynamics Simulations
Abstract
:1. Introduction
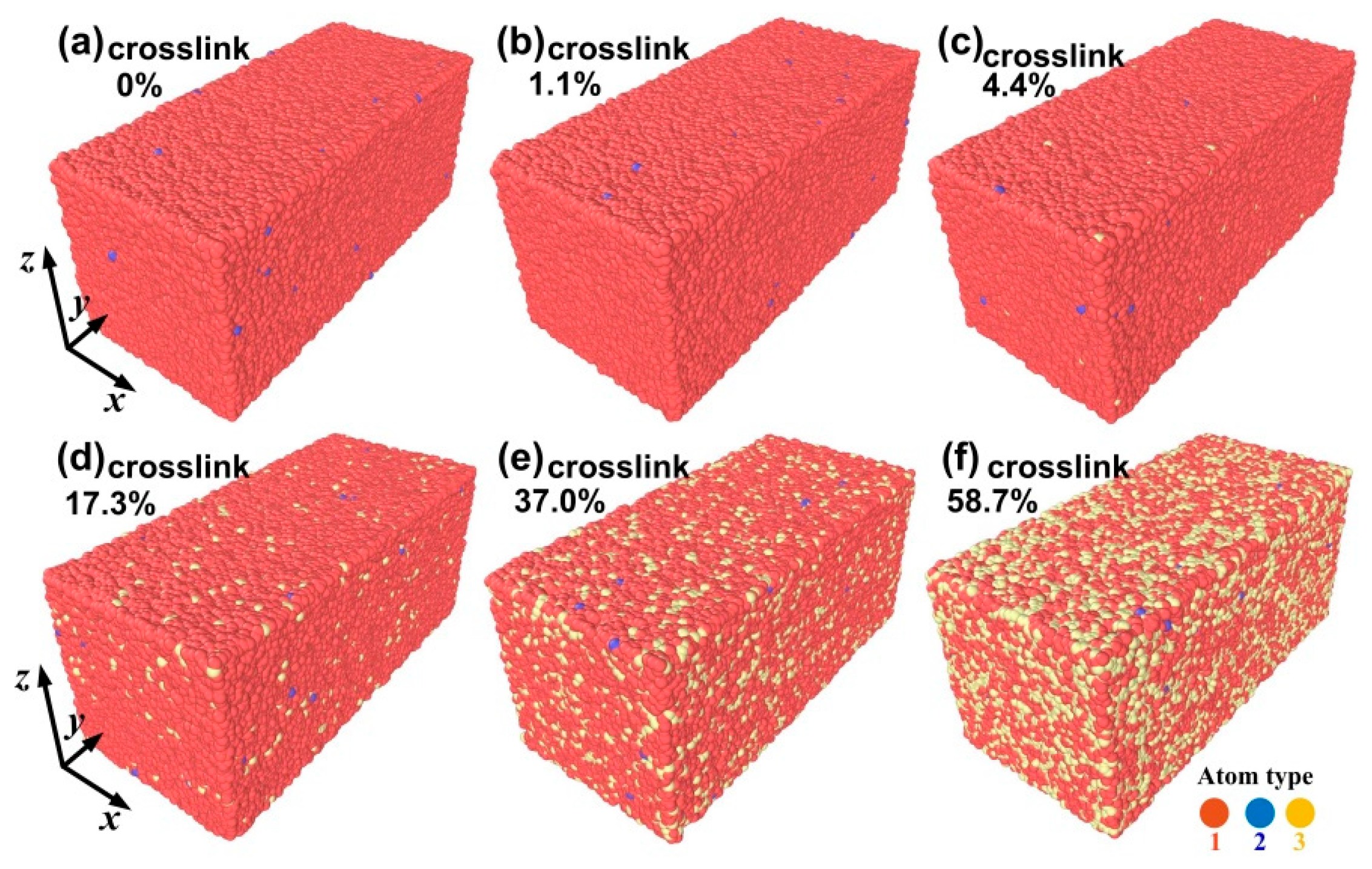
2. Simulations Details
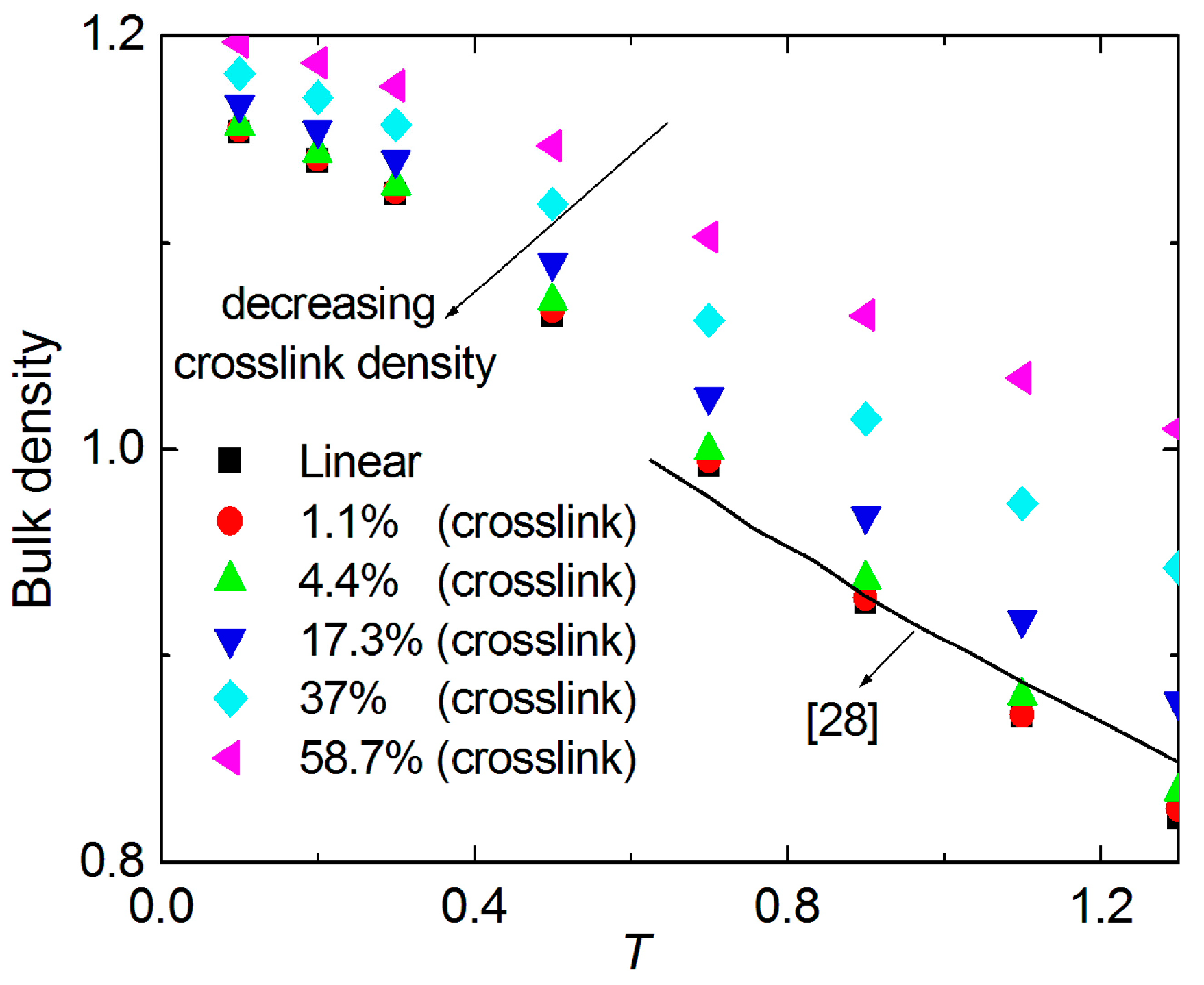
3. Results and Discussion
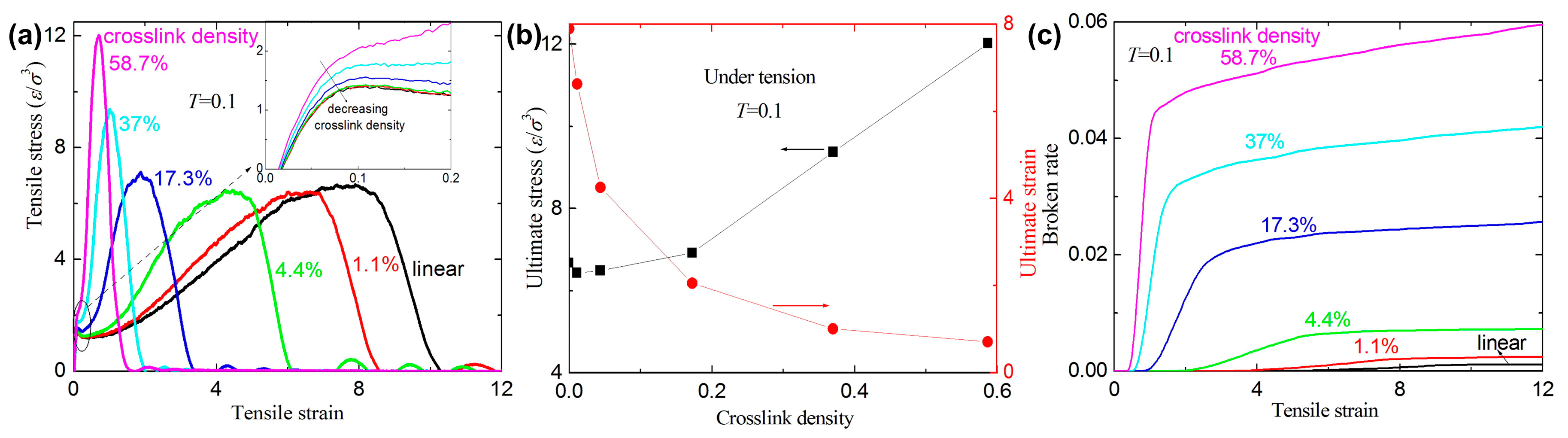
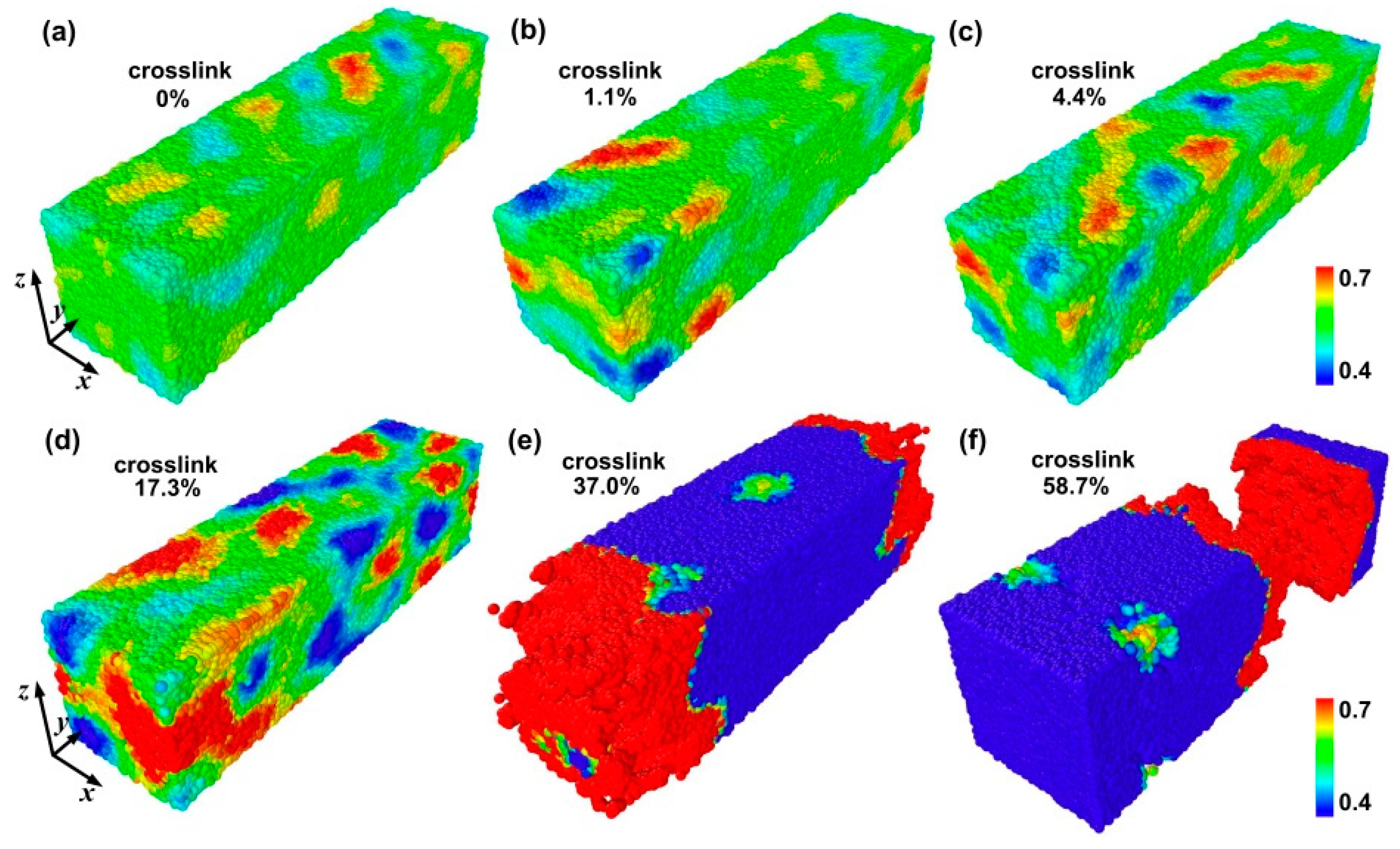
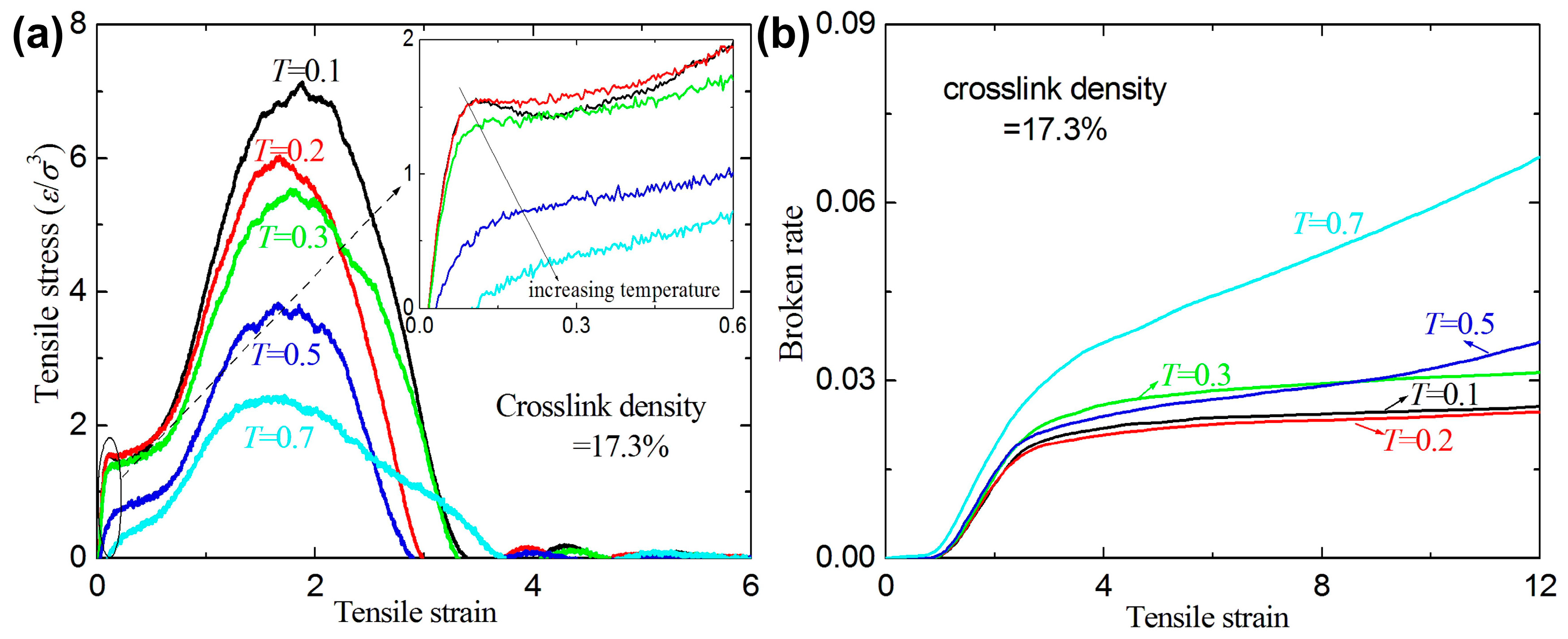
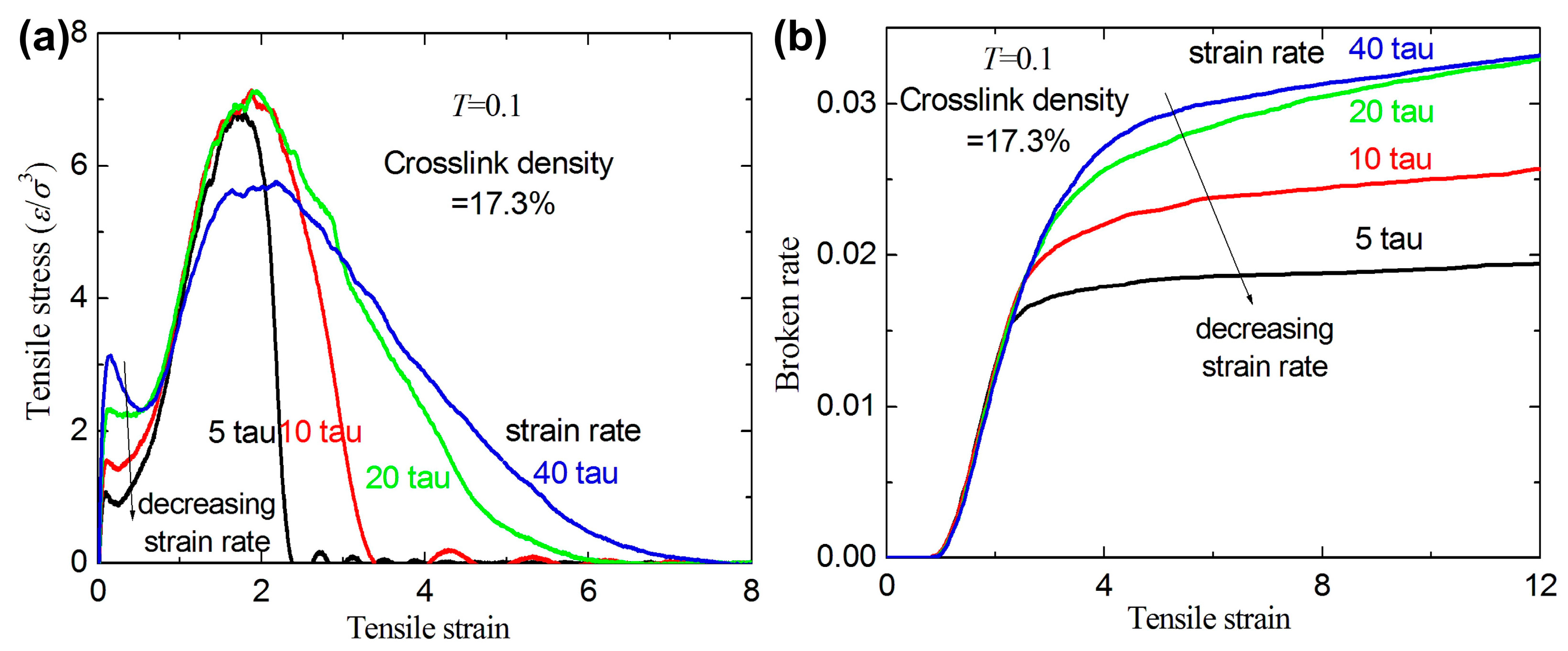
3.1. Failure Behavior under Uniaxial Tension
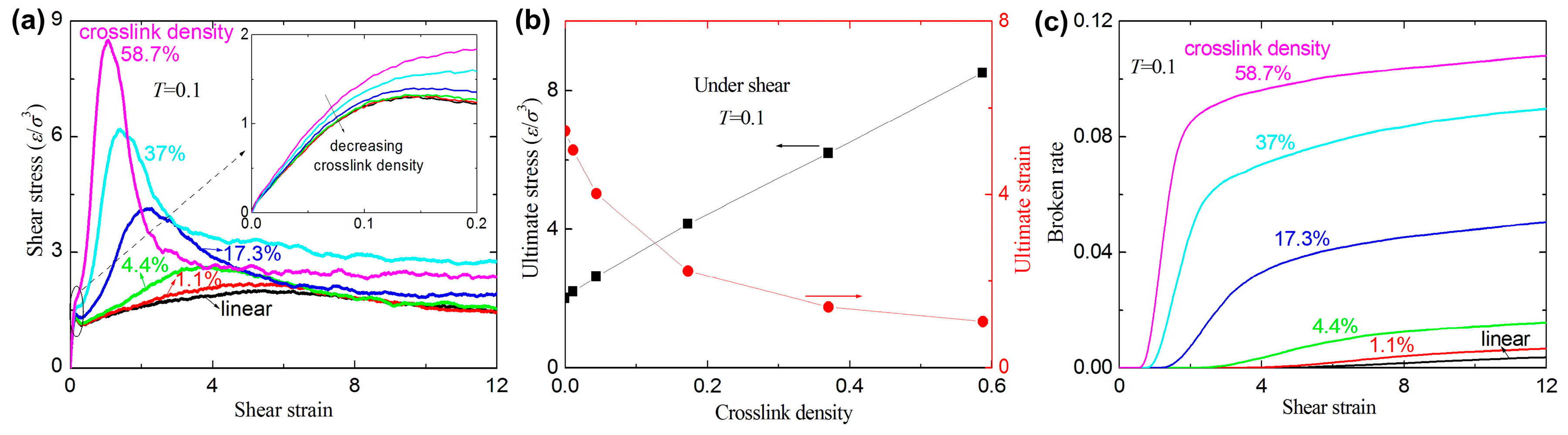
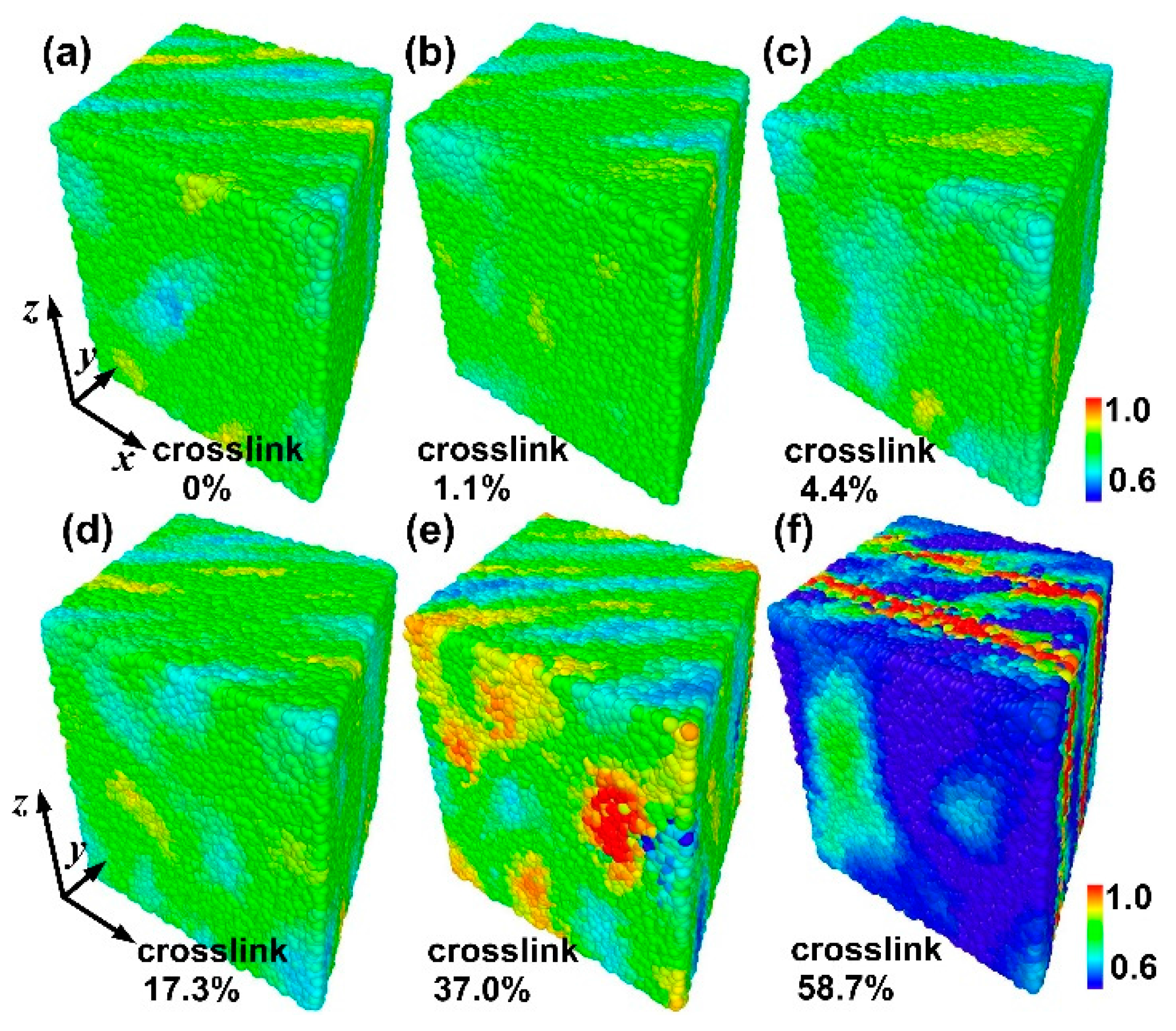
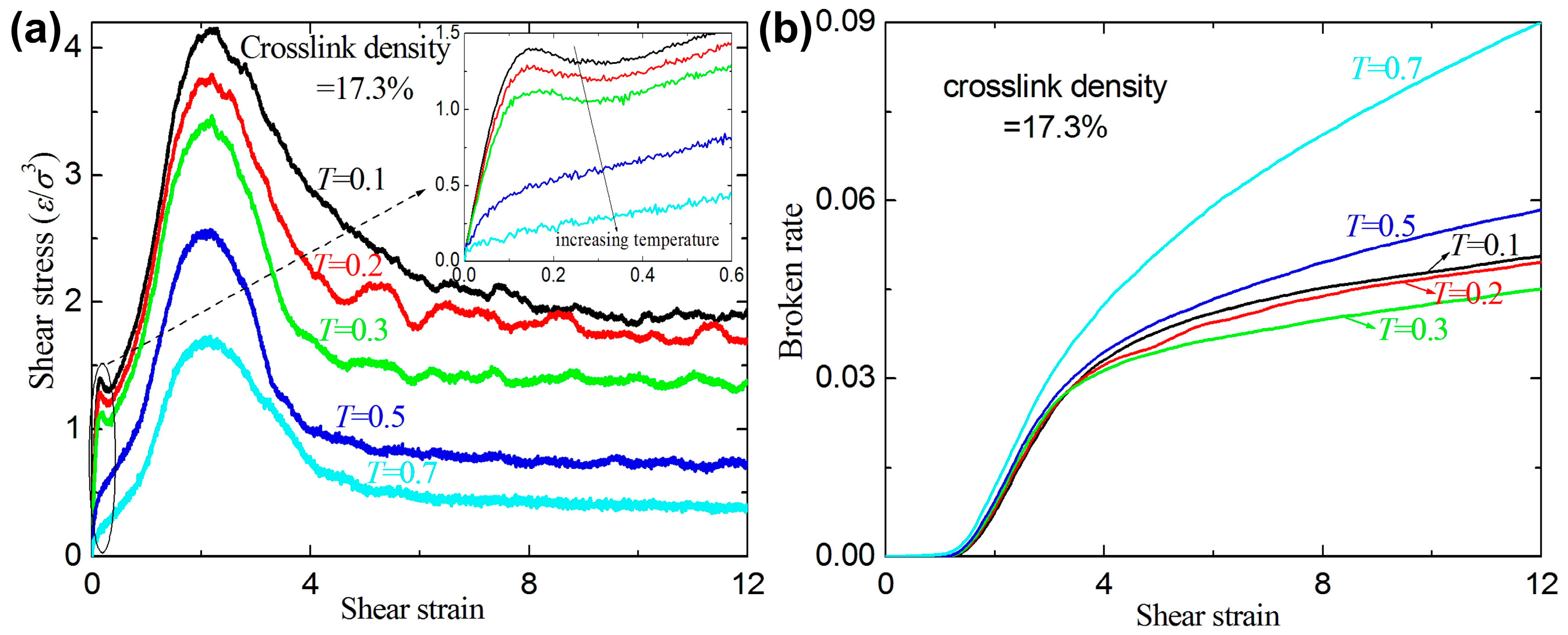
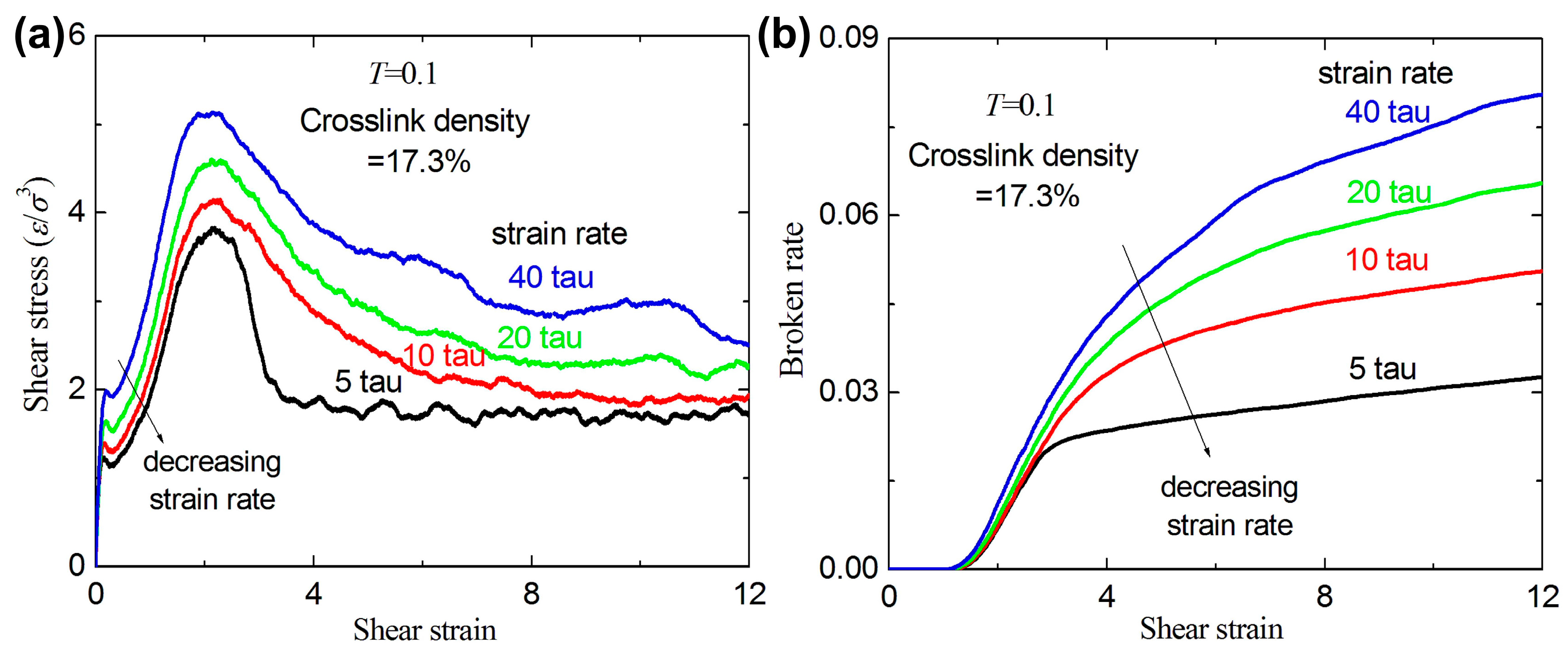
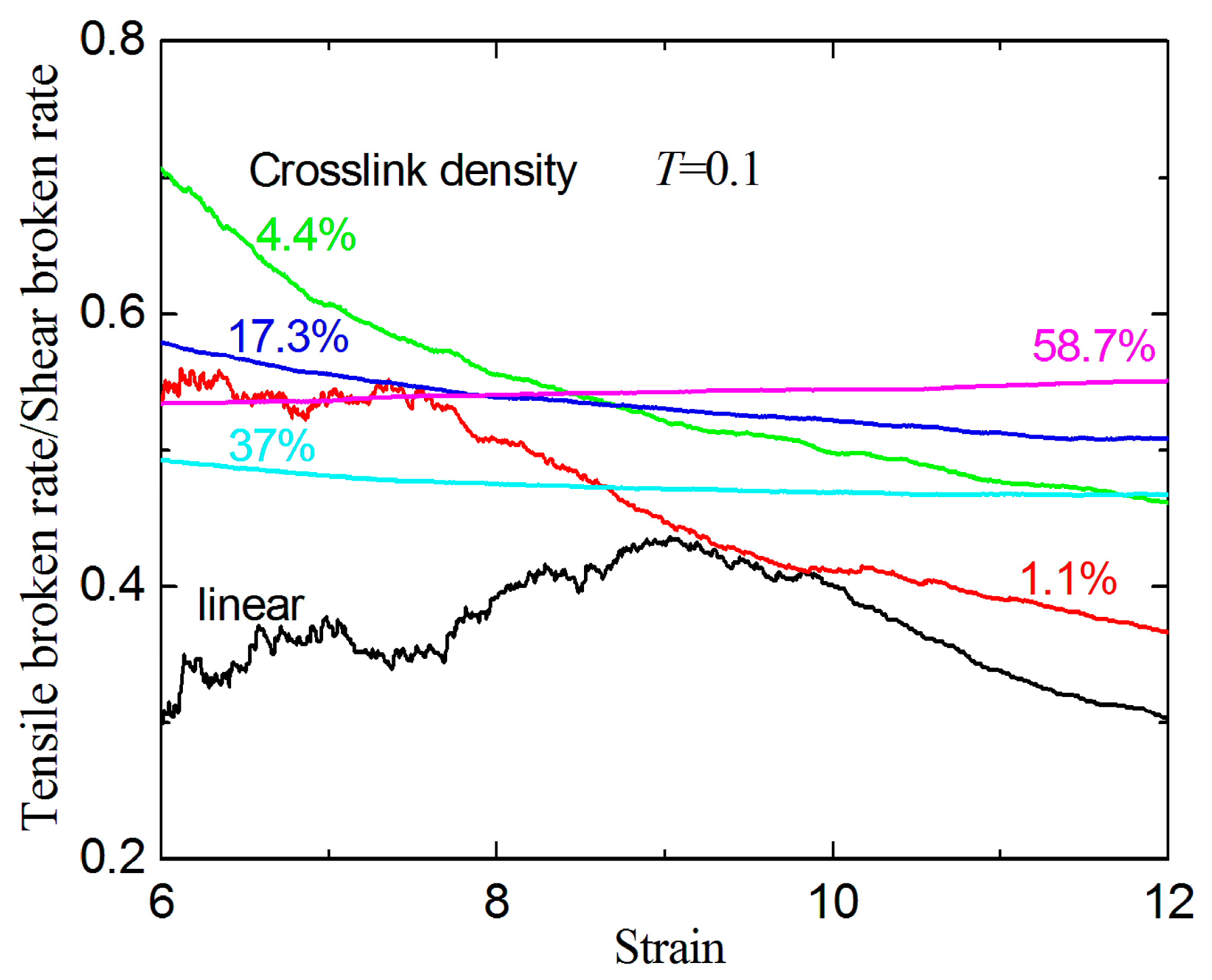
3.2. Failure Behavior under Shear
4. Conclusions
- (1)
- The crosslink density strongly affects the mechanical response of the amorphous polymers. The ultimate stresses and the broken ratios increase with increasing crosslink density under tension and shear, while the ultimate strains decrease with increasing crosslink density.
- (2)
- For a given crosslink density and temperature, the broken ratios and ultimate stresses increase with increasing strain rate.
- (3)
- For a large given strain, the broken ratios under uniaxial tension are always smaller than those under shear.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boyd, R.H.; Gee, R.H.; Han, J.; Jin, Y. Conformational dynamics in bulk polyethylene: A molecular dynamics simulation study. J. Chem. Phys. 1994, 101, 788–797. [Google Scholar] [CrossRef]
- Pant, P.V.K.; Han, J.; Smith, G.D.; Boyd, R.H. A molecular dynamics simulation of polyethylene. J. Chem. Phys. 1993, 99, 597–604. [Google Scholar] [CrossRef]
- Shepherd, J.E. Multiscale Modeling of the Deformation of Semi-Crystalline Polymers. Ph.D Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2006. [Google Scholar]
- Lu, J.; Wool, R.P. Sheet molding compound resins from soybean oil: Thickening behavior and mechanical properties. Polym. Eng. Sci. 2007, 47, 1469–1479. [Google Scholar] [CrossRef]
- Thompson, J.I.; Czernuszka, J.T. The Effect of Two Types of Cross-Linking on Some Mechanical Properties of Collagen. Biomed. Mater. Eng. 1995, 5, 37–48. [Google Scholar] [PubMed]
- Sun, J.Y.; Zhao, X.H.; IlleperumaW, R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z.G. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Liu, Y.; Ding, Y.; Zheng, Q.; Xu, Z. Mechanics of carbon nanotube networks: Microstructural evolution and optimal design. Soft Matter. 2011, 7, 10039–10047. [Google Scholar] [CrossRef]
- Wang, C.; Xie, B.; Liu, Y.; Xu, Z. Mechanotunable Microstructures of Carbon Nanotube Networks. ACS Macro Lett. 2012, 1, 1176–1179. [Google Scholar] [CrossRef]
- Carnford, S.W.; Buehler, M.J. In silico assembly and nanomechanical characterization of carbon nanotube buckypaper. Nanotechnology 2010, 21, 265706. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, J.L.; Wu, W.L. Influence of Cross-Link Density on the Thermal Properties of Thin Polymer Network Films. Langmuir 2003, 19, 4863–4865. [Google Scholar] [CrossRef]
- Zhao, J.; Nagao, S.; Odegard, G.M.; Zhang, Z.; Kristiansen, H.; He, J. Size-dependent mechanical behavior of nanoscale polymer particles through coarse-grained molecular dynamics simulation. Nanoscale Res. Lett. 2013, 8, 541. [Google Scholar] [CrossRef] [PubMed]
- Prucker, O.; Christian, S.; Bock, H.; Rühe, J.; Frank, C.W.; Knoll, W. On the glass transition in ultrathin polymer films of different molecular architecture. Macromol. Chem. Phys. 1998, 199, 1435–1444. [Google Scholar] [CrossRef]
- Rottach, D.R.; Curro, J.G.; Budzien, J.; Grest, G.S.; Svaneborg, C.; Everaers, R. Permanent Set of Cross-Linking Networks: Comparison of Theory with Molecular Dynamics Simulations. Macromolecules 2007, 40, 131–139. [Google Scholar] [CrossRef]
- Yang, L.; Tan, C.H.; Hsieh, M.J.; Wang, J.; Duan, Y.; Cieplak, P.; Caldwell, J.; Kollman, P.A.; Luo, R. New-generation amber united-atom force field. J. Phys. Chem. B 2006, 110, 13166–13176. [Google Scholar] [CrossRef] [PubMed]
- Hoy, R.S.; Robbins, M.O. Strain hardening of polymer glasses: Entanglements, energetics, and plasticity. Phys. Rev. E 2008, 77, 031801. [Google Scholar] [CrossRef] [PubMed]
- Hoy, R.S.; Robbins, M.O. Strain Hardening in Polymer Glasses: Limitations of Network Models. Phys. Rev. Lett. 2007, 99, 117801. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, J.; Odegard, G.M.; Zhang, Z. Effect of chain architecture on the compression behavior of nanoscale polyethylene particles. Nanoscale Res. Lett. 2013, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Zhang, Z.L.; Kristiansen, H.; Redford, K.; Fonnum, G.; Modahl, G.I. Crosslinking effect on the deformation and fracture of monodisperse polystyrene-co-divinylbenzene particles. Express Polym. Lett. 2013, 7, 365–374. [Google Scholar] [CrossRef]
- Cukierman, S.; Halary, J.L.; Monnerie, L. Dynamic mechanical response of model epoxy networks in the glassy state. Polym. Eng. Sci. 1991, 31, 1476–1482. [Google Scholar] [CrossRef]
- Heux, L.; Halary, J.L.; Lauprêtre, F.; Monnerie, L. Dynamic mechanical and 13C n.m.r. investigations of molecular motions involved in the β relaxation of epoxy networks based on DGEBA and aliphatic amines. Polymer 1997, 38, 1767–1778. [Google Scholar] [CrossRef]
- Zhao, J.; Nagao, S.; Zhang, Z. Thermomechanical properties dependence on chain length in bulk polyethylene: Coarse-grained molecular dynamics simulations. J. Mater. Res. 2010, 25, 537–544. [Google Scholar] [CrossRef]
- Faulon, J.L. Stochastic generator of chemical structure. 4. Building polymeric systems with specified properties. J. Comput. Chem. 2001, 22, 580–590. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, J.W.; Wei, N.; Zhang, Y.; Rabczuk, T. Thermal conductivity dependence on chain length in amorphous polymers. J. Appl. Phys. 2013, 113, 184304. [Google Scholar] [CrossRef]
- Capaldi, F.M.; Boyce, M.C.; Rutledge, G.C. Molecular response of a glassy polymer to active deformation. Polymer 2004, 45, 1391–1399. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, L.; Rabczuk, T. The tensile and shear failure behavior dependence on chain length and temperature in amorphous polymers. Comput. Mater. Sci. 2014, 96, 567–572. [Google Scholar] [CrossRef]
- Stevens, M.J. Interfacial Fracture between Highly Cross-Linked Polymer Networks and a Solid Surface: Effect of Interfacial Bond Density. Macomolecules 2001, 34, 2710–2718. [Google Scholar] [CrossRef]
- Kremer, K.; Grest, G.S. Dynamics of entangled linear polymer melts: A molecular-dynamics simulation. J. Chem. Phys. 1990, 92, 5057–5086. [Google Scholar] [CrossRef]
- Bennemann, C.; Paul, W.; Binder, K.; Dünweg, B. Molecular-dynamics simulations of the thermal glass transition in polymer melts: α-relaxation behavior. Phys. Rev. E 1998, 57, 843–851. [Google Scholar] [CrossRef]
- Panico, M.; Narayanan, S.; Brinson, L.C. Simulations of tensile failure in glassy polymers: effect of cross-link density. Model. Simul. Mater. Sci. Eng. 2010, 18, 055005. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yu, P.; Dong, S. The Influence of Crosslink Density on the Failure Behavior in Amorphous Polymers by Molecular Dynamics Simulations. Materials 2016, 9, 234. https://doi.org/10.3390/ma9040234
Zhao J, Yu P, Dong S. The Influence of Crosslink Density on the Failure Behavior in Amorphous Polymers by Molecular Dynamics Simulations. Materials. 2016; 9(4):234. https://doi.org/10.3390/ma9040234
Chicago/Turabian StyleZhao, Junhua, Peishi Yu, and Shuhong Dong. 2016. "The Influence of Crosslink Density on the Failure Behavior in Amorphous Polymers by Molecular Dynamics Simulations" Materials 9, no. 4: 234. https://doi.org/10.3390/ma9040234
APA StyleZhao, J., Yu, P., & Dong, S. (2016). The Influence of Crosslink Density on the Failure Behavior in Amorphous Polymers by Molecular Dynamics Simulations. Materials, 9(4), 234. https://doi.org/10.3390/ma9040234




